Advances in Animal and Veterinary Sciences
Research Article
In vivo Study of Mutagenic Activity of the Complex Antibacterial Medication of Mastitis in Mice Model
Sergey V. Shabunin, Galina A. Vostroilova, Liliya V. Cheskidova, Dmitriy I. Shabanov, Anastasiya A. Korchagina*
All-Russian Veterinary Research Institute of Pathology, Pharmacology and Therapy, 394087, 114B, Lomonosova Str, Voronezh, Russia.
Abstract | Complex antimicrobial medications containing penicillin group antibiotics in combination with glucocorticoids are often used intramammary for the treatment of mastitis in lactating cows. However, there is insufficient data on the presence of negative and positive effects of their interaction. In this regard, we set the goal of identifying the potential mutagenic properties of the complex medication containing amoxicillin, cloxacillin and prednisolone as active substances. For this purpose, the samples of metaphase chromosomes were made from the bone marrow cells of white mice. The number of cells with chromosomal aberrations was counted. The micronucleus test was carried out in polychromatophilic erythrocytes of peripheral blood obtained from the tail vein of mice. As a result, it was found that subcutaneous administration of the complex medication to white mice for 7 days at a dose of 80 mg/kg of body weight and a single administration at a dose of 560 mg/kg of body weight did not have a mutagenic effect. This is confirmed by the absence of a significant increase in the proportion of bone marrow cells with chromosomal aberrations and the number of polychromatophilic erythrocytes with micronuclei relative to the values of the control group.
Keywords | Complex medication, Genotoxicity, Mice, Penicillins, Prednisolone
Received | May 06, 2021; Accepted | June 30, 2021; Published | August 15, 2021
*Correspondence | Anastasiya A. Korchagina, All-Russian Veterinary Research Institute of Pathology, Pharmacology and Therapy, 394087, 114b, Lomonosova str, Voronezh, Russia; Email: [email protected]
Citation | Shabunin SV, Vostroilova GA, Cheskidova LV, Shabanov DI, Korchagina AA (2021). In vivo study of mutagenic activity of the complex antibacterial medication of mastitis in mice model. Adv. Anim. Vet. Sci. 9(10): 1601-1607.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.10.1601.1607
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Shabunin et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Mastitis of cattle remains one of the main problems of dairy farming (Pal, 2018; Ruegg and Petersson-Wolfe, 2018). The introduction of antibacterial drugs into the affected lobes of the udder, which ensures that the drug enters the area of the inflammation focus and affects the cause of the inflammatory process, is considered to be the most common way of treatment for this disease (Royster and Wagner, 2015; Hyde et al., 2019).
Currently, complex drugs that combine antimicrobial and anti-inflammatory effects are widely used in veterinary medicine (Reader et al., 2020). Drugs for the treatment of cow mastitis that contain β-lactam antibiotics in combination with corticosteroids, as a rule, occupy a large part of market. Medications for intramammary administration often use substances from the group of penicillins (penicillin, ampicillin, cloxacillin, amoxicillin) and prednisolone as active agent (Sipka et al., 2013; Royster and Wagner, 2015).
The assessment of the mutagenicity of a drug should be carried out in case of suspicion of genotoxicity of at least one of its substances, however, the complex effect of β-lactams and corticosteroids on the animal organism has not been studied enough (Turkez et al., 2017; Ahmad et al., 2018; Hartwig et al., 2020). However, it is known that the use of several substances in combination as one drug can increase the likelihood of side effects. In this regard, within the framework of this study, we set the goal of identifying the potential mutagenic effects of the medication containing semisynthetic penicillin, aminopenicillin and a glucocorticosteroid. The study of mutagenicity was carried out by accounting for chromosome aberrations in bone marrow cells and calculating the frequency of micronuclei in polychromatophilic erythrocytes in the blood of outbred mice. Since the genotoxicity of some substances can manifest itself with the accumulation of a drug in the organism after repeated admission, we evaluated the mutagenic effect of a complex drug when simulating a course treatment in mice.
MATERIALS AND METHODS
Ethical approval
The experiments on animals were approved by the Ethics Commission of All-Russian Veterinary Research Institute of Pathology, Pharmacology and Therapy (Protocol No. 02-06-2020).
