Advances in Animal and Veterinary Sciences
Research Article
Efficacy of Aerosol Vaccination of Adjuvantd Pigeon Pox Vaccine in Pigeon
Ayatollah I Bassiouny, Kafafy MH, Heba M El Naggar*
Veterinary Serum and Vaccine Research Institute (VSVRI), Agriculture Research Center (ARC),El-Seka-Beda Street, Abbasia, 131, Cairo, Egypt.
Abstract | The current study was conducted to evaluate the adjuvanted aerosol vaccination of pigeon pox vaccine (PPV) with Gel 01 in comparison with feather follicle non adjuvanted route of vaccination. In order to determine the best aerosol field dose of PPV, challenge was done for 3 groups of pigeon (group no 2, 3 and 4) aerosolly vaccinated with non adjuvanted prepared live attenuated Vero cell tissue culture adapted PPV different field dose ( Log 10 2,5, 3.5, and 4.5 TCID50) the protection percent were 75%, 80% and 80% respectively as field dose of 10 3.5TCID50 was the field dose of choice to be used with different concentrations of Gel 01 adjuvant. Four groups of pigeon were vaccinated with PPV; group (1) vaccinated via feather follicle route in addition to other 3 groups of pigeon ( group 5, 6 and 7) vaccinated via aerosol route with different Gel 01 adjuvant ( 5%, 10% and 15% respectively ). Serum samples were collected weekly for 6 weeks. The highest level of induced antibodies detected by virus neutralization test (VNT) and ELISA were (3.0, 2.0, 2.5 and 2.5 NI respectively) and ( 1.9, 1.5, 1.8 and 1.7 S/P respectively) at the 3rd week post vaccination. The level of antibodies reduced dramatically in pigeon groups vaccinated via aerosol route group (5, 6 and 7) reached (1.5, 1.75 and 2.0 NI respectively ) and (1.2 , 1.3 and 1.4 S/P respectively ) at 6th week on contrast to group (1) vaccinated with feather follicle route has a slight decrease in antibody level reached 2.75 NI and 1.8 S/P at the 6th week post vaccination. Negative result for VNT and ELISA were found in control non vaccinated group all over the period of study. All vaccinated four pigeon groups and non vaccinated control group were challenged by inoculation of the virulent PPV after 3 weeks from vaccination showing protection percent of 95%, in group (1) vaccinated by feather follicle while it was 85%, 90%and 90% in groups vaccinated via aerosol route with different Gel 01 percent ( 5%, 10% and 15% respectively ) showing that 10 % Gel 01 is the concentration of choice to be used with PPV via aerosol route. On contrast, the control non- vaccinated pigeons showed 0% of protection. This study recorded the efficacy of aerosol vaccination of tissue culture adjuvanted PPV in pigeon using Gel 01 comparison to usual routine vaccination with unadjuvanted PPV by feather follicle route to establish a more safe route of vaccination in valuable oriental and racing pigeons and easier route of vaccination of large pigeon flock with recommendation of booster vaccination after 3 weeks in order to maintain a high level of protection and more duration of immunity.
Keywords | Pigeon Pox, Aerosol, Gel 01, ELISA , Vaccine.
Received | August 29, 2021; Accepted | September 05, 2021; Published | November 01, 2021
*Correspondence | Heba M El Naggar, Veterinary Serum and Vaccine Research Institute (VSVRI), Agriculture Research Center (ARC),El-Seka-Beda Street, Abbasia, 131, Cairo, Egypt; Email: [email protected]
Citation | Bassiouny A, Kafafy MH, El Naggar HM (2021). Efficacy of aerosol vaccination of adjuvantd pigeon pox vaccine in pigeon. Adv. Anim. Vet. Sci. 9(12): 2116-2123.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.12.2116.2123
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 El Naggar et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Pigeon pox is a serious virus disease in pigeons especially in young pigeon as causing high percentage of mortality due to (diphtheritic form) fibrino-necrotic lesion in the mucous membrane of the upper respiratory tract and (cutaneous form) the development of discrete proliferative nodular skin lesions (Hemanth et al., 2014).
