Advances in Animal and Veterinary Sciences
Research Article
Macroanatomy and Angiography of Coronary Arteries in Equus asinus
Aya S. El-Sonpaty1, Karim M. Khalil1, 2, Mohammed A. Salem3, Medhat Ahmed El-Ayat1 *
1Anatomy and Embryology Department, Faculty of Veterinary Medicine, Cairo University, Giza 12211, Egypt; 2College of Applied & Health Sciences, A’Sharqiyah University, Ibra, Oman; 3Department of Radiology, Faculty of Medicine, Cairo University, Giza 12211, Egypt.
Abstract | The present study was investigated the morphological features of the coronary arteries in the donkey. The study was carried on the hearts of thirty adult donkeys. The specimens were injected with red gum milk latex/barium suspension through the right and left coronary arteries. The injected hearts were imaged then preserved in 10% acidified formalin solution before the dissection. The coronary artery is a bilateral coronary type. No anastomosis was observed between both left and right circumflex branches. A high incidence of right coronary dominance was observed. The present study traced rare cases of arterial trifurcation of the left coronary artery along with the presence of a left diagonal branch. The conus arteriosus had supplied by two main branches, R. coni arteriosus dextra from A. coronaria dextra and R. coni arteriosus sinister from A. coronaria sinistra and they were anastomosed together at the margo cranialis. The myocardial bridge as a congenital heart defect was recorded in five specimens. In conclusion, the coronary circulation of donkeys was more similar to those of horses and mules.
Keywords | Equus asinus, Arteria coronaria, Coronarography, Coronary circulation, Heart.
Received | July 22, 2021; Accepted | August 02, 2021; Published | October 01, 2021
*Correspondence | Medhat Ahmed El-Ayat, Anatomy and Embryology Department, Faculty of Veterinary Medicine, Cairo University, Giza 12211, Egypt; Email: [email protected]
Citation | El-Sonpaty AS, Khalil KM, Salem MA, El-Ayat MA (2021). Macroanatomy and angiography of coronary arteries in equus asinus. Adv. Anim. Vet. Sci. 9(11): 1964-1972.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.11.1964.1972
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 El-Ayat et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
The donkeys are versatile animal, used in ride, light draught work, car traction in rural areas, for anatomy teaching purposes as well as a cheap source for meat consumption for wild animals (Norris et al., 2021). There is a shortage of information about the donkey and it is often imperfectly treated as a small horse (Senior, 2013). The anatomy of the coronary arteries among animal kingdom is fascinating and most varied. In quadruped, the heart is supplied via coronary circulation; the right coronary artery [arteria (a.) coronaria dextra] and the left coronary artery (a. coronaria sinistra). Coronary vessels have been studied in many animal species including laboratory animals (Icardo and Colvee, 2001, Sans-Coma et al., 1993, Vicentini et al., 1991), pet animals (Barszcz et al., 2016, Barszcz et al., 2013; Dursun, 1979), and farm animals (Bertho and Gagnon, 1964; George et al., 1969; Ghazi and Tadjalli, 1993; Hegazi, 1962; Karadag and Soyguder, 1989; Kupczyńska et al., 2015; Tipirdamaz, 1987; Yuan et al., 2009).
On revising the literature that dealt with the coronary arteries anatomy in equine found to be very scarce (El-Bakary et al., 1993; Gómez et al., 2017a; Gómez et al., 2017b; Ozgel and Dursun, 2005; Ozgel et al., 2004; Rawlings, 1977), thus motivated our attention to shed a light on this subject specially to determine the origin, course and distribution of the coronary arteries in donkey. The present work could be a guide for further anatomical and clinical researches as radiographs, scans, angiography as well as surgical interventions.
Materials and Methods
Specimens collection and preparation
A total of 30 hearts of adult healthy donkeys of different sex (15 males and 15 females) were collected from the local dissecting hall in Faculty of Veterinary Medicine in Giza and from slaughterhouse of Giza Zoo after the competent authorities’ approval. Each heart specimen was washed using warm water. After a gross inspection of each heart, the openings of the coronary arteries were identified at the commencement of the ascending aorta. The arteries were rinsed by warm isotonic physiological solution NaCl 0.9% containing a small addition of heparin calcium to remove the blood residual in the blood vessels. The flushing process was continued until the returned fluid appeared pure and clear.