Medication
The complex medication for the treatment of mastitis in cows during lactation, containing amoxicillin trihydrate –.0%, cloxacillin sodium –5.0%, prednisolone -0.3% and excipients.
Animals
Detection of the potential mutagenicity of a complex antibacterial medication were conducted at the Department of Experimental Pharmacology on white outbred mice (n=42). For the experiment, we used 3 month old females with a weight of 22-24 g of vivarium breeding of “All-Russian Veterinary Research Institute of Pathology, Pharmacology and Therapy”. All the works were carried out in accordance with the Guidelines for conducting preclinical studies of drugs (Durnev et al., 2012).
The experimental animals were kept in standard vivarium conditions (T air +18-23°C, relative humidity 45-60%). Ad libitum access to water and feeds. The manipulations with the animals in the framework of the experiment were carried out in accordance with the provisions of the European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes (Strasbourg, March 18, 1986).
Experimental design
The animals from group I (n=12), which was a negative control, were subcutaneously injected with 0.1 ml of vaseline oil for 7 days with intervals of 24 hours. The animals from group II (n=12) (positive control) were once intraperitoneally injected with cyclophosphamide (Baxter, Germany) at a dose of 20 mg/kg of body weight (Durnev et al., 2012). Mice from the experimental group III (n= 12) were injected with the complex antibacterial drug at a dose of 80 mg/kg, similarly to the animals in the negative control group. The mice of the experimental group IV were once subcutaneously injected with the drug at a 7-fold increased therapeutic dose of 560 mg/kg (n=6).
Frequency of chromosome aberrations
We carried out a study of the frequency of chromosome aberrations in bone marrow cells of mice in 6 animals in each group. Twenty-four h after the last injection of the drugs, the animals were euthanized by cervical dislocation; 2.5 h before this, they were injected with 0.025% colchicine solution (PanEko, Russia). Then, bone marrow cells were removed from the femur, and the resulting cell suspension was incubated for 25 min. in 0.075 M hypotonic KCl solution at 37 °C, then the cells were fixed with acetoalcohol (methanol: acetic acid - 3:1) and bone marrow cell samples were prepared with Romanowsky-Giemsa staining (Preston et al., 1987). The samples were viewed using BIOSKOP-1 microscope (× 1000; LOMO, Russia). We counted at least one hundred metaphase plates per animal. The frequency of cells with chromosome aberrations was determined, single and paired fragments, chromatid and chromosome exchanges, achromatic gaps and breaks along the centromere, as well as the cells with multiple pathologies (more than 5 per cell) were taken into account (Durnev et al., 2012).
Micronucleus test
The frequency of micronuclei was assessed in polychromatophilic erythrocytes (PCE) of the peripheral blood obtained from the tail vein of mice before drug injection and on days 2, 5, 7 and 9 after the start of the experiment in groups I and III, as well as on day 2 in group II (n=6). For the animals of group IV, the study of the frequency of micronuclei was not carried out (Mavournin et al., 1990; Durnev et al., 2012). The sample preparation of the peripheral blood of mice was carried out by a conventional method with the staining of blood smears according to Romanowsky-Giemsa. The samples were viewed using BIOSKOP-1 microscope (× 1000; LOMO, Russia); as micronuclei, we took into account rounded uniformly colored clearly outlined objects lying in the polychromatic erythrocytes (PCE) plane (Heddle and Salamone, 1981). The number of micronuclei was taken into account in 2000 PCE per animal, and the ratio of the number of PCE to normochromatic erythrocytes (NCE) was also taken into account (Durnev et al., 2012).
Statistical analysis
Statistical analysis was carried out using the statistical software package STADIA 8.0 (MSU, Russia). Values (Mean ± SE) were considered significantly different at P < 0.05.
RESULTS AND DISCUSSION
Accounting for chromosome aberrations in the bone marrow cells of mice is one of the main highly sensitive methods for studying the mutagenic effect of substances. In our study, the introduction of cyclophosphamide induced a twelvefold increase in the frequency of cells with chromosome aberrations to 12.0±1.62% (at P<0.0005 relative to the negative control), primarily due to a significant increase in the number of single fragments, which was more than 18 times higher than their number in the negative control, paired fragments were also encountered by 11 times more often and the number of exchanges was 9 times more frequent.
