Advances in Animal and Veterinary Sciences
Short Communication
Cell Culture Establishment from Zebra Finch Embryonic Fibroblasts
Maria Kulak, Olga Takki, Svetlana Galkina*
Saint Petersburg State University, Universitetskaya Emb. 7/9, Saint Petersburg, Russia, 199034.
Abstract | The zebra finch Taeniopygia guttata is a model object of neurobiology, ethology, cell and developmental biology. We describe a detailed protocol for successful establishment and maintaining of primary cell lines of the zebra finch using enzymatic method. The cells obtained are of various morphology resembling keratinocytes, fibroblasts, and melanocytes. Zebra finch cell lines derived in this way can be cryopreserved and successfully recovered to provide a source for cytological and cytogenetic studies. The protocol can be used as a basis for cell culture establishment for any songbird.
Keywords | Taeniopygia guttata, Somatic cells, Primary cell culture, Enzymatic method, Fibroblasts
Received | October 07, 2020; Accepted | December 03, 2020; Published | January 15, 2021
*Correspondence | Svetlana Galkina, Department of Genetics and Biotechnology, Biological Faculty, Saint Petersburg State University, Universitetskaya Emb. 7/9, Saint Petersburg, Russia, 199034; Email: [email protected]
Citation | Kulak M, Takki O, Galkina S (2021). Cell culture establishment from zebra finch embryonic fibroblasts. Adv. Anim. Vet. Sci. 9(3): 344-347.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.3.344.347
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Kulak et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
The zebra finch Taeniopygia guttata (Aves, Passeriformes, Estrildidae) is an effective model object of neurobiology used for investigation both the neurophysiological (singing, recognition of acoustic signals, learning) and behavioral (communication, choice of a partner, caring for offspring) aspects of the brain functioning. Its genome was sequenced in 2010 and became the second deciphered avian genome after the chicken one (Warren et al., 2010). The interest in zebra finch cell and developmental biology was promoted by the discovery of an additional chromosome, which is present only in the germ cell line (Pigozzi and Solari, 1998; Torgasheva et al., 2019).
Cell culture makes it possible to obtain and preserve the genetic material of the studied object in unlimited quantities. Maintenance of cell lines, preparation of cytological and cytogenetic preparations are standard methods that have become routine in many laboratories. However, the method of obtaining a primary cell culture (i.e. cells obtained directly from animal tissues) requires optimization for each object. It concerns mainly the concentration of antibiotics used for the decontamination of animal tissue pieces and the temperature of cultivation. The body temperature in different bird species varies from 38.54±0.96 to 43.85±0.94°C, while the lower temperature is typical for larger birds (Prinzinger et al., 1991).
The most convenient material for obtaining avian primary cell culture is developing embryos. Zebra finch is an altricial species. The period of its embryonic development is about two weeks. Immediately after oviposition, the embryo is at the early/middle blastula stage, which corresponds to the EGK-VI/VIII stage (Eyal-Giladi and Kochav, 1976; Mak et al., 2015). It is convenient to manipulate with 8-9 days embryos (stage 33-34, Murray et al., 2013). If the embryo is older, we advise to take only skin and connective tissues samples and not to use dense structures (eyes, cartilage, feathers) for cell culture. If the embryo is underdeveloped, the eggs should be incubated in a humidified air incubator at +39°C. It is also possible to obtain cells from a biopsy material of an adult bird (e.g., pieces of skin). In this case it is necessary to carefully decontaminate samples with antibiotics and antimycotics cocktail (e.g. 1000 μg/ml streptomycin, 1000 U/ml penicillin, 25 μg/ml amphotericin B, 250 μg/ml gentamicin, diluted in culture medium).
In general, the procedure for obtaining a primary cell culture consists of three stages: preparation of the workplace and material for obtaining cells, trypsinization of animal tissue samples, inoculation of cells into a culture flask. The protocol below describes how to obtain cell culture of zebra finch fibroblasts, and it can be used as a basic protocol for cell culture establishment for any songbird.