Gum-milk latex technique
The flushed specimens were used for Gum milk latex technique (Sary et al., 2020). Both coronary arteries were separately injected by 5% latex/barium suspension that made by mixing 5g of barium sulphate in 100 mL latex colored with red Rotering® ink. An angiocatheter was inserted into each ostium and loosely secured with a string. A syringe containing the suspension then attached to the angiocatheter. Approximately 15 to 20 milliliter of the suspension was then injected under the hand pressure until slight resistance was encountered. The injected hearts were imaged where the exposure factors were 70 cm. FFD, with 45 KVP and 105 MAS (Amrad Static X-Ray machine, Amarad Medical, USA). The injected specimens then left to preserve in 10% acidified formalin solution for 7 days before they subjected to dissection for exposing the arteries and tracing the various twigs. Measurements of the external diameters of coronary arteries and their branches were taken and recorded in millimeters (mm) using the Vernier’s digital caliper. The nomenclature was adopted according to Nomina Anatomica Veterinaria 6th edition (2017).
Result
The heart of donkey is mainly supplied by two coronary arteries, the right (A. coronaria dextra) and left (A. coronaria sinistra). The right coronary artery is the larger, dominance and supplies most of the myocardium mass as well as the extreme terminal part of the interventricular septum.
Arteria coronaria dextra
The right coronary artery (Fig.1, 3, 4/a) springs from the aorta opposite to the cranial aortic sinus and above the edge of valvula semilunaris dextra. Its mean diameter measured 10 mm at its origin. The right coronary artery leaves its origin, proceeds cranially between the truncus pulmonalis and auricula dextra then sharply bends right to gain the coronary groove in which it persuading a characteristic distorted convoluted course look-like a vermiform appearance, that`s why it seems long in its length. The mean length ranges between 180 - 200 mm. On a level with the caudal edge of the auricula dextra the right coronary artery splits abruptly into two terminal branches, ramus (r.) circumflexus dexter (Fig. 1/c) and r. interventricularis subsinuosus (Fig.1/b). Along its course it gives off three atrial branches and four ventricular branches. (Fig.1). In general, a. coronaria dextra provides nourishment to the atrium dextra, ventriculus dextra and interventricular septum.
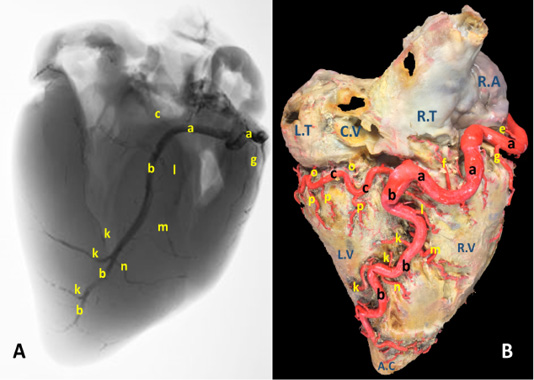
Figure 1: A) A radiograph and B) latex injection of donkey heart showing the distribution of the right coronary artery and its branches on the atrial surface.
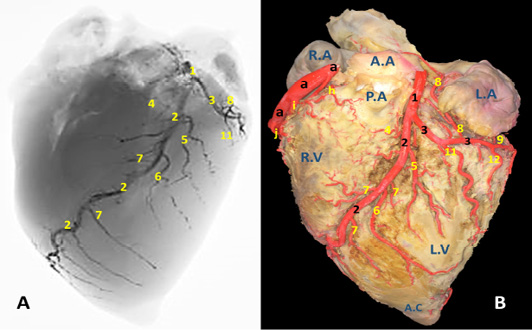
Figure 2: A) A radiograph and B) latex injection of donkey heart showing the distribution of the left coronary artery and its branches on the auricular surface.