The administration of the studied medication to animals did not cause an increase in the proportion of bone marrow cells with chromosomal abnormalities (Figure 1). According to the data obtained, the proportion of the cells with chromosome aberrations in animals of the experimental group III did not differ significantly from the negative control mice (Table 1). The frequency of the cells with chromosome abnormalities in animals after a single injection of the medication at a 7-fold therapeutic dose also did not have statistically significant differences relative to the negative control group. These findings were as consistent with the studies of İstifli and Topaktaş (2010), where amoxicillin in human lymphocyte cell culture even at high doses (1 mg/ml) did not induce cytogenetic instability determined by the methods of accounting for chromosome aberrations, sister chromatid exchanges and micronuclei.
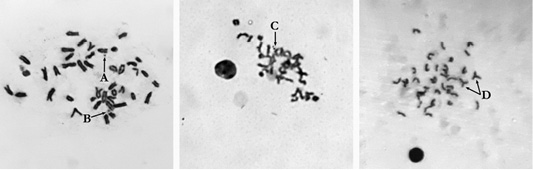
Figure 1: Metaphase plate mouse bone marrow cells: A: Single fragment (×1000); B: Gap (×1000); C: Paired fragments (×800); D: Exchanges (×800).
Another study demonstrated a dose-dependent increase in the frequency of chromosome aberrations in the Allium cepa test system of widely used glucocorticoids, where prednisolone was studied at doses of 5 mg/L, 20 mg/L and 60 mg/L (De Oliveira et al., 2017).
The micronucleus test is the most commonly used genotoxic test in in vivo studies due to its simplicity and accuracy (Hayashi, 2016). Micronuclei are formed as a result of the non-inclusion of whole chromosomes or their fragments into the main nucleus of the cell due to the pathologies of mitosis, obtaining their own membrane. These formations are able to persist for several divisions and act as a diagnostic sign of mutagenic factors of various nature (Araldi et al., 2015; Baderna et al., 2019).
It was found that the clastogenic effect of cyclophosphamide manifested itself in a significant increase (P<0.002) in the proportion of cells with micronuclei relative to the indicators of other groups up to 9.9±1.69 ‰. In animals from the positive control group, we found PCE containing from one to five micronuclei.
At the same time, the introduction of the complex drug did not induce an increase in the number of polychromatophilic cells with micronuclei in the blood of mice (Figure 2). The proportion of the cells with micronuclei in the experimental group and the negative control group did not have statistically significant differences (Figure 3; Table 2). Single micronuclei were mainly observed in the cells.
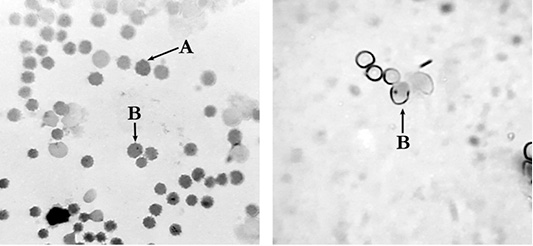
Figure 2: Polychromatophilic erythrocytes of mouse peripheral blood: A: polychromatic erythrocyte (PCE); B:– micronucleated polychromatic erythrocytes (MNPCE); magnification ×800.
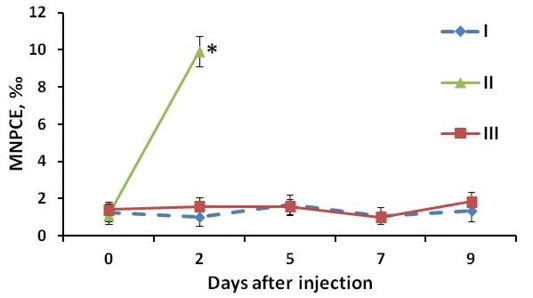
Figure 3: MNPCE frequency in mouse blood: I: negative control group (vaseline oil); II: positive control group (Cyclophosphamidum 20 mg/kg); III: experimental group (study drug 80 mg/kg). At the y-axis (MNPCE) - the frequency of polychromatophilic erythrocytes of the peripheral blood with micronuclei, ‰ - ppm. At the x-axis (Day after injection) - the number of days after the start of drug administration, days; 0 - the day of the first administration of the drug, blood samples were obtained before injection. *, statistically significant difference relative to the negative control group at P<0.05.