Equipment
Instruments (all instruments that come in contact with the cells must be sterile)
Reagents (All solutions that come in contact with the cells must be sterilized by autoclaving or filtration)
Before starting, clean working area with disinfectant and sterilize with UV light, switch on a laminar flow hood and allow the airflow to stabilize.
- 1. Prepare complete cell culture medium adding FBS to final concentration of 10%, warm it to 39°C in a water bath / air incubator. Thaw and warm an aliquot of 0.25% trypsin.
- 2. Thoroughly wash the eggshells in warm soapy water. Care is required, the eggshells can be very thin. Transfer the eggs to a laminar, blot additionally with a paper towel soaked in 70% ethanol or spray with 70% ethanol from a spray bottle.
- 3. Cut the eggshell with sterile scissors from the blunt end (where the air chamber is located) (Figure 1a), remove the embryo into a Petri dish with ~1ml of sterile 1xPBS, decapitate the embryo (to get rid of nerve cells, if possible). Wash the embryo from the blood 2-3 times in 3 ml of 1xPBS. When working with several embryos, use a separate Petri dish for each sample.
- 4. Transfer the washed embryo to a new Petri dish and macerate it using sterile scissors and / or a 5 ml syringe without a needle. Add 0.8-1.5 ml 0.25% trypsin. Using a sterile Pasteur glass pipette, transfer the resulting suspension into a sterile weighing bottle, put a sterile magnetic stir bar inside. Place the vessel on a magnetic stirrer in a CO2 incubator at 39°C and stir for 10-20 min depending on the size of the tissue pieces.
- 5. At the end of trypsinization, transfer the vessel with the cell suspension to the laminar and add 1-2 ml of the culture medium with FBS to inhibit the action of trypsin.
- 6. Transfer the cell suspension to a centrifuge tube, add culture medium to 10 ml, and centrifuge for 5-10 min at 1000 rpm.
- 7. Remove supernatant leaving 0.5-1 ml. Resuspend the pellet delicately with a sterile Pasteur glass pipette. Lightly press it to the bottom to avoid foaming.
- 8. If there is a small number of cells in the pellet, transfer the cells to a 25 cm2 flask with 5 ml of the medium; if there are a lot of cells, transfer them to a 75 cm2 flask with 10 ml of medium (Figure 1b). Sign on the flask sample name, passage number, and date. Place flask in a CO2 incubator (39°C, 5% CO2).
- 9. The next day, change the culture medium to remove floating (dead) cells. Add 10 ml complete culture medium to a 25 cm2 flask and 20 ml to a 75 cm2 flask.
- 10. Regularly check the flasks, pay attention to the turbidity and color change of the medium. Check the condition of cells using an inverted phase-contrast microscope paying attention to their morphology, viability, appearance of floating dead cells.
- 11. If phenol red added to the standard cell culture medium changes color from bright red (pH 7.4) to yellow (low pH) or purple (high pH) due to the accumulation of cell metabolites, the medium should be replaced. On average, the procedure is performed every 2-3 days. If there are few cells, we recommend to change half the volume of the culture medium to keep the cellular mediators released by the cells into the media during growth. The time to establish primary cell culture depends on the initial density of cells attached to the bottom of the culture flask (Figure 1c).
- 12. When cells form a subconfluent monolayer, they should be passaged. To do this: remove the medium, wash the bottom of the flask twice with 1xPBS or Hank’s solution, add ~ 1 ml of 0.05% trypsin, incubate for 1 min at 39°C. Observe the cells with an inverted phase-contrast microscope - avian cells are very sensitive to trypsin. When the cells become round, hit the sidewall of the flask with your palm, transfer the flask to the laminar and inhibit the action of trypsin by adding 1.5-2 ml of cell culture medium with FBS. Gently resuspend the contents of the flask with a sterile Pasteur glass pipette.
