Advances in Animal and Veterinary Sciences
Research Article
Advances in Animal and Veterinary Sciences 2 (5): 261 – 263Status of Mycobacterium Avium Subspecies Paratuberculosis Infection in an Indian Goshala Housing Poorly or Unproductive Cows Suffering with Clinical Bovine Johne’s Disease
Tarun Kumar1, Ran Vir Singh1, Deepak Sharma1, Saurabh Gupta2, Kundan Kumar Chaubey2, Krishan Dutta Rawat2, Naveen Kumar2, Kuldeep Dhama3, Ruchi Tiwari4, Shoor Vir Singh2*
- Division of Genetics and Breeding, Indian Veterinary Research Institute (IVRI), Izatnagar, Bareilly–243122, Uttar Pradesh, India
- Microbiology Laboratory, Animal Health Division, Central Institute for Research on Goats (CIRG), Makhdoom, PO–Farah, Mathura– 281122, Uttar Pradesh, India
- Division of Pathology, Indian Veterinary Research Institute (IVRI), Izatnagar, Bareilly–243122, Uttar Pradesh, India
- Department of Veterinary Microbiology and Immunology, College of Veterinary Science and Animal Husbandry, DUVASU, Mathura –281001, Uttar Pradesh, India
*Corresponding author: shoorvir.singh@gmail.com; shoorvir_singh@rediffmail.com
ARTICLE CITATION:
Kumar T, Singh RV, Sharma D, Gupta S, Chaubey KK, Rawat KD, Kumar N, Dhama K, Tiwari R, Singh SV (2014). Status of Mycobacterium avium subspecies paratuberculosis infection in an Indian goshala housing poorly or unproductive cows suffering with clinical Bovine Johne’s disease. Adv. Anim. Vet. Sci. 2 (5): 261 – 263.
Received: 2014–03–21, Revised: 2014–04–25, Accepted: 2014–04–26
The electronic version of this article is the complete one and can be found online at
(
http://dx.doi.org/10.14737/journal.aavs/2014/2.5.261.263
)
which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
ABSTRACT
In the present study status of Mycobacterium avium (MAP) subspecies paratuberculosis (MAP) was estimated in cows belonging to a Goshala located at the Holy town of Barsana in Mathura district, wherein cows were suffering with clinical Bovine Johne’s Disease (BJD). Randomly 118 cows were sampled and screened for MAP. Of 118 fecal samples screened by microscopy and culture, 45 (38.1%) and 10 (8.4%) were positive for MAP infection, respectively. While screening of 118 serum samples by ‘Indigenous ELISA kit’, 32 (27.1%) were positive. Sensitivity of ‘fecal microscopy’ with respect to culture and Indigenous ELISA kit was 90.0 and 65.0%, respectively. Comparison of culture and Indigenous ELISA with ‘fecal microscopy’ revealed substantial agreement between two tests. Study showed that in the absence of sensitive and specific field based screening tests, microscopy would be a cost effective and useful test for the large scale screening of the domestic ruminants against BJD.
INTRODUCTION
Bovine Johne’s disease (BJD) caused by Mycobacterium avium subspecies paratuberculosis (MAP) leads to development of granulomatous enteritis primarily in domestic ruminants though it also infects wildlife and human beings (Chiodini et al., 1984; Beard et al., 2001; Hermon–Taylor, 2009; Singh et al., 2010). BJD has major impact on the farm economy and has been frequently reported from dairy farms and farmer’s herd worldwide (Kumar et al., 2007; Singh et al., 2013a; Singh et al., 2014a). Globally, India is the leading milk producer (124.85 million tonnes per 116.0 million milking animals), however, low per animal productivity of 218.0 million cattle heads is the major concern of Indian dairy industry (FAO, 2014). More than 70% of US dairy cattle herds are infected with MAP causing annual loss to the tune of $200–250 million while total losses to the US dairy industry exceed $1.5 billion/year (Ott et al., 1999). Though every cow exposed to MAP may not become clinically infected (Chiodini et al., 1984), however, may continue to remain asymptomatic shedder of MAP in feces and milk (Ayele et al., 2001). Inter–species transmission and prolonged survival in environment are crucial factors for control and eradication of the disease (Singh et al., 2010; Larsen et al., 1956; Singh et al., 2012). Major hindrance in eradication of BJD is lack of sensitive tests for detecting infected animals at early stage of disease (Singh et al., 2014b). Cow slaughter is prohibited in India therefore management of BJD is critical for the success of dairy industry in the country. Study aimed to investigate status of BJD in a newly established Goshala located at the Holy town of Barsana in Mathura district and suffering with clinical BJD. Study also evaluated comparative efficacy of two screening tests for the large scale testing of animals for the BJD.