Atrial branches of A. coronaria dextra
R. proximalis atrii dexter (Fig. 3 /d) stems from the deep caudal surface of a. coronaria dextra opposite to the base of the auricula dextra. It has a mean diameter determined as 0.2 – 0.4 mm, while Its mean length is measured 13 mm. It leaves its origin then proceeds caudally being concealed by the auricula dextra along the cranial wall of the right atrium in which it is ramified via delicate 2-3-minute atrial rami. R. intermedius atrii dexter (Fig. 1/e) emerges from the caudal aspect of a. coronaria dextra opposite to the free border of right atrium. Its measurements are determined to have an estimated diameter of 0.25 – 0.35 mm and 10 - 15 mm length. It leaves its origin and proceeds caudo-dorsally, crosses the sulcus coronaria to gain the superficial surface of the right atrium in which it is dispersed. R. distalis atrii dexter (Fig.1/f) arises from the dorsal surface of a. coronaria dextra just before its bifurcation and opposite to the sulcus interventricularis subsinuosus. Its mean diameter is measured 0.17 mm. while its length is determined 16 mm. It proceeds caudally opposite to the sulcus interventricularis subsinuosus and ramifies in the caudal part of the right atrium and the initial commencements of the caudal vena cava.
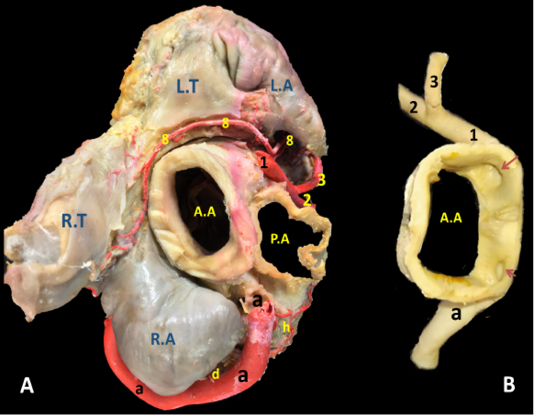
Figure 3: The cardiac base of donkey heart showing A) the origin and directions of the coronary arteries and B) the position of the right and left coronary ostia inside the aorta.
Ventricular branches of A. coronaria dextra
R. marginis convexi (Fig. 1, 4 /g) originates from the ventral surface of a. corona dextra during its passage on the cranial border of the heart being opposite to the margo ventricularis dexter. It proceeds ventrally along margo ventricularis dexter of the heart. Its mean diameter is determined as 0.4 mm, while its length is ranged between 35 - 55 mm. It terminates in the upper third of margo cranialis in 75% of examined cases, in the middle third or in the distal third in 25% of specimens. The vessel shows slight tortuosity during its course. It supplies fine twigs to the right ventricle as well as the cranial border of the heart. R. coni arteriosi dexter (Fig. 2, 3 /h) are originated separately or by a common stem with r. proximalis ventriculi dextri from the cranial aspect of a. coronaria dextra opposite to the auricula dextra. Its mean diameter calculated as 0.25 mm. It leaves its origin opposite to the pulmonary trunk then proceeds ventrally on the cranial wall of the right ventricle then bends left to reach the wall of the conus arteriosus in which it ramifies and anastomoses with r. coni arteriosi sinister. Moreover, it gives small twigs that dispersed into the caudal part of the truncus pulmonalis as well as the aorta. R. proximalis ventriculi dextri (Fig. 2/i) is emanates from the ventral surface of the parent trunk opposite to the origin of the R. Marginis convexi. Its mean diameter is determined as 1.5 - 1.8 mm. It leaves its origin and runs ventrally and caudally through the texture of the auricular surface of the right ventricle then divided into 2-3 branches that ramify on the wall of the right ventricle. R. distalis ventriculi dexter (Fig. 2/j) stems from the ventral surface of a. coronaria dextra. Its average width equal 1.78 mm. Along its course it gives 3-4 fine branches to the wall of the right ventricle near the margin of sulcus interventricularis paraconalis.
Terminal branches of A. coronaria dextra
R. interventricularis subsinuosus (Fig. 1/b) is one of the two terminal branches of a. coronaria dextra. From point of view, this vessel is larger in both length and width than the right circumflex branch. It is considered as the direct continuation of the parent trunk (a. coronaria dextra). It emanates from the right coronary artery with an approximate diameter of 6.84 mm at its commencement. It leaves its origin and proceeds ventrally along the sulcus interventricularis subsinuosus then crosses the margo caudalis to gain the left ventricle. It continues its course ventrocaudally and terminated 20-30 mm far from the apex cordis. Its mean length is determined between 140-150 mm long.