Pigeon pox disease is caused by pigeon pox virus (PPV) within family Poxviridae subfamily Chordopoxvirinaeand genus Avipoxvirus (Andraw, 2012). The economic importance of the disease caused by the losses of infected pigeons, also due to cutaneous diphtheritic or systemic form as the lesions around the eyes and mouth with ulcerated oral cavity making pigeons unable to drink or eat causing dehydration or starvation leading to death (Hemanth et al ., 2014).
Vaccination is the ideal method for protection of pigeons from pigeon pox infection PPV vaccine can be used against pigeon and fowl poxvirus infection and also (Simon and Morten, 2011).
In Egypt, first pigeon pox vaccine was prepared by adaptation of pigeon pox virus (Hungarian strain) on the chorioallantoic membrane (CAM) of 9-11 day-old embryonated chicken eggs (Helmy et al., 1967). But recently alternative PPV vaccine was prepared by using locally isolated strain attenuated, adapted and propagated on Vero cell line (Kafafy et al ., 2018).
The using of feather follicle method as a method for bird vaccination may have disadvantage as the post vaccinal effect and tick may reduce the values of some viable racing and demestic pigeons, in addition to the great efforts in manually bird vaccination (Gottstein et al., 2004). So the using of PPV vaccine can be given by aerosol route for protection against a pathogenic strain of virus given cutaneously (Mockett et al., 1990) especially its know that the common mode of infection for pigeon pox is via respiratory tract (OIE 2018). The antigen delivery to mucosa enhances local and systemic immune responses. So it provides an advantageous for vaccination on contrast to antigen injection (Kang et al ., 2004).
Vaccination via mucosa by spray is a rapid and preventive method of vaccination in outbreaks in endemic areas and it should be suitable for mass application even applied by spray or aerosole and effective after single application (de Geusa et al ., 2011; Shakya and Nandakumar ., 2012) mucosal vaccination through inhalation of antigen alone may cause a tolerance state by contacting to respiratory tract mucosa so using virus antigen causes low immunogenic response than using a polymer based adjuvant Montanide™ Gel 01 ST (Gel 01) (gel particles of sodium polyacrylate in water) which trigger the immune response of mucosal vaccination and overcome the problems of tolerance (Tseng et al., 2009).
Recently, Gel 01 polymer adjuvant is used to formulate mucosal bivalent inactivated Newcastle and Avian Influenza (H9N2) administrated through spray route produce protective humoral immune and cell-mediated response as well as challenge (Heba M E et al., 2017).
The present study aimed to prepare adjuvanted PPV vaccine with Gel 01 and evaluate the efficacy of aerosol vaccination with the prepared vaccine in pigeons.
Material and Methods
Viruses
Vaccinal strain: Pigeon pox vaccine was prepared on Vero cell line supplied by Pox Department, Veterinary Serum and Vaccine Research Institute (VSVRI). It was used for preparation of adjuvant PPV with Gel 01 with virus titer of 105.5 TCID50/ml.
Virulent pigeon pox: Virulent PPV was kindly supplied by Pox Vaccine Department, (VSVRI), Abbasia, Cairo. It had a titer of 105EID50 / ml and used for challenge of experimentally vaccinated pigeons.
Adjuvant: Montanide™ Gel 01 ready to dilute polymeric adjuvant was used. It contains gel particles of sodium polyacrylate in water.
Susceptible pigeons: One hundred and sixty (160)susceptible pigeons of 45 day - old were used in this study. These pigeons were divided as follows: Table (1).
Vaccination
Feather follicle vaccination: It was done for the attenuated tissue culture pigeon pox vaccine 10 5.5TCID50/ ml using a field dose of 10 2.5TCID50/ field dose using a feather follicle route acciording to OIE 2018 and Kaffafy et al. (2018).
