Advances in Animal and Veterinary Sciences
Research Article
Oxidative Stress, Hemogram, Hepatorenal Function Evaluation and Molecular Diagnosis of Babesiosis in Crossbred Cows Naturally Infected with B. bigemina
Emad Abdel-Hamied1, Waleed Arafa2, Mourad Mahmoud Mahmoud1*
1Department of Animal Medicine, Faculty of Veterinary Medicine, Beni-Suef University, Beni-Suef 62511, Egypt; 2Department of Parasitology, Faculty of Veterinary Medicine, Beni-Suef University, Beni-Suef 62511, Egypt.
Abstract | This study was conducted to describe the clinical picture, hemato-biochemical changes, and lipid peroxidation/antioxidant status in cows affected with clinical babesiosis for understanding disease pathogenesis, perfect diagnosis, and evaluation of severity and prognosis of the disease. Thirty-eight crossbred cows were involved in this study. Twenty-six diseased crossbred cows showed typical signs of babesiosis and another 12 apparently healthy cows as control were enrolled in the current work. The selected animals were subjected to thorough clinical examination. Giemsa stained blood films were prepared from all cows and microscopically examined for parasitic piroplasm. Molecular demonstration of Babesia bigemina using PCR was performed. Blood samples from examined animals were collected and the hematologic, biochemical, lipid peroxidation and antioxidant alterations were estimated. High fever, inappetence, depression, congested mucous membranes and often dark red urine were the most consistent findings in acute cases. Labored breathing, pale to icteric mucous membranes and general weakness were recorded in prolonged cases. Hematological findings showed significant reductions in RBCs, Hb, hematocrit and neutrophils accompanied with significant elevations in lymphocytes and monocytes in affected animals. Significant reductions in albumin and A/G ratio along with significant increase in globulin, bilirubin, urea, creatinine, iron and copper levels were the most consistent biochemical changes observed in babesiosis affected cows. Excessive lipid peroxidation along with decreased total antioxidant status was observed in cows with babesiosis. The obtained findings indicated that babesiosis is a serious disease has a very characteristic clinical picture in which high fever and hemoglobinuria are the main aspects. Hematologic and biochemical findings indicated that the babesiosis affected cows suffered poor blood picture, severe anemia, liver and kidney dysfunction and significant oxidative stress which are implicated in the severity, pathogenesis and prognosis of bovine babesiosis.
Keywords | Babesiosis, Hemato-biochemical, MDA, Oxidative biomarkers, PCR
Received | June 11, 2020; Accepted | September 3, 2020; Published | November 15, 2020
*Correspondence | Mourad Mahmoud Mahmoud, Department of Animal Medicine, Faculty of Veterinary Medicine, Beni-Suef University, Beni-Suef 62511, Egypt; Email: [email protected]
Citation | Abdel-Hamied E, Arafa W, Mahmoud MM (2020). Oxidative stress, hemogram, hepatorenal function evaluation and molecular diagnosis of babesiosis in crossbred cows naturally infected with B. bigemina. Adv. Anim. Vet. Sci. 8(12): 1402-1409.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.12.1402.1409
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Abdel-Hamied et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Bovine babesiosis is considered one of the most economically important and serious tick-borne disease affecting cattle especially in the tropical and subtropical regions (Jongejan and Uilenberg, 1994). Haemoprotozoan diseases gain their importance due to the severe economic losses and the harmful effects on the immune status of the affected animals (Urquhart et al., 1996). Babesiosis is caused by an intra-erythrocytic haemoprotozoan attacks susceptible animal red blood cells (Zintl et al., 2003). In Egypt, bovine babesiosis is mainly caused by Babesia bigemina and B. bovis species (Adham et al., 2009; Mahmoud et al., 2015). Inappetence, Fever, hemolytic anemia, icterus, emaciation and hemoglobinuria are the most observed clinical findings in cattle with babesiosis. Moreover, in pregnant cattle, diarrhea and abortion may occur (Mosqueda et al., 2012; Decaro et al., 2013). Hypochromic to normochromic anemia with alterations in the serum biochemical indicators were recorded in bovine babesiosis (Sharma et al., 2016). Oxidative stress has been reported in animals with babesiosis (Kumar et al., 2019). Oxidative stress arises from disparity between scavenging mechanisms and radical generating mechanisms (Omar et al., 2015). When the reactive oxygen species exacerbate the antioxidant system ability, free radicals could harm the different tissues and cells, so oxidative process ensues (Zaidi et al., 2005). Erythrocytes are the most susceptible to lipid peroxidation because of abundant membrane lipids which are the most sensitive molecules to oxidative damage. Lipid peroxidation resulting in increased red cells fragility and cellular lysis and subsequently anemia (Wagner et al., 1988; Saluja et al., 1999). Under field conditions, tentative diagnosis of haemoprotozoal diseases depends usually upon the clinical examination findings and demonstration of ticks on the diseased cases. Confirmation of field diagnosis is usually based on microscopic detection of the causative protozoan in Giemsa-stained blood smears from affected animals (Aktas et al., 2006; OIE, 2008). Molecular diagnosis using PCR allow the detection of faint parasitemia that couldn’t be detected by the classical methods used to identify blood protozoa especially in carrier states with low parasietemia (Almeria et al., 2001; Altay et al., 2008). Therefore, this work was designed to point out the clinical picture, hemato-biochemical alterations and oxidant/antioxidant status associated with babesiosis in naturally affected crossbred cows with special emphasis on molecular diagnosis of such disease.
MATERIALS AND METHODS
Animals and experimental design
This study was carried out in Beni-Suef province, Egypt (Coordinates: 29°04′N 31°05′E) during the period from June 2019 to October 2019. A total of 38 lactating crossbreed cows of three to five years old were enrolled in current work. Twenty-six cows showed typical clinical signs of bovine babesiosis were selected for the present study. Another 12 apparently healthy cows were kept as a control group. A comprehensive clinical examination of all cows was performed. Based on microscopic examination and PCR findings, the diagnosis was confirmed. Hemato-biochemical alterations, lipid peroxidation status and antioxidant capacity in babesiosis affected cows were estimated.
Clinical examination and blood sampling
All animals were subjected to thorough clinical examination and the clinical findings were recorded (Radostits et al., 2000). Paired blood samples were drawn from control and babesiosis affected cows (during the acute stage of the disease) by jugular vein puncture into two clean sterile tubes. The first one was collected into EDTA containing tube to obtain whole blood for hematologic examination and molecular detection of the protozoan. A total of 500uL of whole blood was kept at -20 °C for the extraction of DNA. The second sample was collected into plain tubes for harvesting non-haemolysed serum. Serum samples were kept in a clean dry Eppendorf tubes at -20 °C for biochemical analyses.
Microscopic demonstration of Babesia spp. in Giemsa stained blood films
On clean glass slides, three thin blood films were prepared from each animal from ear veins and let to dry. The films were fixed in absolute methanol for a minute then Giemsa stained for a half-hour. Slides were examined for the presence of intra-erythrocytic babesia (Rampersad et al., 2003).
Molecular detection of Babesia bigemina using PCR
DNA extraction was carried out using Geneaid, New Taipei, DNA extraction kit. From each blood sample about 200µl blood was used for DNA extraction. DNA extracts were kept at −20 °C in order to perform the PCR amplification. Polymerase chain reaction of B. bigemina by using apical membrane antigen primers (F 5/-TACTGTGACGAGGACGGATC- 3/, R5/-CCTCAAAAGCAGATTCGAGT-3) was performed as per Sivakumar et al. (2012). PCR reaction 25µL of 12.5µL 2X master mix, 1µl of the Forward primer (10pmol/µl), 1µl of the Reverse primer (10pmol/µl), 3 µlDNA, and 7.5µl nuclease free water. Initial denaturation at 95 ºC for 5 min, denaturation (37 cycles) at 95 ºC for 30 s, annealing at 54 ºC for 60 s and elongation for 1 min at 72 ºC were the PCR cycling conditions. Then the final extension at 72 ºC for 7 min. Amplified products were detected on a agarose gel 1.5% under UV transillumination after being stained with ethidium bromide.
