Advances in Animal and Veterinary Sciences
Research Article
Microbiological, Cytological and Immunolgical Investigation of Endometritis in Arabian Mares
Mona MH Soliman1*, Eman Ragab2, Sally Ibrahim3, Youssef Ahmed3
1Department of Microbiology and Immunology, Veterinary Research Division, National Research Centre, Doki, Egypt; 2Department of Microbiology, Faculty of veterinary medicine, Cairo University, Cairo, Egypt; 3Department of Animal Reproduction and A.I, Veterinary Research Division, National Research Centre, Dokki, Egypt.
Abstract | Endometritis is the principal reason of reduced reproductive efficiency in female horses. Bacteriological, mycological and cytological investigations are the backbone of detecting endometritis in mares. Interleukin-6, Prostaglandin E2 and Prostaglandin F2𝛼 are pro-inflammatory cytokines and their determination in serum of mares is important to predict early stages of endometritis to prevent its detrimental effect on fertility of mares. Low volume lavage (LVL) fluid was microbiologically examined (bacteria and fungi) together with cytological smears from endometrial swabs. Microbial growth on culture of the LVL pellet revealed that diseased mares had Gram-negative bacteria such as E. coli (75%), Klebsiella spp. (40%), Gram-positive bacteria such as Staphylococcus aureus (60%) and Staphylococcus epidermidis (55%). For mycological results; there were 25 (25%) diseased mares showed positive results for yeasts and moulds as 13 were positive for yeasts and 12 for moulds. Microbial examination together with cytological results revealed that endometritis was diagnosed in 80 out of 100 mares. Serum evaluation of IL6, PGE2 and PGF2𝛼 revealed their higher levels in diseased mares (83%) compared with control ones. In conclusion, microbiological examination of endometrial Low volume uterine lavage is of great importance to diagnose precisely the causative agent either bacteria or fungi. Microbiological together with cytological smears and clinical finding are crucial when evaluating mares. The IL6, PGE2 and PGF2𝛼 determination in serum can detect early stages of endometritis to quickly treat those cases.
Keywords | Bacteriology, Cytology, Mare endometritis, Mycology, Pro-inflammatory cytokines
Received | June 20, 2021; Accepted | September 02, 2021; Published | October 15, 2021
*Correspondence | Mona MH Soliman, Department of Microbiology and Immunology, Veterinary Research Division, National Research Centre, Doki, Egypt; Email: [email protected]
Citation | Soliman MMH, Ragab E, Ibrahim S, Ahmed Y (2021). Microbiological, cytological and immunolgical investigation of endometritis in Arabian mares. Adv. Anim. Vet. Sci. 9(12): 2077-2083.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.12.2077.2083
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Soliman et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
The determination of endometritis is basic within the veterinarian’s endeavors to cure infertility in mares. Methods which are accessible to detect endometritis include clinical investigation, transrectal palpation, ultra-sonography of the genital tract, uterine culture, vaginal speculum inspection, endometrial biopsy and cytology (Riddle et al., 2007).
Subclinical and clinical endometritis are the principal causes of decreased reproductive performance in mares. Clinical endometritis is somewhat simple to detect during regular physical and ultrasonographic reproductive checking, while the diagnosis of subclinical endometritis needs more detailed work-up (Aitken, 2012).
Bacterial endometritis is assumed the foremost crucial reproductive illness in the mare and is related with server financial losses. On the other hand, in non-venereal bacterial endometritis, hazard determinants such as reduced uterine defense techniques or bad perineal configuration, instead of the bacterial elements, form the principal trouble (Causey, 2006).
Fungal endometritis is not frequent condition in mares, with a reported incidence of less than 5% of diagnosed endometritis. The prognosis of fungal endometritis in mares is mainly poor, either because the fungal elements resist eradication or because they afterwards return. The principal reasons for treatment failure are proposed to include the resistance of invasive shapes to intrauterine treatment, failure to eradicate the predisposing determinants or recontamination from a reservoir in the caudal reproductive system. On the other hand, some cases of fungal endometritis recover suddenly or without particular treatment, probably since the disturbances that permitted fungal colonization have been adjusted (e.g., expulsion of a necrotic focus) (Stout, 2008).