Aerosol route: It was done for the attenuated tissue culture pigeon pox vaccine 10 5.5TCID50/ ml with different field doses 10 2.5,3.5 and 4.5TCID50/ field dose according to Mockett et al. (1990) as Twenty five pigeons were placed in a cardboard container (460 x 460 x 580 mm) and a fine aerosol created using a spray gun were used.
| Groups | No. of pigeons | Adjuvant | Route | TCID/dose |
| 1 | 20 | NA | by feather follicle |
102.5TCID50/ field dose |
| 2 | 20 | NA | by aerosol |
102.5TCID50/ field dose |
| 3 | 20 | NA | by aerosol |
103.5TCID50/ field dose |
| 4 | 20 | NA | by aerosol |
104.5TCID50/ field dose |
| 5 | 20 | Gel 01(5%) | by aerosol |
103.5TCID50/ field dose |
| 6 | 20 | Gel 01(10%) | by aerosol |
103.5TCID50/ field dose |
|
7 |
20 | Gel 01(15%) | by aerosol |
103.5TCID50/ field dose |
| 8 | 10 | NA | NA | NA |
| 9 | 10 | Gel 01(10%) | by aerosol | 3X |
Group (1): pigeons were vaccinated with PPV vaccine by feather follicle route.
Group (2): pigeons were vaccinated with PPV vaccine by aerosol without using of Gel 01 (using of 10 2.5TCID50/ field dose)
Group (3): pigeons were vaccinated with PPV vaccine by aerosol without using of Gel 01 (using of 10 3.5TCID50/ field dose)
Group (4): pigeons were vaccinated with PPV vaccine by aerosol without using Gel 01 (using of 10 4.5TCID50/ field dose)
Group (5): pigeons were vaccinated with PPV vaccine by aerosol with using Gel 01 (5 %).
Group (6): pigeons were vaccinated with PPV vaccine by aerosol with using Gel 01 ( 10%).
Group (7): pigeons were vaccinated with PPV vaccine by aerosol with using Gel 01 (15 %).
Group (8): were kept as negative control non vaccinated.
Group (9): Ten susceptible pigeons were vaccinated with 10x PPV vaccine by aerosol with using Gel 01 (10%). For safety test
Table 2: Mean NI, S/P ratio and challenge percent of vaccinated pigeons with different field doses via spray
| Group no | Mean NI after 21 days | Mean S/P ratio After 21 days | No. of birds showing lesion post challenge in 20 birds | Protection percent (%) | ||
| 5dpc | 7dpc | 10dpc | ||||
|
Group (2) 10 2.5TCID50/ field dose) |
1.5 | 1.1 |
1 |
22 | 22 | 75% |
|
Group (3) 10 3.5TCID50/ field dose |
1.75 | 1.3 |
0 |
2 | 2 | 80% |
|
Group (4) 10 4.5TCID50/ field dose) |
2.00 | 1.3 |
0 |
3 | 1 |
80% |
Titer of choice is group no (3) 10 3.5TCID50/ field dose
Neutralization Index (NI) calculated as Virus Titer (VT) minus Serum Virus Titer (SVT)
NI ≥ (1.5) consider as positive results
Serum
Pigeon pox hyper-immune sera: Hyper-immune serum against PPV was obtained from Pox Research Department VSVRI and used in Serum Neutralization Test as a positive control
Serum samples: Serum samples were collected from all birds weekly before and after vaccination and challenge for detection of antibody levels by Serum Neutralization Test.
Vero cell culture
Vero cell line were kindly supplied from Pox Research Department, VSVRI and used for SNT
Earle’s Minimum Essential Medium (MEM).
It was obtained from Sigma Chemical Company, USA and used as growth medium containing 10 % Newborn calf serum or as maintance medium containing 2% Newborn calf serum to use for SNT.
Evaluation of the adjuvanted Gel 01 pigeon pox vaccine (Quality control):
Sterility: It was carried out according to OIE (2018), where the mixed adjuvanted PPV vaccines were inoculated separately into tubes of nutrient agar, Sabouraud agar and thioglycolate medium and mycoplasma medium too.
