Advances in Animal and Veterinary Sciences
Research Article
Effects of Eugenol and Vitamin E as a Supplement to Semen Extender on Chilled Canine Sperm Quality
Nguyen Van Vui*, Nguyen Thi Kim Quyen, Nguyen Thuy Linh, Nguyen Thi Mong Nhi
Tra Vinh University, No. 126 Nguyen Thien Thanh Street, Ward 5, Tra Vinh City, Tra Vinh Province, Vietnam.
Abstract | Canine sperm are susceptible to oxidation due to the unbalance between the high level of ROS and inadequate antioxidant protection during preservation. To improve the quality of chilled canine sperm, the supplementation of eugenol and vitamin E in the Tris-citric-fructose-mineral salts egg-yolk extender was conducted to evaluate the effects them on canine sperm quality during storage. Twelve ejaculates from three American Bully dogs were used. The sperm motility parameters were analysed by using computer-assisted sperm analysis (CASA), whereas the plasma membrane integrity, acrosome membrane integrity, and mitochondrial membrane potential parameters were identified by using a confocal laser scanning microscope. Thiobarbituric acid (TBA) assay was used to determine the level of sperm lipid peroxidation. The results showed that the sperm quality parameters in all the semen extenders decreased gradually during the whole storage time of 12 days. Besides, there was no significant difference in the values of chilled canine sperm motility parameters among all the treatments (P>0.05). Moreover, the percentage of the intact plasma membrane, intact acrosome membrane, and high mitochondrial membrane potential parameters in the eugenol treatment were better than those in the rest treatments (P<0.05). In conclusion, the eugenol is greater than the vitamin E to protect canine sperm during cooling preservation.
Keywords | Eugenol, Vitamin E, Antioxidants, Semen extenders, Canine sperm
Received | March 12, 2021; Accepted | March 17, 2021; Published | June 01, 2021
*Correspondence | Nguyen Van Vui, Tra Vinh University, No. 126 Nguyen Thien Thanh Street, Ward 5, Tra Vinh City, Tra Vinh Province, Vietnam; Email: [email protected]
Citation | Vui NV, Quyen NTK, Linh NT, Nhi NTM (2021). Effects of eugenol and vitamin e as a supplement to semen extender on chilled canine sperm quality. Adv. Anim. Vet. Sci. 9(7): 964-970.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.7.964.970
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Vui et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
During sperm preservation, canine sperm is highly sensitive to oxidative stress which induces biochemical and functional damages to the sperm. Because canine sperm plasma membrane involves a rich amount of polyunsaturated fatty acids (Darin Bennett et al., 1974), they are prone to lipid peroxidation as exposed to reactive oxygen species [ROS] during the process of cooling (Vieira et al., 2017). In physiological concentrations, ROS can assist sperm function in hyper-activation, acrosome reaction, capacitation, and sperm oocyte penetration (Aitken, 2017), while the high concentration of ROS can induce sperm lipid peroxidation which leads to changes in membrane fluidity and damage to sperm structures as well as subsequent sperm death (Lucio et al., 2016; Aitken, 2017). Fortunately, the enzymatic antioxidants in seminal plasma of canine semen including superoxide dismutase, glutathione peroxidase, phospholipid hydro-peroxide glutathione peroxidase, and catalase (Neagu et al., 2011; Angrimani et al., 2014), can prevent or minimize the harmful effects of oxidation caused by ROS (Ighodaro and Akinloye, 2017). However, the useful effects of these enzymatic antioxidants are no longer available because the seminal plasma of canine semen must be removed during the sperm preservation process (Hori et al., 2017). This may reduce the available antioxidants for sperm protection. Thus, the supplementation of antioxidant substances in the semen extenders may improve the quality of chilled canine sperm. In previous studies, several enzymatic and non-enzymatic antioxidants have been added to improve the quality of canine sperm during storage (Michael et al., 2007; Beccaglia et al., 2009; Neagu et al., 2009; Michael et al., 2009; Sahashi et al., 2011; Wittayarat et al., 2013; Ogata et al., 2015; Lucio et al., 2016; Andersen et al., 2018), but varied results were found depending on the type and concentration of antioxidants as well as the kind of semen extenders. Therefore, finding the appropriate antioxidant substances for chilled canine sperm is required.