Hematological investigations
For estimation of hematological alterations associated with babesiosis, erythrocytic count, hemoglobin content (Hb), hematocrit, red blood indices and total and differential leukocytic counts were determined in blood samples from both healthy and babesiosis affected cows according to the standard methods described by Feldman et al. (2000).
Biochemical analyses
Total proteins, albumin, total and conjugated bilirubin, ALT, AST, glucose, urea, creatinine, Iron, and Copper levels were estimated using commercial chemical test kits (Bio-Diagnostic Company in Egypt). The analyses were done using spectrophotometer following manufacturer instructions.
Lipid peroxidation status and antioxidant capacity
Malondialdehyde (MDA) as lipid peroxidation indicator and total antioxidant capacity (TAC) as indicator of total antioxidant status were determined in the serum of the control and affected cows using commercial kits (Bio-Diagnostic Company in Egypt). These parameters were analyzed using spectrophotometer following manufacturer instructions.
Statistical analysis
Data were analyzed statistically using SPSS program. Two sample t-test was used to compare the differences in mean values between both groups (Snedecor and Cochran, 1994). All values were expressed as mean and standard deviation of mean (SD), and the observed differences were significant when the P value was less than 0.05.
Results and Discussion
Clinical examination findings
High fever (40.90±0.42°C in diseased cows vs. 38.67±0.22°C in controls), inappetence, depression, congested mucous membranes, cessation of rumination, and often dark red to brown urine were the most consistent clinical signs in babesiosis affected cows in acute stages. Coffee-colored urine was a characteristic clinical feature in severely affected cases. General weakness, labored breathing (43.20±4.30 cycle/min in diseased cows vs. 23.12±2.44 cycle/min in controls), tachycardia (84.20± 2.41 beat/min in diseased cows vs. 54.30±1.1.96 beat/min in controls) and pale to deep yellow mucous membranes were recorded in more prolonged cases. In advanced stages, diseased cases suffered severe weakness, incoordination followed terminally by recumbency, coma, and death. On the other hand, clinical examination showed that control cows were clinically healthy and free from any abnormal findings.
Microscopic examination findings
Microscopical examination of Giemsa-stained slides from diseased cases revealed babesia as pear-shaped forms inside RBCs. The parasitemia level of B. bigemina was high (> 10%) in the diseased cases (Figure 1). Whereas blood films from the clinically healthy animals were free from babesia.
PCR findings
PCR results revealed that DNA extracts of whole blood samples from diseased cases were positive for B. bigemina where specific bands were detected at 211 bp (Figure 2).
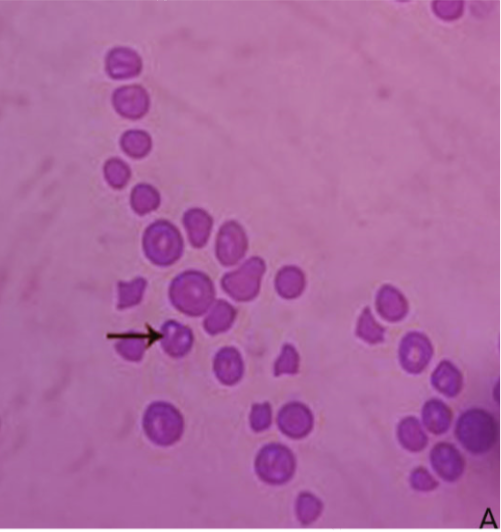
Figure 1: Blood film stained with Giemsa from infected cattle (Group B). Arrow showing B. bigemina infected RBCs.
