Advances in Animal and Veterinary Sciences
Research Article
Community Composition of Parasitic Nematodes of Cyprinion Macrostomum from North and Mid West Regions In Iraq
Azhar A. Al-Moussawi1, Harith Saeed Al-Warid2*
1Iraq Natural History Research Center and Museum, University of Baghdad, Baghdad, Bab AL-Muadham 10047, Baghdad, Iraq; 2Department of Biology, College of Science, University of Baghdad, Al-Jadriyah Campus, Baghdad 10071, Iraq.
Abstract | The present study was undertaken to investigate the community composition and the infection patterns of parasitic nematodes among preserved Cyprinion macrostomum specimens (n=50) which were collected previously from the Tigris and Euphrates rivers at two different regions in Iraq (North region and Midwest region). Ninety-four nematodes (adults and late stage larvae) were counted from fish specimens. The overall prevalence of Rhabdochona spp. nematodes found highest among examined fishes (n=19, 38%). Prevalence of Rhabdochona spp. (adults and larval stages) differed significantly between fishes from North region and fishes from Midwest region. Only two Rhabdochona species nematodes were found prevalent among examined fish included Rhabdochona denudata and Rhabdochona tigridis. Both adult stages of Rhabdochona denudata and Rhabdochona tigridis showed significant differences in their prevalence between Midwest region and North region. Fish from Midwest region showed higher prevalence (42.9%) for each Rhabdochona denudata and Rhabdochona tigridis compared with to north region. Mean intensity did not differ significantly between fishes from North and Midwest regions for Rhabdochona denudate and Rhabdochona tigridis.
Keywords | Parasitic nematodes, Prevalence, Cyprinion macrostomum, Rhabdochona denudata, Rhabdochona tigridis
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | October 25, 2018; Accepted | December 06, 2018; Published | January 07, 2019
*Correspondence | Harith Saeed Al-Warid, Department of Biology, College of Science, University of Baghdad, Al-Jadriyah Campus, Baghdad 10071, Iraq; Email: [email protected]
Citation | Al-Moussawi AA, Al-Warid HS (2019). Community composition of parasitic nematodes of cyprinion macrostomum from north and mid west regions in iraq Adv. Anim. Vet. Sci. 7(3): 214-217.
DOI | http://dx.doi.org/10.17582/journal.aavs/2019/7.3.214.217
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2019 Moussawi and Warid. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
The Tigris kingfish Cyprinion macrostomum is one of the most important fish species in riverine in Iraq (Coad, 2010). It has wide distribution in the neighbored countries of Iraq especially Iran, Syria and Turkey (Nasri et al., 2013). Cyprinion macrostomum feeds on some zooplanktons and phytoplanktons (Nasri et al., 2018). More than sixty parasite species have been recorded from C. macrostomum in Iraq such as Dactylogyrus macrostomi, D. mascomai, D. pulcher and Dogielius molnari (Mhaisen et al. 2013, Mhaisen et al., 2017, Mhaisen et al., 2018).
Rhabdochona denudata was described for the first time as a new species by Rahemo and Kasim (1979) with the name Rhabdochona mesopotamica. It has subsequently been considered as a synonym of Rhabdochona denudata by Moravec et al. (1991). for this, Rhabdochona denudata has been adopted for this nematode. Rahemo (1978) presented Rhabdochona tigridis as a new species under the name Rhabdochona tigrae. Later on Moravec et al. (2009) considered it as a synonym of Rhabdochona tigridis.
The present study was undertaken to investigate the community composition and the infection patterns of parasitic nematodes among preserved Cyprinion macrostomum specimens which were collected previously from the Tigris and Euphrates rivers at two different regions in Iraq (North region and Midwest region).
MATERIALS AND METHODS
Preserved fishes (n=50) from the Iraq Natural History Museum were used to examine the community composition of some parasitic nematodes All fishes were identified as Cyprinion macrostomum by fish specialist from Natural History Research Center and Museum. The museum records showed that these fishes had been collected from different locations in North and Midwest regions of Iraq. Fish carcasses were examined after having been preserved in 4% formalin for up to 44 years. Gastrointestinal tracts of fishes were removed, opened longitudinally, nematode specimens were isolated and preserved in 70% ethanol, cleared by lactophenol and identified morphologically according to Rahemo and Kasim (1979) and Nasri et al. (2013).