R. interventricularis subsinuosus gives off 2-3 relatively fine branches that supply the apex cordis. Along its course it gives off several branches. Rr. septales dextri interventricularis (Fig. 1/k) are represented by 2-3 branches where the larger one has an average mean diameters determined as 2.60 mm. They arise approximately at right angles from the caudal aspect of the Subsinuosal interventricular branch and proceed deeply to gain the cranial surface of the interventricular septum in which they ramified. R. collaterales dexter proximales (Fig. 1/l) arises from the cranial surface of r. interventricularis subsinuosus at a mean length 3 mm from its origin and opposite to r. circumflex dexter. Its mean diameter is determined as 0.4 mm. R. collaterales dexter intermedeus (Fig. 1/m) proceeds from the cranial surface of r. interventricularis subsinuosus at a distance of 15-20 mm. Its mean diameter is determined as 0.2 mm. It leaves its origin and proceeds cranioventrally to disperse in the wall of the right ventricle. R. collaterales dexter distalis (Fig. 1/n) originates from cranial aspect of r. interventricularis subsinuosus at a distance 20 mm beyond the preceding vessel. Its mean diameter is recorded as 0.3 mm. It pierces the wall of the right ventricle 30 mm far from the cardiac apex and ramifies in the myocardium of the right ventricle.
R. circumflexus dexter (Fig. 1, 4/c) is one of the couple terminal branches of a. coronaria dexter. It traverses caudally through the right portion of the coronary sulcus, crossing the margo caudalis to gain the left surface of the heart. Its mean length is determined as of 55 mm and have an approximate diameter of 5.76 mm at its origin. It supplies the caudal aspect of the left ventricle especially its obtuse margin. On a level with the margo ventricularis sinister, it is divided into two fine branches which supply the wall of the left ventricle. No anastomosis is observed with the same branch of the left side. Along its course it gives off atrial (Rr. atrialis) and ventricular (Rr. ventriculares) branches.
Rr. atrialis of r. circumflex dexter (Fig. 1/o) are three in number with a mean diameter ranges between 0.2 - 0.4 mm. The first branch is a considerable vessel arises at the commencement of the parent trunk and dedicated for nourishment of the atrioventricular node. The rest of the atrial branches arise from the dorsal surface of the parent artery being concealed beyond the caudal vena cava. The mean distance between these branches ranges between 10 - 15 mm. The atrial branches irrigate the wall of atrii dexter as well as the base of the caudal vena cava.
Rr. ventriculares of r. circumflex dexter (Fig. 1/p) are three considerable branches arise from the ventral surface of the right circumflex artery, proceed caudoventrally gain the middle third of the right ventricle to which it ramify then continues caudally to reach the margo caudalis where they arborize in the texture of the left ventricle.
Arteria coronaria sinistra
The left coronary artery (Fig. 2, 3, 5 /1) is a short vessel, smaller in length and width if compared with the right one. Its measurements are determined as 45 mm in length and 0.8 mm in width. The left coronary artery emanates from left aortic sinus of the ascending aorta behind the left semilunar valve. It leaves the aorta, soon bends left then proceeds in a caudoventral direction between left auricle and pulmonary trunk. On gaining the sulcus coronarius, it bifurcates into two terminal branches: r. interventricularis paraconalis (Fig. 2, 3, 5 /2) and r. circumflex sinister (Fig. 2, 3, 5 /3). The former vessel is the larger in width and length. It descends in a ventrocranial direction through sulcus interventricularis paraconalis while the r. circumflex sinister proceeds caudally to occupy its location in the left portion of the sulcus coronarius. Generally, a. coronaria sinistra through its branches supplies the wall of left atrium and ventricle.
R. interventricularis paraconalis (Fig. 2, 3, 4, 5 /2) is the direct continuation of A. coronaria sinistra. It extends obliquely in a caudoventral direction toward the apex cordis. It continues in the paraconal interventricular groove accompanying the vena cordis magna toward the cranial border of the heart. Its mean length was determined as 86.50 mm. while its mean width was recorded as 6.5 mm at its origin. At a distance of approximately 45-50 mm from the apex cordis the paraconal interventricular branch terminates by two terminal branches named r. terminalis dorsalis et ventralis.