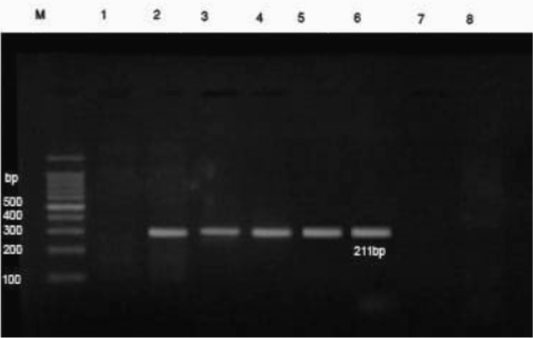
Figure 2: PCR findings of B. bigemina infected samples. M- Ladder of 100 base pair. (1) Control -ve, (2) B. bigemina control +ve, (3-6) positive babesiosis samples of 211 bp, 7 and 8 negative samples. The positive babesiosis samples showed the specific amplicon size of B. bigemina apical membrane antigen 1 (AMA-1) of 211bp.
Hematological findings
Hematological findings are summarized in Table 1. Significant reduction (P<0.05) of RBCs count, hemoglobin concentration, hematocrit and neutrophils along with significant elevation (P<0.05) of lymphocytes and monocytes were recorded in diseased group. No significant differences in the total leukocytic count between the two groups were observed. The obtained data showed that the babesiosis affected cows suffered a pronounced normocytic normochromic anemia.
Table 1: Hemogram in healthy and babesiosis affected cows (Mean±SD).
| Parameter | Controls | Babesiosis affected cows | P-value |
|
TEC (106/ul) |
7.37±0.55 | 3.92±1.29 |
˂0.001** |
| HB (g/dl) | 12.22±1.32 | 5.73±0.99 |
˂0.001** |
| PCV (%) | 35.58±3.56 | 17.19±2.96 |
˂0.001** |
| MCV (fl) | 49.53±9.09 | 48.21±18.47 | 0.696 (NS) |
| MCH (pg) | 16.78±2.40 | 16.07±6.16 | 0.7 (NS) |
| MCHC (%) | 33.64±0.69 | 33.19±1.58 | 0.522 (NS) |
|
TLC (103/ul) |
6.79±1.07 | 5.18±2.22 | 0.088 (NS) |
| Neutrophils (%) | 35.40±4.26 | 23.50±6.86 |
˂0.001** |
| Lymphocytes (%) | 57.40±4.68 | 67.50±4.78 |
˂0.001** |
| Eosinophils (%) | 3.61±1.62 | 3.67±1.92 | 0.644 (NS) |
| Monocytes (%) | 3.59±1.21 | 5.33±1.56 | 0.024* |
*Healthy and diseased cows significantly different at p<0.05. **Significant at p<0.001. NS: Non-significant; TEC: Total erythrocytic count; Hb: Hemoglobin; PCV: Packed cell volume; MCV: Mean corpuscular volume; MCH: Mean corpuscular haemoglobin; MCHC: Mean corpuscular hemoglobin concentration; TLC: Total leukocyte count.
Table 2: Biochemical findings in healthy and babesiosis affected cows (Mean±SD).
| Parameter | Controls | Babesiosis affected cows | P-value |
| Total protein(g/dl) | 6.21±1.42 | 5.71±2.07 | 0.09 (NS) |
| Albumin (g/dl) | 3.96±0.31 | 2.98±0.36 |
˂0.001** |
| Globulin (g/dl) | 2.18±0.48 | 3.50±0.97 | 0.004* |
| A/G ratio | 1.77±0.27 | 0.96±0.44 |
˂0.001** |
| ALT (U/L) | 17.40±4.16 | 21.99±9.05 | 0.91 (NS) |
| AST (U/L) | 29.44±2.50 | 32.53±14.45 | 0.216 (NS) |
| Total bilirubin(mg/dl) | 0.40±0.19 | 3.50±1.15 |
˂0.001** |
| Direct bilirubin(mg/dl) | 0.33±0.15 | 0.99±0.50 | 0.001** |
| Indirect bilirubin(mg/dl) | 0.10±0.05 | 2.51±0.11 |
˂0.001** |
| Glucose (mg/dl) | 69.56±11.30 | 73.19±12.91 | 0.247 (NS) |
| Urea (mg/dl) | 14.86±3.56 | 22.69±6.06 | 0.002* |
| Creatinine (mg/dl) | 2.17±0.16 | 3.08±0.41 |
˂0.001** |
| Iron (ug/dl) | 145.23±15.50 | 211.71±30.32 | 0.004* |
| Copper (ug/dl) | 113.71±10.19 | 173.23±25.51 | 0.002* |
*Healthy and diseased cows significantly different at p<0.05. **Significant at p<0.001. NS: Non-significant.