Vitamin E is a lipid-soluble antioxidant that can scavenge the oxygen radicals and inhibit the free radical-induced lipid peroxidation as a chain-breaking antioxidant (Dad et al., 2006). In previous studies, the adding of vitamin E in the semen extenders has been carried out to improve the quality of sperm in canine studs (Michael et al., 2009), bulls (Asadpour, 2011), boars (Jeong et al., 2009; Satorre et al., 2012), rams (Abdi-Benemar et al., 2015), roosters (Moghbeli et al., 2016), and stallions (Almeida and Ball, 2005; Vasconcelos Franco et al., 2016).
Moreover, eugenol is an amphiphilic antioxidant which has the ability to scavenge the free radicals (Farhath, 2013; Mahapatra and Roy, 2014). However, until now, no study has investigated the effect of eugenol on mammalian sperm as an antioxidant. Hence, adding eugenol to the extender can improve chilled canine sperm quality by reducing sperm lipid peroxidation during storage.
In our previous study, we have revealed that the Tris-citric-fructose-mineral salts egg-yolk extender had the beneficial effects on chilled canine sperm quality during storage. Therefore, the aim of the present study was to investigate the effects of vitamin E and eugenol as a supplement in the Tris-citric-fructose-mineral salts egg-yolk extender on chilled canine sperm quality during preservation.
MATERIALS AND METHODS
Reagents
All chemicals were purchased from Sigma-Aldrich (Singapore), and sterile distilled water was used to prepare all solutions. The antioxidants were used in this study including vitamin E (T3251) and eugenol (E51791).
Animals and semen collection
Twelve ejaculates from three healthy male American Bully dogs aged 2 to 5 years were collected to use in this study. Ejaculates were obtained once a week from each dog by digital manipulation according to the previous technique (Linde-Forsberg, 1991). All dogs were trained to ejaculate and proven fertility before studying. This study was performed under the guidelines of the Institutional Animal Care and Use Committee of the Tra Vinh University, Vietnam.
Initial evaluation of semen quality
Before pooling, each ejaculate was analysed to determine the semen quality including volume, concentration, progressive motility, viability and abnormal morphology. Sperm progressive motility and sperm concentration were evaluated using computer-assisted sperm analysis (CASA). Sperm morphology and viability were estimated using eosin-nigrosin staining (Tamuli and Watson, 1994). The ejaculates were used in this study with the quality criteria of progressive motility ≥70%, sperm concentration ≥200×106 sperm/mL, sperm abnormal morphology ≤5%, and sperm viability ≥90%.
Preparation of extenders
Different extenders in this study contained the basis extender of Tris-citric-fructose-mineral salts-egg yolk extender and one of the following antioxidants: vitamin E (50µg/mL) and eugenol (50µg/mL). The concentration of vitamin E and eugenol (50µg/mL) was based on our preliminary study (not show data). The basis extender without antioxidants was as a control. DMSO was used as a solvent to dilute eugenol, whereas vitamin E was diluted in ethanol before diluting continuously in DMSO. The final level of DMSO in each extender was 0.8%. The composition of these extenders is given in Table 1.
Semen processing and experimental design
After collection, semen from three dogs was pooled and divided into three equal aliquots. Then, the seminal plasma was removed by centrifuging at 720×g for 5 minutes (Rijsselaere et al., 2002). The sperm were diluted with three extenders to reach a sperm concentration of 100 x 106 sperm/mL. After that, sperm samples were placed in a styrofoam box containing water at 25oC and cooled down gradually (0.3oC/min) to 5oC by adding the ice for 1 hour. After cooling, samples were stored in the fridge (5oC). A repeated measurement in the completely randomized design with four replicates and each replicate being a pool of three ejaculates was conducted for experimental design in this study.