R. terminalis dorsalis is one of the two terminal branches of r. interventricularis paraconalis. Its mean diameter is determined as 0.35 mm while its mean length is approximately 17 mm. It proceeds cranioventrally along the ventriculus dexter in which it ramifies. R. terminalis ventralis is the other terminal branch. It is larger than its partner with a mean width 0.42 mm. It leaves its origin and extend cranioventrally toward the apex cordis. Along its course it gives off 3-4 branches that dispersed in the myocardium of the right ventricle as well as the apex cordis.
R. interventricularis paraconalis gives off several branches along its course. R. coni arteriosi sinister (Fig. 2, 5 /4), unlike all other branches which arise from the caudal surface, exits from the opposite cranial side of the parent trunk. It proceeds in a craniodorsal direction to be distributed in the left aspect of the conus arteriosus as well as the base of the truncus pulmonaris. On reaching the cranial border of the heart it is divided to several branches that anastomose with those of the right conal branch. The mean diameter and length of R. coni arteriosi sinister is determined as 3.70 mm and 35.20 mm, respectively. R. interventricularis paraconalis gives rise to three branches. R. collaterales sinister proximales (Fig. 2, 5 /5) emanates from the caudal surface of the paraconal interventricular branch and its diameter is recorded as 1.6 mm. It proceeds ventrally and caudally for 20-30 mm then bifurcates and distributed in the wall of the left ventricle, the interventricular septum as well as the papillary muscles. R. collaterales sinister distales (Fig. 2, 5 /6) is a stout vessel, originates from the caudal surface of the distal one-third of R. interventricularis paraconalis and continues its course ventrally and caudally along the wall of the left ventricle to gain the apex cordis in which it ramified. Rr. septales sinister (Fig. 2, 5 /7) are 4-5 fine branches arise from the cranial surface of R. interventricularis paraconalis near the apex cordis. These branches leave its origin and extend cranially to gain the right ventricle then pierce its wall to reach the cranial surface of the interventricular septum in which it ramified. Along their course give off minute branches that deeply dispersed through the myocardial texture of the ventricle.
R. circumflexus sinister (Fig. 2, 3, 4, 5 /3) is the smaller terminal branch of the left coronary artery. Its mean diameter is 5.30 mm at its origin from the parent trunk, while its length is measured 40-50 mm. Its course is concealed under the auricula sinistra till it gains the caudal edge of ventriculus sinister where it terminates by two branches: R. marginis concave and r. ventriculis sinister. It is worthy to mention that there is no any vascular anastomosis could be traced between this branch and its counterpart on the right side. Along its course, it gives off four atrial branches and two ventricular branches.
Atrial branches of r. circumflexus sinister. R. sinoatriali (Fig. 2, 3 /8) is a stout long branch arises from the left circumflex at a distance of 20-30 mm from origin. Its mean length is determined as 50-58 mm while its diameter is calculated as 0.5 mm. It is worth noting that this vessel takes a reverse course to the parent trunk as it proceeds in an opposite direction. It leaves its origin and bends dorsally then cranially undercover of the auricula sinistri then continues its course between the right atrium and cranial vena cava to supply the sinoatrial node. R. proximales atrii sinister (Fig. 2 /8) is a stout vessel, springs from the dorsal surface of the left circumflex branch at a mean distance of 37mm from its origin, mean diameter is estimated as 2.80 mm while its length is measured as 15 mm. It supplies the left atrium as well as the auricula sinistra. R. intermedeus atrii sinister (Fig. 2 /9) is relatively minor branch with an estimated width of 1.5 mm. It arises from the dorsal surface of the left circumflex branch at a distance of 25 mm from r. proximales atrii sinistri. It proceeds dorsally along the wall of the left atrium as well as the left auricle. R. distalis atrii sinister (Fig. 4 /10) is a small branch with average diameter of 0.64 mm and length of 16 mm. It leaves the left circumflex branch from its dorsal aspect and extends dorsomedially to gain the base of the left auricle in which it ramifies.
Ventricular branches of r. circumflexus sinister. Ramus proximales ventriculi sinister (Fig.2, 4, 5 /11) originates from the ventral surface of the left circumflex branch. Its mean diameter is estimated as 0.28 mm at its origin. It courses caudoventrally toward the caudal border of the heart. It disperses in the wall of the left ventricle. R. distales ventriculi sinister (Fig.2, 4 /12) leaves the ventral surface of r. circumflexus sinister with a mean width of 1.50 mm. It proceeds caudoventrally toward the caudal border of the heart. Along its course it gives off 2-3 minute branches that pierce the wall of the left ventricle.