Table 3: Results of NI and SP ratio of sera collected from vaccinated pigeons.
| Group (1) | Group (3) | Group (5) | Group (6) | Group (7) | Control Negative group(8) | |||||||
| Weeks | NI | S/P | NI | S/P | NI | S/P | NI | S/P | NI | S/P | NI | S/P |
|
Pre vaccination |
0.50 | 0.4 | 0.50 | 0.50 | 0.50 | 0.3 | 0.50 | 0.3 | 0.50 | 0.5 | 0.50 | 0.3 |
| 1WPV |
1.25± 0.64 |
0.7± 0.46 |
0.75± 0.37 |
0.6± 0.28 |
1.00± 0.34 |
0.7± 0.26 |
1.25± 0.43 |
0.8± 0.3 |
1.25± 0.4 |
0.6± 0.36 |
0.25 | 0.5 |
| 2WPV |
2.25± 0.64 |
1.6± 0.46 |
1.50± 0.37 |
1.0± 0.28 |
1.50± 0.34 |
1.1± 0.26 |
1.75± 0.43 |
1.3± 0.3 |
2.00± 0.4 |
1.4± 0.36 |
0.25 | 0.4 |
| 3WPV |
3.00± 0.64 |
1.9± 0.46 |
1.75± 0.37 |
1.4± 0.28 |
2.00± 0.34 |
1.5± 0.26 |
2.50± 0.43 |
1.8± 0.3 |
2.50± 0.4 |
1.7± 0.36 |
0.25 | 0.5 |
| 4WPV |
2.75± 0.64 |
1.7± 0.46 |
1.75± 0.37 |
1.3± 0.28 |
1.75± 0.34 |
1.2± 0.26 |
2.25± 0.43 |
1.5± 0.3 |
2.25± 0.4 |
1.4± 0.36 |
0.25 | 0.6 |
| 5WPV |
2.75± 0.64 |
1.9± 0.46 |
1.50± 0.37 |
1.1± 0.28 |
1.75± 0.34 |
1.1± 0.26 |
2.00± 0.43 |
1.4± 0.3 |
2.00± 0.4 |
1.3± 0.36 |
0.25 | 0.5 |
| 6WPV |
2.75± 0.64 |
1.8± 0.46 |
1.50± 0.37 |
1.1± 0.28 |
1.50± 0.34 |
1.2± 0.26 |
1.75± 0.43 |
1.3± 0.3 |
2.00± 0.4 |
1.4± 0.36 |
0.25 |
0.4 |
Group (1): susceptible pigeons were vaccinated with PPV vaccine feather follicle route
Group (3): susceptible pigeons were vaccinated with PPV vaccine by aerosol with 10 3.5TCID50/ field dose)
Group (5): susceptible pigeons were vaccinated with PPV vaccine by aerosol with using of Gel 01 (5 %).
Group (6): susceptible pigeons were vaccinated with PPV vaccine by aerosol with using of Gel 01 (10%).
Group (7): susceptible pigeons were vaccinated with PPV vaccine by aerosol with using of Gel 01 (15 %).
Table 4: Protection of vaccinated and control chicks against virulent fowl pox virus
| Challenge Time post vaccination | Birds group | No. of challenged pigeons/group | No. of birds showing lesion post challenge | Protection percent (%) | ||
| 5dpc | 7dpc | 10dpc | ||||
| 1 month | Group (1) | 20 | 0 | 0 | 1 | 95% |
| Group (3) | 20 | 0 | 2 | 2 | 80% | |
| Group (5) | 20 | 0 | 2 | 1 | 85% | |
| Group (6) | 20 | 5 | 1 | 1 | 90% | |
| Group (7) | 20 | 0 | 1 | 1 | 90% | |
| Group (8) | 10 | 2 | 3 | 5 | 0% | |
Group (1): twenty susceptible pigeons were vaccinated with PPV vaccine feather follicle route
Group (3): Twenty susceptible pigeons were vaccinated with PPV vaccine by aerosol without using of Gel 01
Group (5): Twenty susceptible pigeons were vaccinated with PPV vaccine by aerosol with using of Gel 01 (5 %).
Group (6): Twenty susceptible pigeons were vaccinated with PPV vaccine by aerosol with using of Gel 01 ( 10%).
Group (7): Twenty susceptible pigeons were vaccinated with PPV vaccine by aerosol with using of Gel 01 (15 %).