Evaluation of sperm motility
Automated analysis of sperm motility was evaluated using computer-assisted sperm analysis (CASA; HTR-IVOS 14.0; Hamilton Thorne, USA). The technical settings of CASA for canine sperm as the following were used in this study: frames per sec. (Hz), 60; no. of frames, 30; minimum contrast, 30; minimum cell size (pix), 7; cell size (pix), 6; cell intensity, 75; path velocity (VAP) (µm/s), 20; straightness (STR) (%), 40; VAP cutoff (µm/s), 9; and VSL cutoff
Table 1: The composition of the semen extenders used to dilute canine sperm
| Extender ingredients | Extenders | ||
| Control | Eugenol | Vitamin E | |
| Tris (mg) | 900 | 900 | 900 |
| Citric acid (mg) | 500 | 500 | 500 |
| Fructose (mg) | 1250 | 1250 | 1250 |
| NaCl (mg) | 450 | 450 | 450 |
|
KHPO4 (mg) |
60 | 60 | 60 |
| KCl (mg) | 60 | 60 | 60 |
|
CaHPO4 (mg) |
20 | 20 | 20 |
|
MgCl2 (mg) |
10 | 10 | 10 |
| Egg yolk (mL) | 20 | 20 | 20 |
| Eugenol (mg) | - | 5 | - |
| Vitamin E (mg) | - | - | 5 |
| Gentamycin (mg) | 200 | 200 | 200 |
| DMSO (mL) | 0.8 | 0.8 | 0.8 |
| Distilled water (mL) | To 100 | To 100 | To 100 |
| pH | 6.57 | 6.56 | 6.57 |
| Osmolality (mOsmol/kg) | 479 | 486 |
484 |
(µm/s), 20. Before analysing, sperm was diluted with a warmed (38oC) Tris buffer at a rate of 1:1. Then, 5 μL of each sperm sample was mounted into a warmed (38oC) 2X-CEL counting chamber and covered by coverslips. Each sperm sample in 2X-CEL counting chamber was evaluated in at least 5 randomly selected fields. The percentage of total motility and the percentage of progressive motility parameters were collected.
Evaluation of plasma membrane integrity, acrosome membrane integrity, and mitochondrial membrane potential
The plasma membrane integrity, acrosome membrane integrity, and mitochondrial membrane potential parameters of canine sperm were determined using a fluorescent staining combination of propidium iodide (PI), Hoechst 33342 (H342), fluorescein isothiocyanate–conjugated Pisum sativum agglutinin (FITC-PSA), and 5,5’,6,6’-tetrachloro-1,1’,3,3’-tetraethylbenzimidazolyl-carbocyanine iodide (JC-1) according to the method described by Celeghini et al. (2007). Briefly, 10 µL of H342 (40 µg/mL in DPBS) was added to 100 µL sperm sample and incubated in a water bath at 38oC for 10 minutes. After that, the mixture sample was added continuously by 2 µL of PI (0.5 mg/mL in DPBS), 15 µL of JC-1 (153 µM JC-1 in DMSO), and 20 µL of FITC-PSA (100 µg/mL in DPBS). The mixture sample was then incubated for 8 minutes at 38oC. Next, the stained sperm sample was washed by adding 200 µL of DPBS and centrifugation at 800×g for 2 minutes. The stained sperm pellet was re-suspended in 100 µL of DPBS and immediately examined
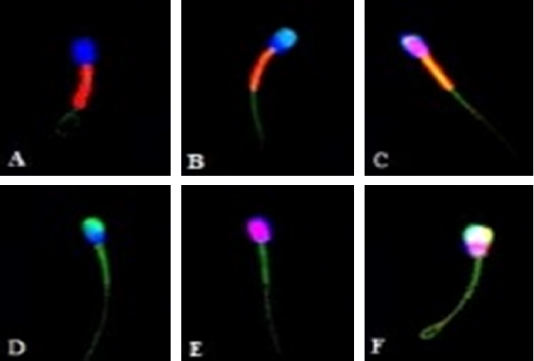
Figure 1: Canine sperm under a confocal laser scanning microscope (600x magnification) after staining with H324, PI, FITC-PSA, and JC-1. (A) Intact plasma and acrosome membrane, and high mitochondrial membrane potential. (B) Intact plasma membrane, damaged acrosome membrane, and high mitochondrial membrane potential. (C) Damaged plasma membrane, intact acrosome membrane, and high mitochondrial membrane potential. (D) Intact plasma membrane, damaged acrosome membrane, and low mitochondrial membrane potential. (E) Damaged plasma membrane, intact acrosome membrane, and low mitochondrial membrane potential. (F) Damaged plasma and acrosome membrane, and low mitochondrial membrane potential.