Mares suffering from mycotic endometritis are often elder, multiparous female horses with a history of constant barrenness. Clinical features may include a purulent vaginal secretion and large amounts of flocculent discharge inside the uterine lumen on palpation and ultrasound investigation though other cases may be subclinical. Differentiation between the diverse shapes of mycotic elements is crucial while choosing remedy and evaluating in vitro sensitivity testing for every isolate is suggested, as sensitivities to commonly utilized antifungals differ accordingly. Moreover, it is common that mycotic infection is opportunistic and can only affect chronically disordered uterine/vaginal environment, pneumovagina, continuous endometritis and frequent intrauterine antimicrobial treatment are known as predisposing issues (Scott, 2020).
Cytological and microbiological investigations of uterine swabs are the backbone in the diagnosis of acute endometritis in female horses (Walter and Wehrend, 2007). With microbiological analysis alone, there is a chance for inaccurate judgment on the examinations because of false-positive and false-negative culture outcome (Moller, 2005; LeBlanc and Causey, 2009). Moreover, a positive culture might not be related with endometritis and decreased fertility (Moller, 2005; LeBlanc et al., 2007).
Subclinical endometritis is affected by the sort of pathogen and the mare’s subsequent immunological reaction (LeBlanc and Causey, 2009; Overbeck et al., 2011). However, isolation of bacterial organisms does not fundamentally assure the existence of endometritis. (Riddle et al., 2007; LeBlanc, 2010).
Cytological investigation of a uterine specimen is a highly valuable diagnostic test, and various scientists have set its sensitivity over that of microbiological investigation (Riddle et al., 2007). There are different approaches to classify the cytological outcome; some researchers report the number of polymorphonuclear leukocytes (PMNs) as a percentage of all cells observed in a slide (Couto and Hughes, 1985; Ricketts and Mackintosh, 1987) whereas others report real numbers of cells observed per microscopic field inspected (Knudsen, 1964; Purswell et al., 1989). Endometritis is most associated with aerobic bacteria (Riddle et al., 2007).
Interleukin-6 is well-known to be a cytokine that created by T cells. T cells are categorized mainly into Th1 and Th2 by the criteria of cytokine generation. The Th1 cell influences cell mediated immunity by creating basically IL-2, TNF-α, and IFN-γ, and the Th2 cell influences antibody generation by producing primarily IL-4, IL-5, IL-6, IL-10 and IL-13 (Mosmann et al., 1986).
Elevated level of IL-6 in the prepartum period tended to predict endometritis. Alteration in the measurements of IL-6 level amid pregnancy is valuable method for anticipating problems in postpartum reproductive disorders as postpartum retained placenta, endometritis and/or follicular cyst (Ishikawa et al., 2004).
Prostaglandins are lipid compounds originated enzymatically from arachidonic acid. The reproductive tract is the main generator of PGE2 and PGF2𝛼.The involvement of prostaglandins in pain, infertility, angiogenesis, tissue remodeling, and cell proliferation in addition to different pathological issues is well studied by different authors (Rakhila et al., 2015). Thus, the present work was planned to diagnose endometritis in Arabian mares using microbiological and cytological examination together with IL6, PGE2 and PGF2𝛼 assessment.
Materials and Methods
A total of 100 Arabian mare specimens aged from ‘8-14’ years old were collected from stud farms, Giza, Egypt. These specimens were gathered just once from each female horse during the estrus, after mares owner’s agreement.
Endometrial swabs
Mare’s tails were held out way to prevent any probable contamination then perineal region was washed with cleanser and washed with clean water for at least thrice to properly clean the area. A while later, the perineal region was dried with expendable tissues. The inspected specialist put a long clean obstetrical sleeve and used a little sum of germ-free lubricator. The double-guarded culture swab (IMV advances, Paris) was directed physically through the genitalia from the vulva, vagina, and cervix and finally inside the uterus. The swab was introduced and delicately twisted for about fifteen seconds, and after that was withdrawn from the guard, as well as one side was rolled onto a clean magnifying lens glass slide for cytological examination. It was carried out according to methods described by (LeBlanc et al., 2007; Bohn et al., 2014).