Terminal branches of r. circumflexus sinister. R. ventriculi sinister (Fig. 4 /14) is one of the two terminal branches of r. circumflexus sinister. It leaves its origin and proceeds caudoventrally to ramify in the wall of the left ventricle near the margo ventricularis sinistri. R. marginis concave (Fig. 4 /13) is the other terminal branches of r. circumflexus sinister. Its mean diameter is estimated as 0.48 mm at the origin. It leaves the ventral surface of the parent trunk and proceeds dorsally and caudally to disperse in the wall of left ventricle.
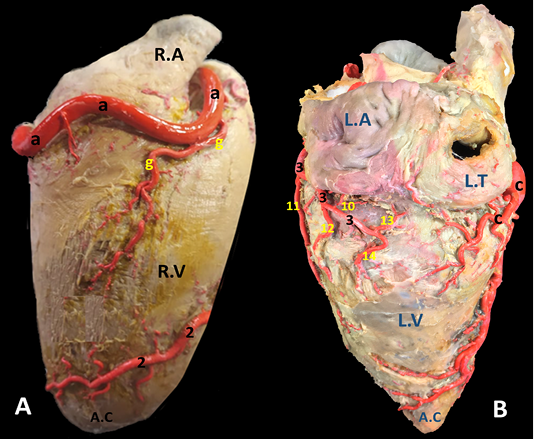
Figure 4: A) Margo ventricularis dexter and B) Margo ventricularis sinister of the donkey heart showing the distribution of coronary arteries.
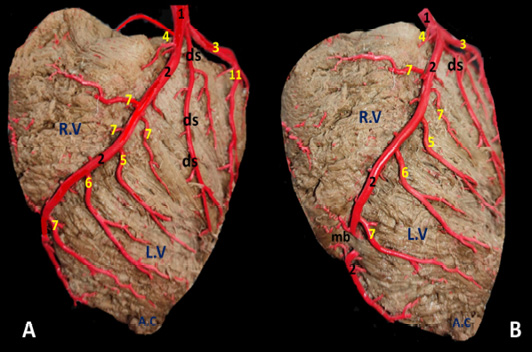
Figure 5: The auricular surface of donkey heart showing A) ramus diagonalis sinister of a. coronaria sinistra and B) myocardial bridge of r. interventricularis paraconalis.
Ramus diagonalis sinister (Fig. 5/ds) is traced in 6 out of 30 (20%) of the examined cases. The existence of such unusual branch led to the trifurcation of the main parent trunk. It has a mean diameter 2.0 mm. and its length is determined as 50.2 mm. It emanates from the A. coronaria sinister at an acute angle between R. circumflexus sinister and R. interventricularis paraconalis. It proceeds ventrally and somewhat caudally along the wall of the left ventricle in which it ramifies.
Myocardial bridge
The myocardial bridge (Fig. 5/mb) is a congenital defect in which one of the coronary arteries underpasses deeply through the myocardium. This defect is rarely traced mostly in ventricles (3 cases left and 2 cases right). The myocardial bridge is elongated in outline and its dimensions is determined 30-40 mm length, 10-20 mm width and 0.3-0.4 mm depth. This defect lead to strangling of the vessel and squeezes to pump blood, where the myocardium wields pressure and compresses the artery, reducing blood flow to the heart.
Legend of figures (1 to 5)
A.A, Aorta. A.C, Apex cordis. C.V, caudal vena cava. P.A, Pulmonary trunk. R.A, right auricle. R.T, right atrium. R.V, right ventricle. L.A, left atrium. L.T, left atrium. L.V, left ventricle.
a, arteria coronaria dextra. b, ramus interventricularis subsinuosus. c, ramus circumflexus dexter. d, ramus proximalis atrii dexter. e, ramus intermedius atrii dexter. f, ramus distalis atrii dexter. g, ramus marginis convexi. h, ramus coni arteriosi dexter. i, ramus proximalis ventriculi dexter. j, ramus distalis ventriculi dexter. k, rami septales dextri interventricularis. l, ramus collaterales dextri proximales. m, ramus collaterales dexter intermedeus. n, ramus collaterales dexter distales o, rami atrialis of ramus circumflex dexter. p, rami ventriculares of ramus circumflex dexter.