using a confocal laser scanning microscope (Nikon/Ni-E, Japan) with a 60x objective lens. For each stained sperm sample, at least 200 sperms were identified. The stained
Table 2: Effects of the antioxidants supplementation in semen extender on the total motility (TM) and progressive motility (PM) of the chilled canine sperm.
| Parameters | Extenders | Day1 | Day3 | Day6 | Day9 | Day12 |
| TM (%) | Control |
93.9±1.1A |
91.7±0.9B |
89.1±2.1C |
75.6±2.0bD |
58.8±5.7bE |
| Eugenol |
95.7±1.2A |
93.3±1.0B |
91.3±1.3B |
83.7±2.5aC |
77.4±4.6aD |
|
| Vitamin E |
94.7±0.7A |
92.9±0.9B |
90.6±1.6B |
77.5±4.3bC |
58.2±7.9bD |
|
| PM (%) | Control |
72.0±0.8A |
68.3±2.3A |
62.2±2.6B |
43.7±6.7C |
23.3±2.6bD |
| Eugenol |
73.8±4.1A |
70.6±4.4A |
65.1±2.6B |
54.2±7.6C |
39.7±2.5aD |
|
| Vitamin E |
71.1±4.3A |
66.7±6.1B |
62.2±5.4B |
43.8±8.9C |
21.4±2.3bD |
Values are mean ± standard deviation. Lowercase superscript letters (a or b) in the same column indicates significant difference among extenders (P<0.05) and uppercase superscript letters (A, B, C, D or E) in the same row indicates significant difference within extenders with different storage time (P<0.05).
sperm with blue-stained in nucleus (H342-positive) and bright red-orange in mid-piece region (JC-1-positive) was intact plasma membrane, intact acrosome membrane, and high mitochondrial membrane potential, while the stained sperm with red-stained in nucleus (PI-positive), yellow-green in acrosome region (FITC-PSA-positive), and bright green in mid-piece region (JC-1-negative) was damaged plasma membrane, damaged acrosome membrane, and low mitochondrial membrane potential. The stained sperm classification under a confocal laser scanning microscope is shown in Figure 1.
Evaluation of sperm lipid peroxidation
The lipid peroxidation of chilled canine sperm was determined using thiobarbituric acid (TBA) assay to measure the concentration of malondialdehyde (MDA) production as described by Maia et al. (2010). Briefly, sperm sample was incubated with 0.24mM FeSO4 to induce lipid peroxidation in a water bath at 38oC for 15 minutes. Then, 1mL TBA reagent (trichloroacetic acid 15% (w/v), thiobarbituric acid 0.375% (w/v) in 0.25N hydrochloric acid) and 1% (v/v) butylated hydroxytoluene solution (50mM) were added to 0.5 mL of the sperm sample. The mixture sample was placed in a water bath (95oC) for 20 minutes and then cooled down immediately. After cooling, the mixture was centrifuged at 1,000×g for 10 minutes to separate the supernatant. The absorbance of sample was calculated using a spectrophotometric plate reader at 535nm. The concentration of MDA in each sample was determined by converting the absorbance of sample with a MDA standard curve. The level of MDA was expressed in nmol MDA/50x106 sperm.
Statistical analysis
Statistical analyses were represented using IBM SPSS statistics for Windows, version 20 (IBM Corp., Armonk, N.Y., USA). Two-factor mixed analysis of variance (ANOVA) was applied to determine the interaction between time and treatment as the main effects, and the Tukey method was used for multiple comparisons of means among groups of each factor (time, treatment). All data are given as mean ± standard deviation (SD). A difference of P<0.05 was considered significant.
RESULTS
Sperm motility
The results of the sperm motility of chilled canine sperm are represented in Table 2. In general, the values of the total motility (TM) and progressive motility (PM) parameters of chilled canine sperm were not noticeable different among the treatments (P>0.05) during the first of 6 days. However, after day 6, the percentage of these parameters in the eugenol extender was higher than that in the control and vitamin E extenders (P<0.05). In addition, the percentages of the TM and PM parameters in all the treatments decreased gradually during 12 days of the whole storage time.