Biochemical findings
Biochemical changes are shown in Table 2. Significant elevations (P<0.05) in the serum globulin, urea, creatinine, total, conjugated and unconjugated bilirubin mean values along with significant reductions (P<0.05) in serum albumin mean values were recorded in babesiosis affected cows in comparison with controls. AST and ALT activities were relatively higher in diseased group than healthy group. Concerning trace elements, the results indicated significant elevation (P<0.05) in serum iron and copper concentrations in babesiosis affected cows when compared with control cows.
Table 3: Lipid peroxidation and antioxidant status in healthy and babesiosis affected cows (Mean±SD).
| Parameter | Controls | Babesiosis affected cows | P-value |
| MDA (nmol/ml) | 3.15 ±0.80 | 9.11±1.73 | 0.002* |
| TAC (mM/L) | 0.27±0.09 | 0.13±0.04 | 0.016* |
*Healthy and diseased cows significantly different at p<0.05. TAC: total antioxidant capacity; MDA: malondialdehyde.
Lipid peroxidation and antioxidant capacity
Significant rise (P<0.05) in the serum malondialdehyde activity (MDA) accompanied by significant reduction (P<0.05) in the total antioxidant capacity was observed in cows with clinical babesiosis when compared to controls (Table 3).
Bovine babesiosis is one of the most economically important and serious tick-borne diseases affecting cattle in Egypt. The great economic losses of bovine babesiosis are due to deaths, reduced meat/milk production, abortions and costs of treatment and control programs (Bock et al., 2004; Radostits et al., 2007). Perfect and early diagnosis with demonstration of hematological and biochemical alterations in cattle with babesiosis are essential for treatment and control measures of such serious disease. In this study, babesiosis was diagnosed based on clinical examination findings, microscopic examination and PCR results.
Concerning clinical examination, the findings showed that babesiosis has a very characteristic clinical picture in which high fever, inappetence, depression, congested mucous membranes, cessation of rumination, and often dark red to brown urine were the most consistent clinical signs in acute cases. Coffee-colored urine was a common sign in severely affected cases. General weakness, labored breathing, accelerated heart rate and pale to deep yellow mucous membranes were recorded in prolonged cases. In advanced stages, diseased cases suffered severe weakness, incoordination followed terminally by recumbency, coma, and death. Similar findings were reported by El-Diasty et al. (2017), Constable et al. (2017) and Hashem et al. (2018).
Hemoglobinuria as a result of the intense hemolysis, high fever due to parasitemia, and hemoglobinemia were the major recorded clinical findings in affected animals in this study. On the other hand, the rest of the observed clinical findings occurred as consequences for both pyrexia and hemolysis. Depression, congested visible mucous membranes, anorexia and cessation of rumination might be linked to the persistent high fever in affected cows. Hypoxia as a result of severe hemolytic anemia caused by intense hemolysis could be the main cause of weakness and accelerated respiratory and cardiac rates in order to compensate the inadequate tissue oxygenation in babesiosis affected cows. The pallor to icteric visible mucous membranes in cows with clinical babesiosis was attributed to the severe hemolytic anemia and hyperbilirubinemia in advanced stages. These results are in agreement with Lakshmi Rani et al. (2010).