Low volume uterine lavage (LVL)
The LVL was done as already explained by (LeBlanc et al., 2007; Bohn et al., 2014). In brief, the inspected specialist passed Equine Catheter (IMV advances, Paris) via the cervix into the uterus, the balloon swelled, and the catheter at that point was situated inside cervical os. Around 300 ml of sterile normal saline (0.9% NaCl) was passed through the uterus. After two minutes, a massage was done to the uterus by means of transrectal palpation for thirty- second and the liquid was hence collected by gravity stream into the original liquid sac. A while later, the initial liquid bag that contained uterine lavage was kept on ice till transportation to the lab for microbiological examination. The characters of the cloudy LVL fluid were evaluated visually into: normal (clear) or abnormal (discolored and debris) was documented.
Microbiological examination
Bacterial isolation and identification
Uterine lavage specimens were sub-cultured into blood agar (Blood agar, 10% defibrinated horse blood in blood agar base), Mannitol salt agar, MacConkey’s agar and were kept at 37ºC for 24 hours. Inoculated plates were inspected for bacterial growth after ‘24-48’ hr for grown colonies; 3-5 colonies from each sort were picked up and were plated on to Blood agar plates for testing hemolytic potential and purification of microbes. The characterization was performed according to cultural, morphological and biochemical properties in accordance to methods described by (Carter, 1984; Holt et al., 1994).
Mycological examination
Mare uterine lavage samples were streaked on Sabouraud Dextrose Agar plates and were kept at 30ºC for ‘3-5’ days. The inoculated plates were inverted to prevent spreaders. The characteristics of yeast colonies that they were of different colors as white, creamy, yellow and pink and they may be regular or irregular in shape but for mould the colonies appeared as star-shaped with different colors. Soliman et al. (2019). Pure colonies were subjected to macroscopic, microscopic and differential biochemical identification assays as described by (Fisher and Cook, 1998; Samson et al., 2010).
Cytological examination
The slide samples collected from mare swabs were fixed and colored with a particular commercial cytological stain; Papanicolaou assay (Biodiagnostic, Egypt), concurring to manufacture’s method. We assessed the slides in respect to the features of cell morphology, cellularity, count of inflammatory cells per 400X field, along with any other notable characteristics (LeBlanc et al., 2007; Bohn et al., 2014).
Determination of IL6, PGE2 and PGF2𝛼 in serum of investigated mares
Serum levels of IL-6 were detected by equine interleukin 6 (IL-6) ELISA kit (SunLong Biotech Co., LTD, Zhejiang, China) and was utilized as reported by the producer’s directions. The test sensitivity and ranges were 0.5 pg/ml and 1.6 pg/ml to 100 pg/ml, respectively.
For PGF2𝛼 concentration, the commercial PGF2𝛼 high sensitivity equine prostaglandin F2𝛼 ELISA kit (SunLong Biotech Co., LTD, Zhejiang, China) was utilized and performed as stated in the producer’s directions. The test sensitivity and range were 0.5 pg/ml and 3 pg/ml to 210 pg/ml. To avoid the destruction of prostaglandins; serum was blended with a 1% stabilizing solution 0.3 M ethylenediaminetetraacetic acid (EDTA) (Sigma) and 1% aspirin (Sigma).
Statistical analysis
The obtained data were statistically analyzed for descriptive statistics using SPSS 14. For analysis of serum IL6, PGE2 and PGF2𝛼 in mares, the unpaired t test with Welch’s correction (GraphPad Software, Inc., San Diego, CA, USA) was used for statistical analysis of IL-6, PGE2 and PGF2𝛼 between control healthy and diseased (endometritis) groups. The P values ≤ 0.05 were constituted statistically significant. Graph Pad Prism 9.0 was utilized for performing statistical investigation.
Results and Discussion
Microbiological results
Results of aerobically growing bacteria from uterine lavage specimens of the examined mares revealed that diseased mares had Gram negative-bacteria such as Escherichia coli (75%), Klebsiella spp. (40%), Gram positive-bacteria as Staphylococcus aureus (60%) and Staphylococcus epidermidis (55%).