Plasma membrane integrity, acrosome membrane integrity and mitochondrial membrane potential
The results of the plasma membrane integrity, acrosome membrane integrity, and mitochondrial membrane potential of chilled canine sperm are given in Table 3. Overall, the proportions of the intact plasma membrane, intact acrosome membrane, and high mitochondrial membrane potential in the eugenol treatment were superior to those in the rest treatments (P<0.05). Although the percentages of these parameters of chilled sperm in the eugenol extender were the highest, they were not significantly different compared with those in the vitamin E extenders (P>0.05) during the first of 6 days.
Moreover, Table 4 presents the percentage of the sperm with intact plasma membrane, intact acrosome membrane, and high mitochondrial membrane potential. The results of this parameter were parallel with those of the intact plasma membrane, intact acrosome membrane, and the high mitochondrial membrane potential parameters. This means
Table 3: Effects of the antioxidants supplementation in semen extender on the intact plasma membrane, intact acrosome membrane, and high mitochondrial membrane potential parameters of the chilled canine sperm.
| Parameters | Extenders | Day1 | Day3 | Day6 | Day9 | Day12 |
| Plasma membrane (%) | Control |
80.0±1.7A |
76.9±2.6A |
64.6±2.1bB |
53.5±2.5bC |
39.7±2.6cD |
| Eugenol |
82.1±1.4A |
78.7±1.6B |
71.1±1.2aC |
64.0±2.2aD |
54.5±2.0aE |
|
| Vitamin E |
81.7±1.9A |
77.9±2.0A |
70.4±2.1aB |
58.3±1.1bC |
48.9±2.1bD |
|
| Acrosome membrane (%) | Control |
70.5±1.5A |
64.4±2.7bB |
54.8±3.8bC |
39.6±3.0cD |
23.3±2.3cE |
| Eugenol |
74.4±2.3A |
71.8±3.3aA |
62.2±2.4aB |
57.9±2.3aB |
46.5±2.4aC |
|
| Vitamin E |
73.1±2.1A |
70.0±3.6aB |
61.6±3.8aC |
47.5±5.4bD |
37.2±1.4bE |
|
| Mitochondrial membrane potential (%) | Control |
84.4±1.1A |
75.8±2.1bB |
65.0±3.0bC |
55.7±1.8cD |
38.2±2.4cE |
| Eugenol |
86.6±1.4A |
83.0±2.4aB |
75.3±2.0aC |
69.4±1.7aD |
60.8±0.7aE |
|
| Vitamin E |
85.7±1.1A |
82.3±1.4aB |
74.2±1.7aC |
61.2±1.0bD |
52.7±1.9bE |
Values are mean ± standard deviation. Lowercase superscript letters (a, b or c) in the same column indicates significant difference among extenders (P<0.05) and uppercase superscript letters (A, B, C, D or E) in the same row indicates significant difference within extenders with different storage time (P<0.05).
Table 4: Effects of the antioxidants supplementation in semen extender on the sperm with intact plasma membrane, intact acrosome membrane, and high mitochondrial membrane potential of chilled canine sperm.
|
Extenders |
Day1 |
Day3 |
Day6 |
Day9 |
Day12 |
|
Control |
70.0±1.6A |
61.5±1.6bB |
50.0±2.0bC |
32.1±2.5cD |
20.1±0.5cE |
|
Eugenol |
72.6±1.8A |
67.7±2.6aB |
56.0±1.4aC |
50.1±2.7aD |
42.1±1.3aE |
|
Vitamin E |
71.6±1.9A |
67.1±2.5aB |
54.5±2.0aC |
41.4±3.2bD |
32.1±1.0bE |
Values are mean ± standard deviation. Lowercase superscript letters (a, b or c) in the same column indicates significant difference among extenders (P<0.05) and uppercase superscript letters (A, B, C, D or E) in the same row indicates significant difference within extenders with different storage time (P<0.05).