Group (8): Ten susceptible pigeons were kept as control non vaccinated.
Safety: Ten susceptible pigeons were vaccinated with 10 x field dose of adjuvanted Gel 01 PPV vaccine using aerosol mode according to OIE (2018).
Potency: Challenge test was applied by inoculation of the virulent pigeon pox virus by feather follicle route in vaccinated and susceptible control pigeons at 3 weeks post vaccination. All birds were subjected to daily observation of gross lesions and collection of serum samples according to OIE (2018).
Serological assay
Serum neutralization test (SNT): Serum neutralization test (SNT) was conducted according to Kaffafy et al. (2018) and Amina and Christine (2020) for the detection of antibody levels after vaccination of different group \and results calculated according to Reed and Muench (1938).
Indirect enzyme linked immunosorbent assay (ELISA): It was carried out according to Kaffay et al. (2015)
Results
Determintion of Ppv Vaccine Field Dose Via Aersol Spray
The results of mean NI and S/P ratio as well as challenge for defferent PPV vaccine field doses administrated via spray shown in Table (2) refer Protective level of antibody represented by NI and S/P ratio in all vaccinated groups while challenging the birds 3 weeks post vacciation with virulent PPV revealed that (5) birds showed post challenging pox lesions and the other 15 birds remained healthy without local or generalized post challenging lesion with protection percent of 75% in group (2) received 10 2.5TCID50/ field dose. While the protective percent against challenge were 80% in both group (3) received 10 3.5TCID50/ field dose and group (4) received 10 4.5TCID50/ field dose as (4) pigeon showed symptoms and the other 16 birds remained healthy without local or generalized post challenging. Table (2).
Quality control of the prepared PPV vaccine
Sterility test: Bacterial culture of prepared adjuvanted PPV vaccine with gel 01 were proved to be free from any bacterial and fungal contamination.
Safety test: Using of 10 times of the recommended dose of adjuvanted PPV vaccine with gel 01 10% proved that the vaccine was safe to be used in pigeons. Where the vaccinated birds did not show any undesirable symptoms refer to the adjuvant or the vaccine virus.
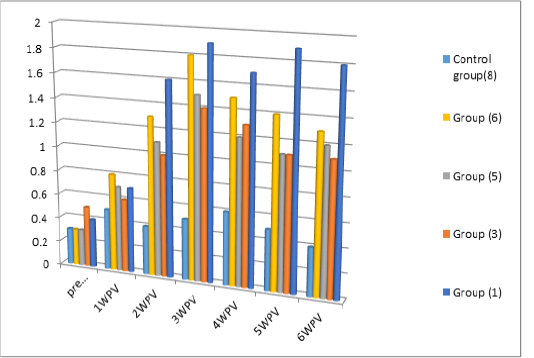
Serum Neutralization test and indirect ELISA: Serum neutralization test as well as Indirect ELISA were carried on the serum samples collected from vaccinated pigeons via spray route without and with different concentrations of adjuvanted Gel 01 (groups no 3, 5, 6 and 7) (0%, 5%, 10% and 15% respectively) PPV vaccines and feather follicle method (group no 1) . The results were expressed as neutralization index (NI) and (SP) ratio. The result which presented in Table (3) Figure (1) revealed that, the level of neutralizing antibodies in all vaccinated Pigeons serum samples as NI values were above 1.5 NI and S/P values above 1 after 2 weeks of vaccination and the highest antibody titers appeared at 3 weeks post vaccination in all groups but higher in group (1) as NI=3 and S/P =1.9 than other groups followed by decrease in antibody level after 3weeks post vaccination which is much more in groups (3, 5, 6 and 7) than group (1). Table (3) and Figure (1).
Result of challenge test in pigeons with virulent pigeon pox virus: Table (4) showed that after 3 weeks post vaccination the protection rate against PPV in group (1) vaccinated by feather follicle was 95% in compared with 80%, 85% , 90% and 90% in birds vaccinated via aerosol mode using different percent of Gel 01 0%, 5% , 10% and 15% respectively while in control group it was 0%. Table (4).