Giemsa-stained blood smears from diseased cases revealed presence of pear-shaped piroplasms of the babesia inside red blood cells. Demonstration of such findings inside red blood cells is confirmative of diagnosis (Singh et al., 2000; Almeria et al., 2001). The parasitemia level of B. bigemina inside the infected RBCs was higher than 10%. This finding coincided with the recorded parasitemia level of B. bigemina (Friedhoff, 1994).
Further confirmation was performed by the molecular detection of the protozoan in blood samples from diseased cases using PCR. In this instance, PCR findings revealed that blood samples from diseased cows were positive for B. bigemina where specific bands were detected at 211 bp (Sivakumar et al., 2012). The high prevalence of B. bigemina among cattle in Beni-Suef district, Egypt was recorded recently (El-Dakhly et al., 2020) that was attributed to the high prevalence of the tick vector R. annulatus in this area (Aboelhadid et al., 2018).
Concerning erythrogram, RBCs count, hemoglobin concentration and PCV were significantly lower in cows with babesiosis than controls. Moreover, MCV, MCH, and MCHC were non-significantly changed and their values were within the normal range in all animals. These results indicated that babesiosis affected cows in this study suffered a pronounced normocytic normochromic anemia during disease course. The results are in agreement with those of Pandy and Misra (1987), Hussein et al. (2007) and El-Diasty et al. (2017).
The significant reduction in TEC, Hb and PCV with the subsequent anemia was attributed to the massive intravascular hemolysis associated with presence Babesia spp. inside RBCs (Callow and Pepper, 1974), production of auto-antibodies directed against circulating erythrocytes (Goes et al., 2007) and increased phagocytosis of parasitized and even unaffected erythrocytes by activated macrophages (Court et al., 2001).
Shifting to leukogram, our data revealed significant increase in lymphocytes and monocytes with a significant decrease in neutrophils% in babesiosis affected group, while the total leukocytic count insignificantly changed (Mohamed, 2017). These changes were attributed to stimulation of phagocytic cells like lymphocytes and monocytes associated with RBCs breakdown for removal of the toxic remnants of damaged erythrocytes (Guglielmone et al., 1996) and activation of body defense mechanisms for antibodies production against the protozoan in response to babesia infection (Court et al., 2001). The significant reduction in neutrophils maybe attributed to neutrophilic sequestration in the spleen, hematopoietic precursor, cell damage, increased neutrophil adherence, or a combination of all (Akel and Mobarakai, 2017).
As shown in Table 2, the obtained data revealed significant biochemical alterations in babesiosis affected cows pointed to impairment of liver and kidney functions in these cows. The intense hemolysis and consequently hemoglobinemia, severe hemolytic anemia and hypoxia in conjunction with the harmful effects of the toxic metabolites of the protozoan results in hypoxic and toxic damage of hepatic and renal tissues and impairment of their functions during disease course (Hamoda et al., 2014).
Concerning liver function, the results showed significantly decreased serum albumin levels along with significantly elevated serum globulin levels in diseased cases compared to controls. The decreased albumin levels were attributed to the disturbed hepatic function and the anorexia that accompanied fever in diseased animals (AL-Aboud et al., 2005), while the elevated globulin levels were attributed to the immune response against Babesia infection (Norimine et al., 2004). Additionally, there were significant elevations in the serum bilirubin levels (indirect, direct and total bilirubin) that was attributed to the intense hemolysis and hepatic dysfunction in diseased animals (Allen and Kuttler 1981; Schwint et al., 2009). Furthermore, AST and ALT serum activities were higher (P>0.05) in the babesiosis affected group than the healthy group. This also was linked to the hepatic cell damage as a result of hypoxia and the produced toxic metabolites in diseased cows (Allen and Kuttler, 1981).
Shifting to renal function, the current study showed significant rise in urea and creatinine serum levels in the diseased cases compared to controls which was attributable to the impaired renal function caused by hypoxic and toxic renal tissue damage as a result of hypoxia and hemoglobinuria in babesiosis affected cows (Camacho et al., 2005; Hamoda et al., 2014).