Table 1: Drug cytogenetic activity in the mouse bone marrow cells.
| Groups | The number of metaphases | Percentage of pathologies per 100 cells | Total percentage of cells with pathologies | ||||
| Gaps | Single fragments | Paired fragments | Exchanges | Multiple pathologies | |||
|
Group 1 |
600 |
0.7±0.23 |
0.7±0.23 |
0.2±0.18 |
0.2±0.18 |
0.0 | 1.2±0.69 |
|
Group 2 |
600 |
0.5±0.24 |
13.0±0.89*** |
2.2±0.66* |
2.8±0.44** |
0.3±0.23 |
12.0±1.62** |
| Group 3 | 600 |
0.8±0.44 |
0.7±0.23 |
0.3±0.23 |
0.0 |
0.2±0.18 |
1.8±1.57 |
|
Group 4 |
600 |
0.8±0.34 |
0.5±0.24 |
0.2±0.18 |
0.3±0.23 |
0.3±0.23 |
2.0±1.00 |
Data represented as Mean ±SE; changes are considered to be statistically significant at *, P<0.02; **, P<0.0005; ***, P<0.00001 relative to the parameters of the group 1.
Table 2: MNPCE frequency in the mouse blood 48 h after the final injection.
| Groups | MNPCE frequency per 1000 PCE, ‰ | PCE/NCE ratio, % |
|
Negative control |
1.3±1.14 |
2.1±0.63 |
|
Positive control |
9.9±1.69* |
3.1±1.10 |
| Study drug | 1.8±1.10 | 2.1±0.60 |
Data represented as Mean ±SE; changes are considered to be statistically significant at *, P<0.002 relative to the parameters of the negative control.
Thus, our data demonstrate no changes in the frequency of cells with chromosome aberrations in the bone marrow and PCE with micronuclei in the peripheral blood of mice who were subcutaneously injected with a combination of β-lactam antibiotics with a glucocorticosteroid for 7 days at a dose of 80 mg/kg of body weight per day and a single administration of a 7-fold daily therapeutic dose, relative to the indicators of animals in the negative control group.
Numerous facts indicate the safety of wide ranges of therapeutic doses of β-lactam antibiotics, which is one of the reasons for their popularity (Bozcal and Dagdeviren, 2017). A number of studies are devoted to the mutagenic potential of β-lactam antibiotics and steroidal anti-inflammatory drugs (Zavarise et al., 1984; Stemp et al., 1989; Arabski et al., 2005; İstifli and Topaktaş, 2010; De Oliveira et al., 2017). However, in publications discussing the negative and positive effects of penicillins and glucocorticoids, there is no data on their interaction (Coondoo and Chattopadhyay, 2013; Van Matre et al., 2018).
According to the literature data, β-lactam antibiotics can have a genotoxic effect on mammalian cells through free radical oxidation of nucleic acid molecules with reactive oxygen species (ROS) (Bozcal and Dagdeviren, 2017), as well as inhibition of the work of DNA polymerase (Metovic et al., 2013).
The ROS-induced DNA damage of lymphocytes and cells of the human gastric mucosa with amoxicillin at a dose of 5 mM/L after its cellular activation was shown using the DNA-comet method. At the same time, the authors believe that the used concentration of the antibiotic is achieved in the stomach only after oral administration and significantly exceeds the possible dose in the blood (Arabski et al., 2005). In addition, the consequences mediated by oxidative stress or other factors of the drug genotoxicity can, to some extent, be leveled out by the reparative systems of the organism (Christmann and Kaina, 2013). Thus, amoxicillin-induced damage to the DNA of lymphocytes was completely restored in 60 minutes (Arabski et al., 2005). It is possible that additional ROS formed during the cellular activation of penicillins (in particular, amoxicillin and cloxacillin) are successfully eliminated by the organism’s antioxidant systems (Deavall et al., 2012).
Zavarise et al. (1984) on the culture of human lymphocytes in vitro using methods of accounting for chromosome aberrations and sister chromatid exchanges have detected that cloxacillin exhibited clastogenic properties only in high concentrations and did not cause chromosome damage at therapeutic doses.
In the study by de Sousa et al. (2019), amoxicillin did not exhibit genotoxicity at doses of 5.13 × 10−3, 10.26 × 10−3, 20.52 × 10−3, 41.05 × 10−3 mM in the micronucleus test on Tradescantia pallida, which was consistent with our results.