- 13. Transfer the cell suspension to a centrifuge tube, bring the volume to 10 ml by adding culture medium and centrifuge for 5 min at 1000 rpm. Remove the supernatant, leaving about 1 ml, and resuspend the pellet. At the first subculture (passage), the cells are transferred into a new flask of the same size (dilution no more than 1: 1), adding 10 ml of complete culture medium to a 25 cm2 flask, and 20 ml to a 75 cm2 flask.
- 14. After 5-7 days, fibroblasts form a monolayer. Now you can subculture the cells at a dilution of 1:2, repeating the procedures in paragraphs 12-13. Don’t forget to sign the flask with the name of the sample, passage number, and date.
After 5-7 passages and performing the necessary tasks (e.g., obtaining cytological and/or chromosomal preparations), it is recommended to freeze cells to keep the resulting culture. Dimethyl sulfoxide (DMSO) used as a cryoprotectant is a very toxic substance for cells, so the contact time with it should be minimized and the work should be carried out as quickly as possible.
To thaw cryopreserved cells
As in the case of cultured cells of other animals, zebra finch cell cultures are used to study the universality of the mechanisms of cell interaction, differentiation and dedifferentiation of cells, and signal transduction. The resulting cells are a valuable source of genetic material for the study of zebra finch somatic genome.
Unlike mammalian cells, zebra finch cells should be cultivated at + 39°C. Also, a lower percentage of trypsin should be used while subculturing due to the greater sensitivity of zebra finch cells. It should also be remembered that the lifetime of primary cell cultures is limited. In optimal cultivation conditions, the most active growth phase occurs between the third and seventh passages. By the 12-15th passage, the primary cell cultures begin to degrade: the cytoplasm of the cells is highly vacuolated, granules appear, the cells become rounded, lose their connection with other cells and the substrate, and eventually die (Figure 1d). Cryopreservation of the obtained cell cultures is not only an opportunity to resume cultivation at any time but also a necessary step to keep cell populations in the most active state, saving reagents and time of a researcher.
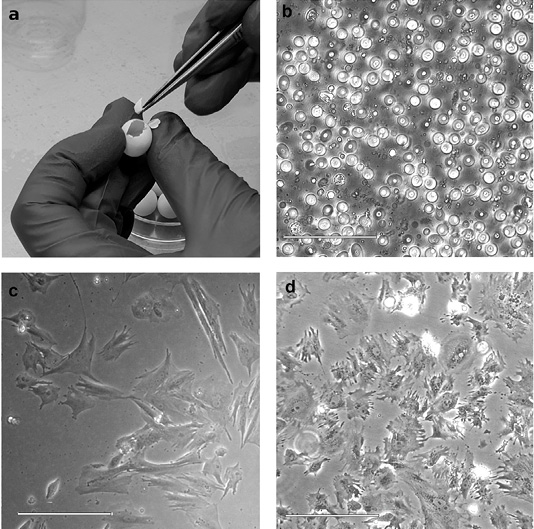
Figure 1: Steps of primary cell culture obtaining from a zebra finch embryo. (a) Embryo isolation from an egg. (b) Cell suspension after mechanical dissociation and trypsinization of the embryo. (c) Primary cell culture of zebra finch Taeniopygia guttata. (d) Degradation of zebra finch cell culture. Highly vacuolated cells are shown. Scale bars are 50 micrometers.
ACKNOWLEDGMENTS
The authors are grateful to Valery Fillon (INRA, Toulouse, France) for the valuable methodical advice. The work was carried out using equipment of the Research Resource Center “Chromas” of St. Petersburg State University and funded by Russian Foundation for Basic Research, project number RFBR 20-04-00967A.
Author’s Contribution
MK carried out experiments. MK, OT, SG wrote the manuscript.
CONFLICT OF INTEREST
All authors have declared no conflict of interest.
Ethics statement
Zebra finches were maintained in a small closed population at the Laboratory of Chromosome Structure and Function (Saint Petersburg State University). Handling followed protocols approved by the Saint Petersburg State University Ethics Committee (statement #131-03-2).
References