Prevalence of infection was calculated as the percent of examined/ infected hosts as defined by Bush et al. (1997). Confidence intervals (95% CI) for prevalence were considered via Quantitative Parasitology 3.0 (Rózsa et al., 2000). Differences in prevalence were examined among regions using Fisher’s exact tests and chi-square test. To evaluate regional variation, individuals were categorized into two regions: North region (Kurdistan and Ninawa, n=36) and Midwest region (Salahuddin and Anbar, n=14). Intensity of infection was calculated according to Bush et al. (1997)as the number of helminths in an infected hosts/ number of infected hosts with 95 % CIs of mean intensity considered using bootstrap tests (Rózsa et al., 2000).
Mean intensity of infection according to regions was calculated, but statistical comparisons across regions were conducted by using a Mood’s median test. Aggregation of helminths among hosts was measured from variance/mean ratios (s2/m). All statistical analyses of intensity and aggregation were analyzed using QP 3.0.
RESULTS AND DISCUSSION
Ninety four nematodes (adults and late stage larvae) were recovered from preserved fish specimens (range= 1-28 nematodes/fish) comprising 27 adult nematodes (29.03%).Two species of nematodes were successfully identified as Rhabdochona denudata (n=17) and Rhabdochona tigridis (n=9). Figure 1A & 1B, Figure 2A & 2B. Remaining 67 nematode samples cannot be identified up to species level as they were all larval stages. These samples were identified to the generic level as Rhabdochona spp. larval stages (n=66) and Capillaria spp. (n=1). Each of species observed in this study have been previously reported in Iraq from different fish species and from different locations across the country (Moravec et al., 1991; Moravec et al., 2009; Moravec et al., 2012; Ali et al., 2014).
The prevalence of Rhabdochona spp. which occurred in 38% (CI = 26.5– 50.6) of the examined fishes. (Table 1). However, no detailed assessments of the prevalence or intensity of infection with Rhabdochona spp. based on necropsy has been previously reported for Iraq in general and for north and midwest regions in particular. Further, former works on fish nematode communities in Iraq conducted in 1970s and 1990s reported the presence of Rhabdochona spp. (Rahemo and Kasim, 1979; Moravec et al., 1991), which suggest the parasite may be spreading in the Iraq.
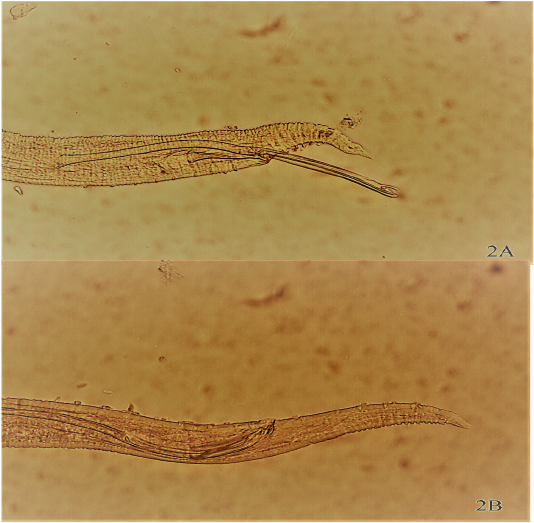
Figure 1: Anterior (A) and Posterior (B) extremity of male Rhabdochona denudata recovered from intestine of Cyprinion macrostomum,(100 X), (40 X) respectively.
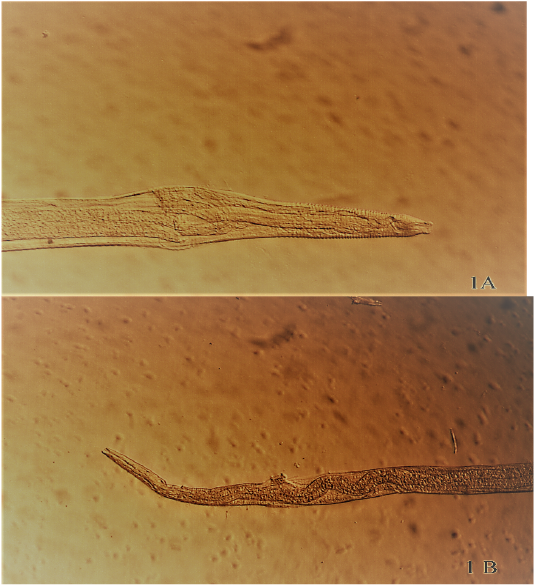
Figure 2: Anterior (A) and Posterior (B) extremity of male Rhabdochona tigridis recovered from intestine of Cyprinion macrostomum, (100 X) for each (A & B).