Discussion
Pigeon pox disease is one of most important viral disease affecting pigeons due to its economic impact on pigeon rearing systems as it causes high fatality rate especially in young birds due to respiratory diphteric form, (Soad et al., 2007; Abdallah and Ola, 2013; OIE, 2018) even it causes cutaneous distortions in valuable oriental racing pigeons and domestic pigeon when infected by skin form (Sudhakara and Sivajothi, 2018). Vaccination is the only effective mean for controlling and preventing pigeon pox disease in pigeon (Amina and Christine, 2020).
Through the present study an innovation of new vaccination route for PPV via aerosol was performed and evaluated through using of adjuvanted Gel 01 PPV aerosol vaccine in comparison to the traditional feather follicle vaccination route in pigeon regarding to that one of most important route for PPV infection is via respiratory inhalation mode (OIE, 2018) in order to establishment an effective rapid and less overwrought method for vaccination of large pigeon flock farm and a safe vaccination way without post vaccinal reaction in oriental viable high price racing pigeon.
Tissue culture propagated pigeon pox vaccine on Vero cell line with virus titer of10 5.5TCID50/ ml was used in this study for aerosol vaccination (Aboul Soud et al., 2018) and (Kaffay et al., 2018), In order to determine the effective field dose 3 susceptible pigeon groups (group 2. 3.and 4) received different field doses of PPV via aerosol without adjuvant (10 2.5TCID50, 10 3.5TCID50 and 10 4.5TCID50/ field dose) followed by antibody detection by (VNT and indirect ELISA) and challenging with virulent PPV at 21 post vaccination the result represented in Table (2) showed that group (3) received 10 35TCID50/ field dose is the field dose of choice with NI 1.75 of SP 1.3 with protection percent of 80% as group 2 showing lower NI 1.5, SP 1.1 and protection percent of 75% while group 4 received higher field dose showing same protection percent of 80% with non-significant increased NI 2.0 and SP 1.3 similar results obtained by Nagy et al. (1990) when they determine the field dose of fowl pox vaccine (FPV) to be used via spray while it was 10 6TCID50/ ml for 100 bird.
The humeral immune response (antibody response) to vaccine is an indirect measure of the immunogenicity of the vaccine and accordingly the immune status of vaccinated pigeons groups vaccinated with PPV via aerosol with different concentrations of Gel 01 (0%, 5%, 10 % and 15%) groups no (3, 5, 6 and 7 respectively) and group no (1) pigeons vaccinated with PPV vaccine through feather follicle was estimated and compared by Serum neutralization test (SNT) and ELISA.
As the observed results in Table (3) showed that protective level of antibody titer in group no 1, 3, 5, 6, and 7 expressed as NI = (2.25, 1.5, 1.75 and 2.0 –S/P values = 1.6, 1, 1.1, 1.3 and 1.4 respectively) appeared 2 weeks post vaccination while the highest level of antibody titer were at 3rd week post vaccination expressed as NI = 3.0,, 1.75, 2.0, 2.5 and 2.5 S/P values =1.9,, 1.4, 1.5, 1.8 and 1.7 respectively. These result is in accordance with (Kaffafy et al ., 2018) and (Amina and Chrestine, 2020), they found that the level of vaccinated pigeon with egg adapted and tissue culture adapted PPV appeared 2 weeks post vaccination reaching its peak at 3 weeks post vaccination also (Nagy et al., 1990; Mockett.et al .,1990) when they used spray mode for FPV vaccine in chicksa compared with different routes of administrated.
From the previous data it was found that Gel 01 best concentration to be added to PPV vaccine used via aerosol was 10% as using 5% Gel 01 showed humeral immune response less than obtained by 10% Gel 01 while using higher concentration of Gel 01 showing non-significant increase iin the humeral immune response these results were in accordance with (Heba et al., 2017) who used 10 % Gel 01 for Preparation of mucosal nanoparticles inactivated vaccine for Newcastle disease and H9N2 AI viruses.