MATERIALS AND METHODS
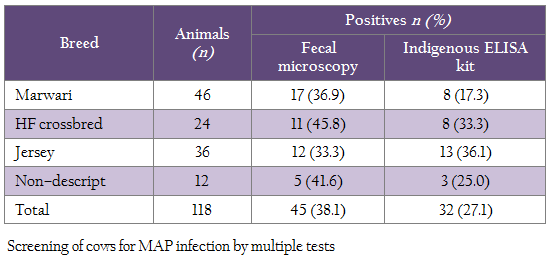
Animal Ethics Entire study and work plan in this study was approved by the Animal Ethics committee of the institute wherein stress on cows was minimum. History A total of 118 cows were screened for the MAP infection from a Goshala (Shri Mataji), established in 2007 and maintained by Shri Maan Mandir Sewa Sansthan, Barsana, Mathura by multiple tests. Initial strength of Goshala was 250, which has been increased to 18,000 in the year, 2013. Goshala was established to conserve indigenous cows and provide protection to stray, old, sick and unprofitable cows and bulls from slaughter. Most of the animals are of Mewati breed from the breeding tract of Kosi/Mewati and few are HF crossbreds and Jersey cows (Table 1). Approximately, 100 animals of Sahiwal breed were purchased in 2011 from its breeding tract in Abohar, Fazillka and Muktsar areas of Punjab state. Physically, cows were weak and was suffering from diarrhea which non–responsive to usual anti–diarrhoeal and anthelmentics (clinical BJD). Nutritional Status and Management of Animals Cows were maintained on nearly optimal nutrition in semi–intensive management system (grazing and dry bhusa with concentrate and occasionally green fodder). Fecal Microscopy Fecal samples were concentrated by centrifugation and stained with Ziehl–Neelsen (ZN) stain as per Singh et al., (2013b) recommendations. Pink coloured acid fast short bacilli indistinguishable to MAP were considered positive for MAP infection. Culture Fecal samples were centrifuged, decontaminated with 0.9% hexadecyl pyridinium chloride and cultured on Herrold’s egg yolk medium (HEYM) with and without mycobactin J as per Whipple et al., (1991) method. Slants were examined for the appearance of colonies at weekly intervals up to 120 days. Indigenous ELISA Kit ‘Indigenous ELISA kit’ initially developed for the screening of goats and sheep at Central Institute for Research on Goats, Mathura, using protoplasmic antigens (PPA) from native ‘Indian Bison type’ biotype (Singh et al., 2007) of goat origin was employed for screening serum samples. Optical densities (OD) were transformed to Sample to Positive (S/P) ratio and animals were negative (0.00–0.09), suspected (0.10–0.24), low positive (0.25–0.39), positive (0.40–0.99) and strong positive (1.0–10.00) for JD status as per Collins, (2002). Statistical Analysis Inter–rater agreement (Kappa) between tests and their sensitivity were compared as per Arizmendi and Grimes (1995).