Although low numbers of fish were collected and only two regions were surveyed in the current study, these results suggest the parasite has predictable widespread in North and Midwest region of Iraq. Prevalence of Rhabdochona spp. (adults and larval stages) differed significantly (Fisher’s Exact test; p ˂ 0.05) between fishes from North region (n = 36; prevalence = 19.4 %; CI = 8.19–36.03) and fishes from Midwest region (n = 14; prevalence = 85.7 %; CI = 57.18–98.23). Both adult stages of R. denudata and R. tigridis showed significant differences in their prevalence between Midwest region and north region (X2= 4.9, p=0.026, X2=10.4, p=0.001, respectively). Fishes from Midwest region showed high prevalence rate (42.9%) for both R. denudata and R. tigridis comparing with the north region (Table 2 and 3). Such strong site-specific prevalence differences in the degree of parasitism, seen in the present study, are widespread in parasitology (Gibson et al., 2016). These patterns may be inclined by environmental features such as habitat and host densities (Vasemägi et al., 2017). On the other hand, results of mean intensity did not vary significantly between fishes from North and Midwest regions for Rhabdochona spp. (adults and larva stages), Rhabdochona denudata (adults) and Rhabdochona tigridis (adults). (Tables 1, 2 and 3).
Table 1: Prevalence of Rhabdochona spp. (adults and larva stages) and mean intensity for all fish examined as well as subsets of the total population.
| Population | Number of fish examined | Number of infected fish (%) | Mean intensity (median) | 95% CI |
| All | 50 | 19 (38) | 4.48 (3) |
26.5- 50.6 |
| North region | 36 | 7 (19.4) | 7.29 (4) |
8.19- 36.03 |
| Midwest region | 14 | 12 (85.7) | 3.42 (2) | 57.18-98.23 |
Table 2: Prevalence of Rhabdochona denudata (adults only) and mean intensity for all fish examined as well as subsets of the total population.
| Population | Number of fish examined |
Number of infected fish (%)
|
Mean intensity (median) | 95% CI |
| All | 50 | 11 (22) | 1.55 (1) | 11.52-35.9 |
| North region | 36 | 5 (13.9) | 1.6 (1) |
4.66- 29.5 |
| Midwest region | 14 | 6 (42.9) | 1.50 (1) |
17.6- 71.1 |
No significant differences were noticed between the prevalence of the adults for both R .denudata and R. tigridis (Table 2 and 3). Also, the relative intensity of these two species was similar across hosts (Figure 3). Such pattern of similarity is likely caused by similarity in host susceptibility or host contact to the parasite in one population or in two close populations (Moravec and Jirků, 2014).
Table 3: Prevalence of Rhabdochona tigridis (adults only) and mean intensity for all fish examined as well as subsets of the total population.
| Population | Number of fish examined |
Number of
infected
fish (%)
|
Mean intensity (median) | 95% CI |
| All | 50 | 7 (14) | 1.14 (1) | 5.81-27.7 |
| North region | 36 | 2 (5.6) | 1 (1) | 0.6-18.6 |
| Midwest region | 14 | 6 (42.9) | 1.17 (1) | 17.6-71.1 |
Table 4: Aggregation of different taxa that parasitized Cyprinion macrostomum from North and Midwest regions of Iraq.
| Taxa | Number of hosts | Variance /mean | |
| Total | Infected | ||
|
Rhabdochona spp. (adults + larvae) |
50 | 19 | 11.43 |
|
R. denudata (adults) |
50 | 12 | 1.63 |
|
R. tigridis (adults) |
50 | 7 | 1.11 |
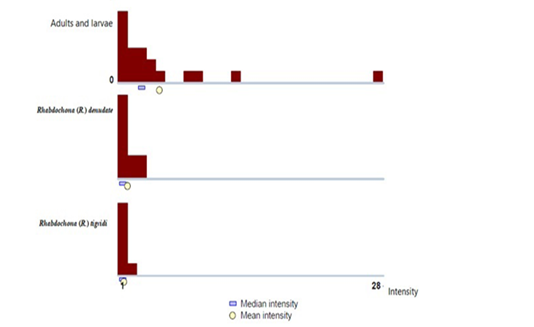
Figure 3: Histograms representing the number of Rhabdochona spp. (adults and larvae), R. denudata (adults) and R. tigridis (adults) collected per Cyprinion macrostomum (n = 50) from North and Midwest regions of Iraq.
For nematode taxa with sufficient sample sizes, variance/ mean for each Rhabdochona spp. (adults and larva stages), adults of both Rhabdochona denudata and Rhabdochona tigridis indicated an aggregated distribution of parasites across examined fishes (Table 4). The aggregation of Rhabdochona spp showed to be high (11.43). The detected patterns of aggregation propose the potential to use investigational and observational methods to assess which environmental and demographic features of individual fish predict best infection status (Wilber et al., 2016). Such analyses are an imperative stage in undertaking informed parasite managing.
conflict of interest
There is no conflict of interest.
authors contribution
All the authors contributed equally.
REFERENCES