We found that the PCE content in the blood did not have statistically significant differences in all the studied groups and did not go beyond the reference values (1-6%) (O’Connell, 2015). The presented data demonstrate the absence of mutagenicity, or its insignificant manifestation in the study of doses of antibiotics exceeding the daily and course doses of amoxicillin -2.4 mg/kg and 16.8 mg/kg and cloxacillin -4.0 mg/kg and 28.0 mg/kg used in the experimental groups.
Glucocorticosteroids can also be mutagenic due to their ability to penetrate into the cells, interact with DNA and regulate gene expression. Structural chemical similarity and mechanism of action suggest slight differences in the manifestation of genotoxicity between steroid drugs (De Oliveira et al., 2017). Thus, a number of synthetic steroids exhibited mutagenic effects in the Ames test. The authors of the study suggest that the genotoxic effect of the drugs is associated with free radical damage to DNA cells (Islam and Ahmad, 1991). At the same time, the Japanese Food Safety Commission published the results of the study in 2016 in which no mutagenic effects of prednisolone were found in vivo (Food Safety Commission of Japan, 2016). Since in some in vitro studies it showed genotoxicity, there was detected the minimum dose of 0.25 g/kg of body weight per day at which adverse effects were observed.
The study conducted by Hayes et al. (2013) showed that prednisolone increased micronuclei in PCE of rat bone marrow at doses of 500, 1000, 1500 mg/kg when orally administered, however, in our study, this trend was not observed, probably due to the use of smaller doses and route of inoculation.
The concentration of prednisolone introduced by us to the animals of groups III and IV was lower than the detected minimum toxic dose by more than 1000 times (0.24 mg/kg) and 148 times (1.68 mg/kg), respectively, which explained the absence of genotoxic action from the glucocorticoid component of the drug.
Thus, the cytotoxic effects observed in some cases caused by β-lactam antibiotics and glucocorticoid drugs were manifested primarily in bacterial, plant or animal models in vitro, and to a lesser extent were found in animal models in vivo, which was probably due to the differences in model objects, the effect of reparative systems and the metabolism of the investigated substances at the level of the organism (Musgrove and Camps, 2012). In addition, it should be noted that the high doses of substances used in the aforementioned sources, which significantly exceed the content of active substances in the complex drug under study. The combination of glucocorticoid and β-lactam antibiotics did not increase the mutagenicity of the composition used. Despite the increasing popularity of in vitro genotoxic tests, largely related to ethical issues (Hayashi, 2016), in our opinion, the study of mutagenicity in in vivo models is mandatory when studying the safety of new veterinary drugs due to the similarity of the organisms in laboratory and farm animals.
CONCLUSIONS AND RECOMMENDATIONS
As a result of our studies, we found that there was no mutagenic effect of the new complex medication containing prednisolone and antibiotics of the penicillin group (amoxicillin and cloxacillin) on the body of white mice when used at a 7-fold daily therapeutic dose once and for 7 days at a therapeutic dose. This is confirmed by the absence of a significant increase in the proportion of bone marrow cells with chromosome aberrations and the number of polychromatophilic blood erythrocytes with micronuclei relative to the values of the negative control group. Probably for the manifestation of the mutagenic effect of the complex medication, it is necessary to use higher doses and longer duration of administration, which requires further study.
ACKNOWLEDGEMENTS
Authors would like to thank Anna Belenko for English language consulting.
NOVELTY STATEMENT
As a result of our studies, we have found that there is no mutagenic effect of a complex antibacterial medication containing antibiotics of the penicillin group (amoxicillin and cloxacillin) and prednisolone on the organism of white mice when used subcutaneous for 7 days at a dose of 80 mg/kg of body weight and single administration at a dose of 560 mg/kg of body weight. This is confirmed by the absence of a significant increase in the proportion of bone marrow cells with chromosome aberrations and the number of blood polychromatophilic erythrocytes with micronuclei relative to the values of the negative control group.
AUTHOR’S CONTRIBUTION
All authors contributed to the study conception, design, material preparation, investigations, data collection and analysis, participated in writing the manuscript and approved the final version.
Conflict of interest
The authors have declared no conflict of interest.
REFERENCES