1, arteria coronaria sinistra. 2, ramus interventricularis paraconalis. 3, ramus circumflex sinister. 4, ramus coni arteriosi sinister. 5, ramus collaterales sinistri proximales. 6, ramus collaterales sinistri distales. 7, ramus septales sinister. 8, rami proximales atrii sinister. 9, rami intermedeus atrii sinister. 10, ramus distalis atrii sinister. 11, ramus proximales ventriculi sinister. 12, ramus distales ventriculi sinister. 13, ramus marginis concave. 14, ramus ventriculi sinister. ds, ramus diagonalis sinister. mb, Myocardial bridge
Discussion
The results revealed that the arterial blood supply of the heart of donkey was performed by two well developed coronary arteries, right and left as mentioned in most domestic animals (Bertho and Gagnon, 1964). However, an additional accessory coronary artery was traced in some cases in dog (Barszcz et al., 2013; Blair, 1961), man (Truex and Angulo, 1952), and monkey (Nikolić et al., 2004). On the other hand, many cases in bovine were reported with only single coronary artery being responsible for the whole heart’s nourishment, presenting only one ostium from the aorta (Karadag and Soyguder, 1989; Kupczyńska et al., 2015). In the same regard, the left coronary artery had referred to be absent in Syrian Hamster (Durán et al., 2009; Sans-Coma et al., 1993), the results which was not recorded in any of the examined specimens in donkey.
Based on the dimensions, as well as the number of the ventricular and atrial branches, and on the origin of the interventricular subsinuosal branch, the right coronary artery was considered the larger and was more dominant in irrigation of donkey heart. The current investigation had asserted that the arterial domination in donkey heart was contributed to the right coronary artery independently as it had possessed larger dimensions in both length and width than the left one, gave off origin for both r. interventricularis subsinuosus and r. circumflexus dexter and supplies most of the myocardial wall of the heart including the apex cordis and left ventricle. Similar observations were reported in buffalo (George et al., 1969), ox (Ocal and Cakir, 1993), camel (Ghazi and Tadjalli, 1993), and pig (Gómez and Ballesteros, 2015a; Sahni et al., 2008; Weaver et al., 1986). On the other hand, Ozgel et al. (2004) and Dursun (1977) gave a contradictory opinion and mentioned that the left coronary artery was the larger and dominant in donkey. The dominance of left coronary artery was also reported in other domestic animals (Aksoy and Karadag, 2002; Dursun, 1979; Karadag and Soyguder, 1989; Teofilovski-Parapid and Kredovitć, 1998; Teofilovski-Parapid et al., 1993). The bilateral coronary pattern was reported in equines (Gómez et al., 2017a; Gómez et al., 2017b), carnivores (Büll and Martins, 2002; Oliveira et al., 2021), and camelids (Taha and Abel‐Magied, 1996).
The present study had revealed that the right and left coronary arteries originate at the same level from the base of the aorta from openings called the coronary ostia lied behind the aortic semilunar valve leaflets. Similar observations were mentioned in earlier studies in camelid (Hegazi, 1962), equines (El-Bakary et al., 1993; Gómez et al., 2017a; Gómez et al., 2017b; Ozgel et al., 2004; Rawlings, 1977), swine (Gómez and Ballesteros, 2015a), carnivore (Oliveira et al., 2021), feline (Barszcz et al., 2016), caprine and ovine (Dumitrescu et al., 2016; Tipirdamaz, 1987). On the other hand, Hadžiselimović et al. (1980) have recorded a rare case in human where the coronary arteries were originated from a single coronary ostium in the right coronary sinus, a result which could not reported in donkey.
The division of a. coronaria dextra into two main branches, r. interventricularis subsinuosus and r. circumflex dexter, was similar to that traced in farm animal species (George et al., 1969; Hegazi, 1962; Noestelthaller et al., 2007) including equines (Gómez et al., 2017a; Gómez et al., 2017b; Ozgel et al., 2004; Rawlings, 1977). The present findings revealed that the left coronary artery and its terminal rami were smaller than their counterpart in the right side. Similar findings were reported in buffalo (George et al., 1969).