RESULTS
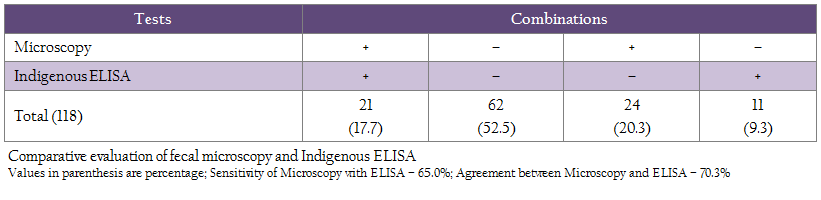
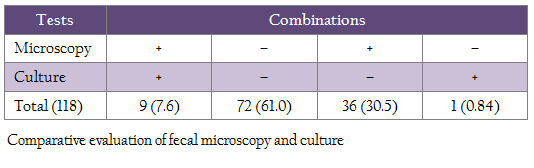
Of 118 cows sampled each for serum and feces were screened using microscopy, ‘Indigenous ELISA’ and culture, 45 (38.1%), 32 (27.1%) and 10 (8.4%) were positive, respectively (Table 1). Comparison of microscopy and ELISA showed, 21 (17.7%) and 62 (52.5%) samples were positive and negative in both the tests (Table 2). Whereas in microscopy and culture, 9 (7.6%) and 72 (61.0%) samples were positive and negative in both the tests (Table 3).
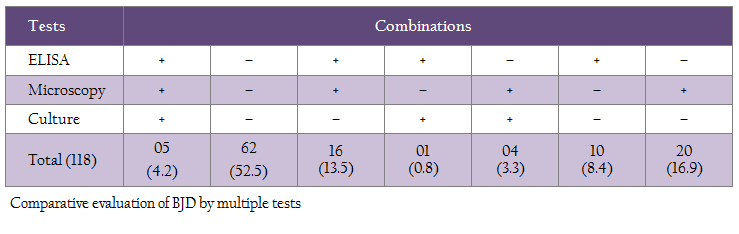
Sensitivity of ‘fecal microscopy’ compared to culture and Indigenous ELISA kit was 90.0 and 65.0%, respectively (Table 2 and 3). Proportional agreement between fecal microscopy and ‘culture’ and ‘Indigenous ELISA’ was substantial (68.6–70.3%) (Table 2 and 3). Using multiple tests, the study showed that at least, 26 (22.0%) cows were positive in two or more than two tests. However, in multiple test regimens, 56 (47.5%) cows were positive in any of the three tests (Table 4).
DISCUSSION
Though Bovine Johne’s disease was first reported in India in 1913 and is still a major health concern both in domestic and wild animals (Chiodini et al., 1984; Beard et al., 2001), however, national estimates on the prevalence of MAP are still not available to a. Earlier studies had also reported endemicity of MAP infection in domestic livestock using microscopy (39.3–68.5%), ELISA (29.0–92.3%), blood PCR (13.1%) and fecal culture (52.5%) (Singh et al., 2013b; Soumya et al., 2009; Vinodh Kumar et al., 2011). Due to poor sensitivity, specificity and non–availability of Johnin, currently there is no alternative field based tests, though a range of diagnostic tests (Johnin, fecal microscopy, fecal culture, blood PCR, ELISA and IFN– γ) are available. No single test could precisely diagnose all cases with 100% accuracy (Singh et al., 2014b; Wadhwa et al., 2012), therefore, three tests were used in the present study to investigate status of BJD in the clinically infected cows.
Earlier studies have also reported higher sensitivity of microscopy as compared to blood PCR. Higher detection of MAP by fecal microscopy may be due to screening of animals with clinical BJD (Singh et al., 2013b). Comparative evaluation of fecal microscopy with ‘Indigenous serum ELISA’ and culture showed that 38.1% cows were positive by fecal microscopy as compared to ‘Indigenous serum ELISA’ (27.1%) and culture (8.4%). Proportional agreement between fecal microscopy and ‘Indigenous ELISA’ was substantial (70.3%) and the sensitivity was 65.0% with 34.3% false negatives. Similar findings have been reported by Singh et al., (2014c) in HF cross–bred cows for MAP infection. Proportional agreement between fecal microscopy and culture was also substantial (68.6%), whereas the sensitivity was 90.0% with 10.0% false negatives. Present study also revealed that at least 26 (22.0%) cows can be considered safely as positives for MAP, since these cows were detected positive either in two or three tests combinations (table 4). However, 56 (47.5%) cows that were positive in any of the three tests can be taken as positive, since disease is endemic in the domestic ruminant population of the country in general and this herd in particular. The 52.5% cows that were negative in three tests may not be true negatives, since all the cows screened were exhibiting clinical symptoms of BJD. The study exposed the limitations of each tests and therefore recommends use of multiple tests. Moreover, the cows were screened only once in one time sampling.