Regarding fungal results; there were 25 (25%) diseased mares showed positive results for yeast and mould growth colonies as 13 were positive for yeasts (13%) and 12 were positive for moulds (12%) and the isolation rates for each isolate were Candida albicans (7%), Candida glabrata (5%), Rhodotorula (1%), Aspergillus fumigatus (5%), Mucer (4%), Penicillium (2%) and finally Alternaria (1%). These diseased mares were also positive for bacterial cultures (mixed infection). The microbiological examination results together with mixed infections are being presented in Table 1.
Cytological examination
The positivity of endometrial cytology was confirmed if (≥ 1 neutrophil for high power field, or ≥1% neutrophil to epithelial cell proportion (1:100). While female horses were considered as having endometritis when the inflammatory cells were basically neutrophils, as clear in (Figures 1 and 2) and in the present study, 80% of the examined mares were positive for endometrial cytology smear.
Table 1: The microbiological examination results together with mixed infections.
| Isolated microorganism | Number of isolates |
| E. coli | 75 (75%) |
|
Klebsiella spp. |
40 (40%) |
| Staphylococcus aureus | 60 (60%) |
| Staphylococcus epidermidis | 55 (55%) |
|
Candida albicans |
7 (7%) |
|
Candida glabrata |
5 (5%) |
|
Rhodotorula |
1 (1%) |
|
Aspergillus fumigatus |
5 (5%) |
|
Mucer |
4 (4%) |
|
Penicillium |
2 (2%) |
|
Alternaria |
1 (1%) |
| Microorganisms combination | |
|
E. coli + Klebsiella spp. |
33 (33%) |
| E. coli + Staphylococcus aureus | 24 (24%) |
|
Klebsiella spp.+Staphylococcus epidermidis |
10 (10%) |
|
Staphylococcus aureus+ Staphylococcus epidermidis |
25 (25%) |
| Bacterial isolates + yeasts | 13 (13%) |
| Bacterial isolates+ Moulds | 11 (11%) |
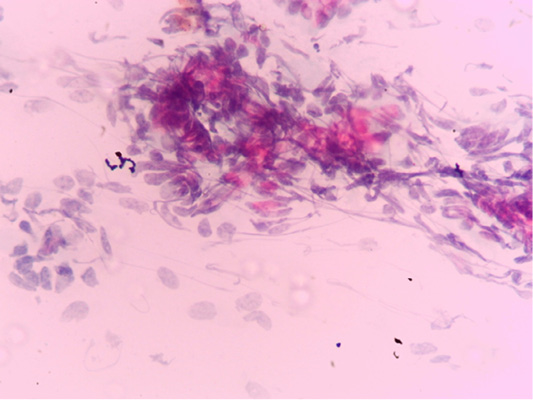
Figure 1: Negative endometrial cytology smear of mares as only endometrium epithelial cells can be seen and no inflammatory cells was observed.
Serum levels of IL-6 and prostaglandins (E2 and F2𝛼) in examined mares
Serum levels of IL-6, PGE2 and PGF2𝛼 in mares with endometritis (diseased) were elevated in 83% from total examined mares when compared with control ones as shown in Table 2. Serum levels of IL-6 were greater (P < 0.001) in diseased female horses (40.5 ± 0.65 pg/ml) compared to the control ones (20 ± 0.41 pm/ml). Also, serum levels of PGE2 showed higher concentrations (P<0.001) in the diseased female horses (40.75 ± 1.11 pm/ml) compared to the healthy ones (13.5 ± 0.65 pm/ml). Concentrations of PGF2𝛼 in serum showed a remarkable raise (P<0.001) in the diseased female horses (208.5 ± 3.18 pg/ml) compared to the healthy ones (125.75 ± 2.17 pg/ml).