The a. coronaria sinister was divided into two branches, paraconal interventricular and left circumflex, similar to the forementioned studies (El-Bakary et al., 1993; George et al., 1969; Gómez et al., 2017b). The present study had traced a rare cases of arterial trifurcation of a. coronaria sinister along with the presence of r. diagonalis sinister in donkey simulating some cases in humans and pigs (Hadžiselimović et al., 1980; Weaver et al., 1986).
The current work had successfully reported the origin and distribution of the sinoatrial node branch in donkey. In pigs and humans this branch has been reported as originating primarily from the right coronary artery, followed by an origin in the left circumflex branch within a range of 30-45% (Crick et al., 1998; Gómez and Ballesteros, 2015a; Sahni et al., 2008). The present study as well as the observation of Ozgel et al. (2004) in donkey have reported Rr. atrii proximalis et intermedius to be originated from r. circumflex sinister and no anastomosis was reported in between. However, Dursun (1977) and Tecirlioglu et al. (1978) have confirmed the existence of such anastomosis.
Many cases of myocardial bridges of variable diminutions were seen embedded in the ventricular myocardium, the result which has not been mentioned in horses (Gómez et al., 2017a; Gómez et al., 2017b). On the other hand, myocardial bridges have been reported in humans, pigs and camels within a range of 20-80% (Gómez and Ballesteros, 2015b; Taha and Abel‐Magied, 1996; Yuan et al., 2009). In the same regards some researchers have considered the myocardial bridges as a risk factor for the development of certain cardiac pathological conditions like angina (Bourassa et al., 2003). We did not observe any anastomosis between both circumflex branches of both sides. Similar observation was reported in donkey (Dursun, 1977; Ozgel et al., 2004) and mule (El-Bakary et al., 1993).
The present results as well as those given by Ozgel et al. (2004) in donkey revealed that the conus arteriosus had supplied by two main branches, r. coni arteriosus dexter from a. coronaria dextra and r. coni arteriosus sinister from a. coronaria sinistra and they were anastomosed together at the margo cranialis. On the other hand, Bertho and Gagnon (1964) revealed that the origin of the r. coni arteriosi dexter from r. circumflexus dexter in dog and pig. A result contrary to what has been observed in donkey.
Regarding the origin and distribution of r. marginis concave, the present work had reported its origin from the ventral surface of r. circumflexus sinister and dispersed in the wall of left ventricle. In hamster and monkey, its origin from the a. coronaria sinistra itself (Sans-Coma et al., 1993; Teofilovski-Parapid et al., 1993). The current study had traced r. marginis concave in donkey to be originated from the ventral surface of a. coronaria dextra during its course in sulcus coronarius. Its mean diameter was 0.4 mm and its mean length was determined 45 mm and supply both surfaces of the right ventricle, a result which is not mentioned in donkey (Ozgel et al., 2004) or in horse (Gómez et al., 2017a; Gómez et al., 2017b).
The current study had dealt with the blood supply of the interventricular septum from, the left and right interventricular septal branches, the former branches originated from r. interventricularis paraconalis near the apex cordis while the right interventricular septal branches originated from r. interventricularis subsinuosus. These branches had seen ramified in cranial and caudal surfaces of the interventricular septum respectively. Similar observation were reported in all farm animal (Christensen, 1962; Karadag and Soyguder, 1989; Ozgel and Dursun, 2005; Rodriguez et al., 1961; Taha and Abel‐Magied, 1996), pet animals (Barszcz et al., 2014; Dursun, 1979), red fox (El-Bably and Abouelela, 2021), and even the monkey (Teofilovski-Parapid et al., 1993).
Conclusion
The arterial blood supply of the heart in the donkey is provided by a bilateral coronary artery, the left and right coronary arteries, with the right coronary artery being dominant and well developed than the left. the coronary circulation of donkeys was more similar to those of horses, mules and small pony.
acknowledgements
The authors are grateful to the general scientific research department of Cairo University, Egypt for support and assistance.
Conflict of interest
The authors declare no conflict of interest.
Ethical statement
The study was conducted at the Faculty of Veterinary Medicine, Cairo University, Egypt (FVMCU). All experimental procedures and management conditions used in this study were approved by the Institutional Animal Care and Use Committee (IACUC; Reference No. Vet CU24112020243).
authors contribution
All listed authors have made substantial contributions to the research design, or the acquisition, analysis or interpretation of data; and drafting the manuscript or revising it critically. All authors have approved the submitted version.
References