Table 5: Effects of the antioxidants supplementation in semen extender on the level of the malondialdehyde (MDA) (nmol/50x106 sperm) of chilled canine sperm
| Treatments | Day1 | Day6 | Day12 |
|
Control |
7.38±0.23aA |
6.76±0.33aB |
7.27±0.51aA |
| Eugenol |
5.99±0.62bA |
5.76±0.40bA |
5.97±0.60bA |
| Vitamin E |
6.20±0.60bA |
5.74±0.24bB |
6.20±0.39bA |
Values are mean ± standard deviation. Lowercase superscript letters (a or b) in the same column indicates significant difference among extenders (P<0.05) and uppercase superscript letters (A or B) in the same row indicates significant difference within extenders with different storage time (P<0.05).
that the eugenol extender was the greatest in this parameter with being higher than the other treatments (P<0.05).
Sperm lipid peroxidation
The levels of the malondialdehyde (MDA) (nmol/50x106 sperm) of chilled canine sperm with the addition of antioxidants are summarized in Table 5. The concentration of the MDA of chilled canine sperm in the control group was higher than that in the eugenol and vitamin E extenders. Although the level of the MDA of chilled canine sperm in the eugenol extender was the lowest, it was not evidently different compared with the vitamin E extender (P>0.05).
DISCUSSION
The present study resulted that the supplementation of eugenol and vitamin E as an antioxidant in Tris-citric-fructose-mineral salts egg-yolk extender could influence in the sperm motility parameters and enhance the quality of chilled canine sperm in the plasma membrane integrity, acrosome membrane integrity, and mitochondrial membrane potential parameters as compared to the control group. In particular, the extender with adding of eugenol was superior to the vitamin E and control extenders. On explanation could be the fact that eugenol has an amphiphilic characteristic (Farhath, 2013; Mahapatra and Roy, 2014), whereas vitamin E is a lipid-soluble substance (Prasanth et al., 2019). Thus, eugenol are not only prone to absorb in the sperm plasma membranes, but also dissolve in the semen extender against lipid peroxidation during cooling and storage (Aitken, 2017), while vitamin E are restricted in the semen extender.
The level of malondialdehyde (MDA) was determined using thiobarbituric acid (TBA) assay which was an important indicator for sperm lipid peroxidation during preservation (Toker et al., 2016; Vieira et al., 2017). The present study indicated that the supplementation of the antioxidants in the extender could prevent the sperm lipid peroxidation by inhibiting the MDA production. Especially, the level of MDA in the eugenol extenders were lower than that in the rest extenders. These results were consistent with the results in the plasma membrane integrity, the acrosome membrane integrity, and the mitochondrial membrane potential parameters. It means that the antioxidant activity of these antioxidants can protect sperm and improve sperm quality during preservation. In this study, the sperm lipid peroxidation parameter was carried out to investigate the lipid peroxidation of the extended sperm. This may indicate that the evaluation was represented in both the semen extender and the sperm. Nevertheless, during preservation both the sperm and the semen extender could have lipid peroxidation (Maia et al., 2010). As a result, the MDA concentration results in this study were the sum of lipid peroxidation occurring in both the sperm and the semen extender. Therefore, the lipid peroxidation in the semen extender could significantly influence in the results of the sperm lipid peroxidation parameter. The present study may suggest that the combination of the hydrophilic antioxidants with the lipophilic or amphiphilic antioxidants supplementation in the semen extender may support a positive interaction between the antioxidants for the sperm and the semen extender.
CONCLUSION
In conclusion, the adding eugenol and vitamin E to the Tris-citric-fructose-mineral salts egg-yolk extender have protective effects on chilled canine sperm quality. In addition, the eugenol is superior to the vitamin E in protection for canine sperm during preservation.
ACKNOWLEDGEMENTS
The authors would like to thank Tra Vinh University for providing facilities to carry out the work.
AUTHOR CONTRIBUTIONS
N.V.V conceived and designed the experiments; N.V.V, N.T.K.Q, N.T.L and N.T.M.N performed the experiments; N.V.V and N.T.K.Q analysed the data; N.V.V, N.T.K.Q and N.T.L wrote the paper; all authors reviewed and approved the final manuscript.
CONFLICT OF INTEREST
Authors declared no conflict of interest.
REFERENCES