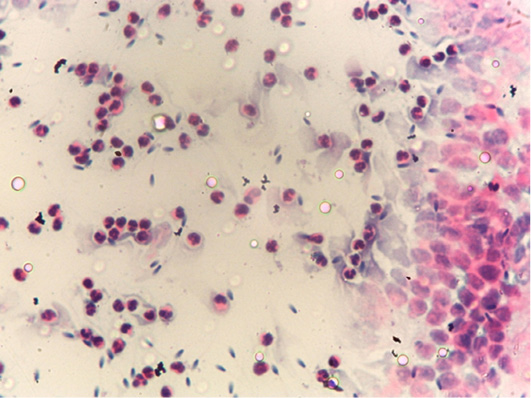
Figure 2: Postive endometrial cytology smear as a few endometrium epithelial cells was observed together with hypersegmented neutrophils.
Table 2: The serum levels of IL-6 and prostaglandins (E2 and F2𝛼) in mares suffering from endometritis (Mean±SE) compared to control ones.
| Tested parameter (pg/ml) | Levels in diseased mares (Mean±SE) | Levels in control mares (Mean±SE) |
| IL6 |
40.5±0.65*** |
20±0.41 |
|
PGE2 |
40.75±1.11*** |
13.5±0.65 |
|
PGF2𝛼 |
208.5±3.18*** |
125.75±2.17 |
***P < 0.001.
In the present study, endometritis was confirmed if the mare presented two or more of the parameters in the following checklist: (1) atypical clinical finding; (2) unusual characteristics of uterine lavage (LVL) liquid; (3) positive endometrial cytology smear; and (4) positive microbial culture of the uterine lavage LVL pellet. Briefly, endometritis was detected in 80 out of 100 female horses that had been tested. Subsequently, it is extremely critical to combine clinical discoveries and laboratory information, when assessing female horses for endometritis (Amorim et al., 2016).
Endometritis has a prominent economic effect. Infected female horses usually have abnormal estrous cycles, need thorough clinical breeding care, and need additional cycles to become pregnant, increasing extra expenses to the owner (Thalheimer and Lawrence, 2001). Those expected high costs are the result of low reproductive performance, and only about 50–60% of mares are anticipated to give a healthy foal after a sole breeding (Bosh et al., 2009).
Endometrial culture and cytology are diagnostic for identifying mares with endometritis, as pregnancy rates were decreased when either culture or cytology were positive (Riddle et al., 2007).
In the present work, a total of 100 samples were gathered from Arabian female horses. Results of aerobically growing bacteria from the Uterine lavage specimens of the examined mares revealed that diseased mares had Gram-negative bacteria as E. coli (75%), Klebsiella spp. (40%), Gram-positive bacteria as Staphylococcus aureus (60%) and Staphylococcus epidermidis (55%) but Riddle et al. (2007) obtained different results as they mainly isolated the beta-hemolytic Streptococcus (78 specimens, 34%), followed by E. coli (17%), Pseudomonas spp. (11.7%) also non-pathogenic microbes (11.3%; Micrococcus, Bacillus and alpha Streptococcus) and Staphylococcus, Klebsiella, Proteus or Enterobacter cloaca were isolated in lower rates. Similar results to ours were obtained by Albihn et al. (2003) as they mostly isolated was E. coli (104 isolates) from total 239 mares also Frontoso et al. (2008) obtained different results from ours as they mostly isolated Beta haemolytic Streptococcus 31.7%, followed by 18.4% E. coli from total 347 isolates from total 586 uterine swab samples from mares suffering from fertility problems and Troedsson (2011) also isolated mostly beta-hemolytic streptococci (Streptococcus equi ssp. zooepidemicus), Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa from mares’reproductive tracts. Other aerobic bacteria are alpha-hemolytic streptococci, Staphylococcus spp., Corynebacterium spp., Acinetobacter spp., Enterobacter spp., Citrobacter spp. and Proteus spp.
Fungi are classified into yeasts and moulds. Certain mycotic microorganisms like Candida species are dimorphic and have the capability to live in both yeast and mould forms under certain conditions (Beltaire et al., 2012).