Concerning trace elements, the results in the present study revealed significant increase in serum iron and copper levels in babesiosis affected cows as compared to controls. Several researchers suggested that the higher concentration of serum iron and copper was attributed to the massive intravascular hemolysis (Pandy and Misra, 1987; Kozat et al., 2003; Hussein et al., 2007). During hemolytic anemia serum iron concentration is increased due to the increased iron transfer from machrophages to plasma (Kaneko et al., 1997; Watanable et al., 1998). Moreover, abnormal erythrocytes are recognized and phagocytosed by the macrophages in the spleen, marrow and/or liver. Hemoglobin is degraded to globin, heme and iron within macrophages therefore, serum iron increased (Stockham and Scott, 2002).
Oxidative stress has been reported in babesiosis affected animals (Kumar et al., 2019). In Bovine babesiosis, activation of inflammatory cells caused by Babesia infection is an important part of the host defense against the parasite (Bock et al., 2004; Saleh, 2009) resulting in excessive pro-inflammatory cytokines production from mononuclear cells/macrophages (Shoda et al., 2000; Goff et al., 2002).The overproduced cytokines activate oxidant-generating enzymes in inflammatory cells causing production of high levels of reactive oxygen and nitrogen species that primarily attack and kill the parasite (Beckman and Koppenol, 1996; Goff et al., 2002). The reactive species induce damage to membrane, nucleic acid and protein of these parasites causing their death (Stich et al., 1998; Kumar et al., 2006; Saleh, 2009). Uncontrolled release of excessive amounts of reactive species and the inability of antioxidants to overwhelm them may induce oxidative damage to host cells and tissues (Zaidi et al., 2005). Lipids especially polyunsaturated fatty acids are the major oxidation targets. Because of abundant polyunsaturated fatty acids in RBCs membrane, they are highly susceptible to oxidative stress and lipid peroxidation resulting in decreased membrane stability, increased membrane permeability, increased RBCs morphological changes and fragility and accordingly anemia (Wagner et al., 1988; Saluja et al., 1999).
Malondialdehyde is an end product of lipid peroxidation. It is a sensitive and commonly used lipid peroxidation biomarker (Chiu et al., 1982). In the current work, significant elevations in MDA levels in cows with babesiosis in comparison with controls were observed. This significant rise of MAD associated with clinical babesiosis pointed to excessive lipid peroxidation with oxidative cellular damage in affected animals during disease course. Similar findings have been reported in previous studies (Saleh, 2009; Salem et al., 2016; Kumar et al., 2019).
The TAC represents the aggregate status of all antioxidants. The results revealed significantly decreased TAC in affected cows in comparison with controls that might be attributed to depletion of antioxidants during neutralization of excessive reactive species generated during course of clinical babesiosis in affected group (Salem et al., 2016; Kumar et al., 2019). The significant rise in lipid peroxidation with reduction of the total antioxidant status in diseased animals in this study proved that babesiosis affected cows experienced severe oxidative stress during disease course. Oxidative stress and lipid peroxidation appear to be involved in the pathobiology and progression of bovine babesiosis. Therefore, the administration of antioxidants as supportive therapy beside Babesicidal drugs is recommended for better and fast recovery.
Conclusions and Recommendations
Babesiosis is a serious disease negatively affects cow health and production. The disease has a characteristic clinical picture in which high pyrexia and hemoglobinuria are the main clinical aspects. Babesiosis in cattle is associated with poor blood picture, severe anemia, disturbed liver and kidney functions and severe oxidative stress which contribute to the disease severity, pathogenesis, and prognosis.
Acknowledgements
Authors thank Hanan E. Saeed, assistant lecturer of clinical pathology department, faculty of veterinary medicine, Beni-Suef University for her help.
Author’s Contribution
Abdel-Hamied E., and Mahmoud M.M., designed and performed the experiment. Arafa W., made the parasitological examinations and PCR assay. All authors participated in discussing the results and writing the final manuscript.
Conflict of interest
The Authors have declared no conflict of interest.
References