Complete elimination of the bacilli is not practical in the country due to ban on cow slaughter for ethical and religious reasons. Transmission of disease from adult infected animals to susceptible young calves could not be prevented due to vertical transmission of the disease (Buergelt et al., 2006) and non–applicability of improved hygiene and management practices, segregation of healthy and diseased animals and non–availability of vaccine. Study showed that fecal microscopy could serve as useful techniques for large scale screening of cattle herds against MAP infection, particularly in poor and developing countries, where culture, ELISA, PCR and other molecular tests may not be cost effective or available.
CONFLICT OF INTEREST
No conflict of interest to declare.
ACKNOWLEDGEMENTS
Authors are thankful to CSIR, New Delhi for providing the funds and Director, CIRG, Makhdoom for providing the facilities.
REFERENCES
Arizmendi F, Grimes JE (1995). Comparison of Gimenez staining method and antigen detection ELISA with culture for detecting Chlamydia in bird. J. Vet. Diagn. Invest. 7: 400–401.
http://dx.doi.org/10.1177/104063879500700320
PMid:7578461
Ayele WY, Machackova M, Pavlik I (2001). The transmission and impact of paratuberculosis infection in domestic and wild ruminants. Vet. Med. 46: 205–224.
Beard PM, Daniels MJ, Henderson D, Pirie A, Rudge K, Buxton D, Rhind S, Greig A, Hutchings MR, McKendrick I, Stevenson K, Sharp JM (2001). Paratuberculosis infection of non–ruminant wildlife in Scotland. J. Clin. Microbiol. 39: 1517–1521.
http://dx.doi.org/10.1128/JCM.39.4.1517-1521.2001
PMid:11283080 PMCid:PMC87963
Buergelt CD, Williams BS, Monif GRG, Pinedo P, Decker JH (2006). Nested polymerase chain reaction and prenatal detection of mycobacterium avium subspecies paratuberculosis (Map) in bovine allantoic fluid and fetuses. Intern. J. Appl. Res. Vet. Med. 4: 232–38.
Chiodini RJ, Van Kruiningen HJ, Merkal RS (1984). Ruminant paratuberculosis (Johne's disease): the current status and future prospects. Cornell Vet. 74: 218–262.
PMid:6375961
Collins MT (2002). Interpretation of a commercial bovine paratuberculosis enzyme–linked immunosorbent assay by using likelihood ratios. Clin. Diagn. Lab. Immunol. 9: 1367–1371.
PMid:12414776 PMCid:PMC130105
FAO (2014). Food and Agriculture Organization of the United Nations.
Hermon–Taylor J (2009). Mycobacterium avium subspecies paratuberculosis, Crohn's disease and the Doomsday scenario. Gut. Pathog. 1: 15.
Kumar P, Singh SV, Bhatiya AK, Sevilla I, Singh AV, Whittington RJ, Juste RA, Gupta VK, Singh PK, Sohal JS, Vihan VS (2007). Juvenile Capri–Paratuberculosis (JCP) in India: Incidence and characterization by six diagnostic tests. Small Rumin. Res. 73: 45–53.
http://dx.doi.org/10.1016/j.smallrumres.2006.10.023
Larsen AB, Merkal RS, Vardaman TH (1956). Survival time of Mycobacterium paratuberculosis. Am. J. Vet. Res. 17: 549–551.