It was noticed that higher antibody level in group no (1) (vaccinated via feather follicle) than the other groups vaccinated with aerosol method. Also the dramatically decrease of the level of immune response in all groups vaccinated via aerosol route than group (1) from week 4 till week 6 post vaccination which is also reported by (Heba et al., 2017) who used adjuvanted spray mucosal vaccination by Gel 01 inactivated influenza H9N2 vaccine and explained the effect of adjuvanted aerosol mucosal vaccination on innate immunity rather than adaptive immunity which indicated urgent boster dose 4 weeks post vaccination (Joo et al., 2011).
Challenge is considered the master test to evaluate and assist the immunizing capacity of the vaccine against pox infection, so all vaccinated groups were tested by challenging the immunity of the vaccinated pigeons against the virulent pigeon pox virus. The results represented in Table (4) showed that protection percent in group no (1) vaccinated with feather follicle route was 95% These results were in accordance with (Kaffafy et al., 2018) and (Amina and Chrestine, 2020) they found that protection percent for tissue culture adapted PP were 90% and 95% respectively when they compared with protection percent of PPV adapted on embryonated chicken egg (ECE) 100%, The protection percent in pigeon groups (no 3, 5, 6 and 7) vaccinated via aerosol with different Gel 01 percent (0%, 5%, 10% and 15%) were 80%, 85% 90% and 90% respectively as groups received PPV with 10 % and 15% Gel 01 gave the higher and equal protection percent 90% than other groups similar results obtained by (Nagy et al., 1990) when they vaccinated chicks with fowl pox (FP) vaccine via aerosol also (Mockett et al., 1990) found that no significant difference in protection percent in groups of chickens vaccinated with FPV via spray route and chickens vaccinated with wing web method as they mentioned that the experiment showed that aerosol route is a very effective route for FP vaccination. As a number of avian viruses enter their host by the respiratory route (infectious laryngotracheitis, avian influenza, infectious bronchitis and Newcastle disease viruses). A fowl pox given by the aerosol route would be expected to give rise to local immunity and this may be a very important factor in limiting spread of the avian viruses. Therefore, the aerosol route remains a practical alternative to the wing web route.
The increased level of humeral immune response and protective percent of challenge by adding 10 % Gel 01 to the PPV vaccine via aerosol which is higher than that of using PPV vaccine via aerosol without adjuvant can be explained on the basis of immune enhancing effects of Montanide adjuvants on vaccinated birds immune response which increase the efficacy of mucosal vaccination against avian infectious diseases (Shakya. and Nandakumar, 2012) as Gel 01 can enhance the efficacy of spray delivery of viral vaccines. This regarding to the muco adhesive nature of the polymer compounds which increase contact time with respiratory tract mucosa giving the chance to the innate immune response to take their role in protecting immune response (Adams et al., 2014). Montanide gel 01 induces strong infiltration of monocytes and macrophages, which enhances phagocytosis of the antigen complex with the polymer simultaneously increase the antigen-presenting cells activity and the innate immune response that trigger the acquired immune system to induce a highly specific immune response, so development of adjuvanted Gel 01 mucosal delivered vaccines may induce superior protection against viruses that infect via mucosal surfaces by stimulating the cellular immunity (Jang et al., 2011).
From the obtained results, the present study proved the capability of using PPV vaccine via aerosol route with Gel 01 adjuvant as mucosal vaccine with recommendation of poster 2nd dose after 3 weeks post vaccination.
Conclusion
This study recorded the efficacy of aerosol vaccination of tissue culture adjuvanted PPV vaacine in pigeon using Gel 01 in comparison to the usual routine vaccination with non adjuvanted PPV vaccine by feather follicle route to establish a more safe route of vaccination in valuable oriental and racing pigeons and easier route of vaccination of large pigeon flock with recommendation of poster vaccination after 3 weeks of vaccination in order to maintain a high level of protection and more long duration of immunity.
Acknowledgements
We appreciate the financial support by Veterinary Serum and research institute (VSVRI).
Conflict of interest
The authors declare that there is no conflict of interest for this publication.
Authors contribution
All authors contributed equally to study design, sampling, Methodology, interpretation of results, and writing of the manuscript.
References