Regarding fungal results; there were 25 (25%) diseased mares showed positive results for yeast or mould growth colonies as 13 were positive for yeasts (13%) and 12 were positive for moulds (12%) and the isolation rates for each isolate were Candida albicans (7%), Candida glabrata (5%), Rhodotorula (1%), Aspergillus fumigatus (5%), Mucer (4%), Penicillium (2%) and finally Alternaria (1%). These diseased mares were also positive for bacterial cultures (mixed infection), however, Riddle et al. (2007) isolated yeast from 17% from the examined mares. Beltaire et al. (2012) obtained similar results to ours as they identified 102 mycotic isolates 69% were yeasts (mainly Candida species), 26% moulds with septated hyphae (mainly Aspergillus species (18%) and 5% mould with non-septated hyphae (mainly Mucor species). Albihn et al. (2003) isolated 16 fungal isolates from total 239 mares, 15 isolates were yeasts and only one mould isolate. Lower isolation rate of yeasts was obtained by Frontoso et al. (2008) as they isolated yeasts from 2% from a total of 347 isolates from total 586 uterine specimens from mares with fertility complaint.
Collectively, pregnancy rates were badly influenced by both positive endometrial cytology and culture outcome. Twice as numerous female horses were analyzed as suffering from endometritis using cytology result than by culture technique, with the level of inflammation more decisive in determining infertility than the lowered existence or absence of inflammation (characterized as > 2 neutrophils). Detection of microbes is often related with diminished pregnancy rates, regardless of cytological smear outcome. The uterine inflammatory profile, as recognized in cytological slides, found to vary among microbes, with beta-hemolytic Streptococcus being connected with more number of positives cytology than coliforms. At last, veterinarians ought to continuously interpret their laboratory outcome with carefulness, unless clinical results assure the existence of endometritis (Riddle et al., 2007).
IL-6 is a cytokine that was stated to be elevated in the peritoneal fluid of women with endometritis. This cytokine may be included in inflammatory changes and macrophage arousal (Wu and Ho, 2003).
In the current work, the serum levels of IL-6 were notably elevated in female horses with endometritis compared with healthy ones. Kasimanickam et al. (2013) assured that cows with clinical and subclinical endometritis exhibited elevated serum levels of IL-6 when compared with normal cows (P < 0.05).
During the last decade, intensive analysis on molecular mechanisms causing the pathological course of endometriosis have been handled. Although numerous variables have been described to be included in these processes, prostaglandin E2 (PGE2) no doubt constitute as one of the foremost basic regulators of all. Accumulating data demonstrate that PGE2 controls many critical functions, such as steroidogenesis, angiogenesis, proliferation, and immune suppression that contribute to the pathogenesis of endometriosis (Hsiao et al., 2014).
Prostaglandin E2 is secreted from macrophages and ectopic endometrial cells and it is increased in the peritoneal cavity in cases of endometritis. This prostaglandin is included in the pathophysiology of the illness. Prostaglandin E2 influences leukocyte populations and provokes angiogenesis (Sacco et al., 2012).
Conclusions and Recommendations
Microbiological examination of endometrial low volume uterine lavage is of great importance to diagnose precisely the causative agent either bacteria or fungi to select the proper antimicrobial agent. Cytology technique is rapid, relatively cheap, safe, and simple to apply under field circumstances and facilitates the detection of subclinical endometritis which is difficult to be diagnosed. IL6, PGE2 and PGF2𝛼 are pro inflammatory cytokines, and their determination in serum of mares is important to predict early stages of endometritis to prevent its detrimental effect on fertility of mares.
Novelty Statement
The current work was planned to diagnose endometritis in Arabian mares using microbiological and cytological examination together with IL6, PGE2 and PGF2 assessment to quickly treat those cases so fertility due to endometritis will be decreased accordingly.
Author’s Contribution
All authors contributed to the design and implementation of the research, to the analysis of the results and to the writing of the manuscript.
Funding
This study was financed by Science and Technology Development Fund (STDF), project ID: 33342, Egypt.
Statement of animal rights
The current work was approved by the Ethical Use and Animal Care Committee of Faculty of Veterinary Medicine, Cairo University (Vet. CU28042021264).
Conflict of Interest
The authors have declared no conflict of interests.
References