PMid:13340126
Ott SL, Wells SJ, Wagner BA (1999). Herd–level economic losses associated with Johne's disease on US dairy operations. Prev. Vet. Med. 40: 179–92.
http://dx.doi.org/10.1016/S0167-5877(99)00037-9
Singh AV, Singh SV, Singh PK, Sohal JS (2010). Genotype diversity in Indian isolates of Mycobacterium avium subspecies paratuberculosis recovered from domestic and wild ruminants from different agro–climatic regions. Comp. Immunol. Microbiol. Infect. Dis. 33: e127–131.
http://dx.doi.org/10.1016/j.cimid.2010.08.001
PMid:20832117
Singh SV, Gupta S, Chaubey KK, Rawat KD, Kumar N, Sohal JS, Singh S, Tiwari R, Chakraborty S, Dhama K (2014c). Johne's Disease (JD) in a High Yielding Holstein Friesian Cattle Dairy Farm in India. J. Biol. Sci. 14: 195–203.
http://dx.doi.org/10.3923/jbs.2014.195.203
Singh SV, N Kumar, KK Chaubey, S Gupta, KD Rawat (2013a). Bio–presence of Mycobacterium avium subspecies paratuberculosis infection in Indian livestock farms. Res. Opin. Anim. Vet. Sci. 3(11): 401–106.
Singh SV, Singh AV, Singh PK, Sohal JS, Singh NP (2007). Evaluation of an indigenous ELISA for diagnosis of Johne's disease and its comparison with commercial kits. Indian J. Microbiol. 47: 251–258.
http://dx.doi.org/10.1007/s12088-007-0046-2
PMid:23100673 PMCid:PMC3450340
Singh SV, Singh PK, Gupta S, Chaubey KK, Singh B, Kumar A, Singh AV, Kumar N (2013b). Comparison of microscopy and blood–PCR for the diagnosis of clinical Johne's disease in domestic ruminants. Iran. J. Vet. Res. 14: 345–349.
Singh SV, Singh PK, Singh AV, Sohal JS, Kumar N, Chaubey KK, Gupta S, Rawat KD, Kumar A, Bhatia AK, Srivastav AK, Dhama K (2014a). Bio–load and bio–type profiles of Mycobacterium avium subspecies paratuberculosis infection in the domestic livestock population endemic for Johne's disease: a survey of 28 years (1985–2013) in India. Transbound. Emerg. Dis. 61 (Suppl. 1): 1–13.
Singh SV, Sohal JS, Kumar N, Gupta S, Chaubey KK, Rawat KD, Chakraborty S, Tiwari R, Dhama K (2014b). Recent approaches in diagnosis and control of mycobacterial infections with special reference to Mycobacterium avium subspecies paratuberculosis. Adv. Anim. Vet. Sci. 2(1S): 1–11.
http://dx.doi.org/10.14737/journal.aavs/2014/2.1s.1.12
Singh SV, Tiwari A, Singh AV, Singh PK, Singh B, Kumar A, Gururaj K, Gupta S, Kumar N (2012). Contamination of Natural Resources (Soil and River water) with Mycobacterium avium subspecies paratuberculosis in three districts of Uttar Pradesh: A Pilot study. Haryana Vet. 51: 1–5.
Soumya MP, Pillai RM, Antony PX, Mukhopadhyay HK, Rao VN (2009). Comparison of faecal culture and IS900 PCR assay for the detection of Mycobacterium avium subsp. paratuberculosis in bovine faecal samples. Vet. Res. Commun. 33: 781–791.
http://dx.doi.org/10.1007/s11259-009-9226-3
PMid:19440851
Vinodh Kumar OR, Ganesan PI (2011). Status of Bovine Paratuberculosis in Organized Farms of Tamilnadu. Indian Vet. J. 88: 90–91.
Wadhwa A, Foote RS, Shaw RW, Eda S (2012). Bead–based microfluidic immunoassay for diagnosis of Johne's disease. J. Immunol. Methods. 382: 196–202.
http://dx.doi.org/10.1016/j.jim.2012.06.006
PMid:22705087
Whipple DL, Callihan DR, Jarnagin JL (1991). Cultivation of Mycobacterium paratuberculosis from bovine fecal specimens and a suggested standardized procedure. J. Vet. Diagn. Investig. 3: 368–373.
http://dx.doi.org/10.1177/104063879100300424
PMid:1760476