Advances in Animal and Veterinary Sciences
Case Report
A Case Report of Outbreak Avian Pox Virus from Layer Chickens and a Pigeon in Yogyakarta, Indonesia
Sri Hartati1, Tri Untari2, Annisaa Lulu Nuraini3, Alfarisa Nururrozi1*
1Departement of Internal Medicine, Faculty of Veterinary Medicine, Universitas Gadjah Mada, Yogyakarta, Indonesia; 2Departement of Microbiology, Faculty of Veterinary Medicine, Universitas Gadjah Mada, Yogyakarta, Indonesia; 3Postgraduate Student, Veterinary Science, Faculty of Veterinary Medicine, Universitas Gadjah Mada, Yogyakarta, Indonesia.
Abstract | In recent years, some outbreaks of skin lesions suspected to be avian pox were observed in a farms layer chicken in Yogyakarta Province, Indonesia. This article reports a confirmed case of avian pox virus from three-layer chickens and a pigeon kept in same farm area. Both chickens and pigeon showed nodular lesions on unfeather head skin and fibro-necrotic lesions on the mucus membrane of the oral cavity. Avian pox virus was propagated from nodular and fibro-necrotic lesions by inoculation into the chorioallantoic membranes (CAM) of 10-days-old specific-pathogen-free chicken embryos for 5-7 days. The CAM was examined to observe the pock lesions. The tissue samples from nodular lesions were stained with Hematoxylin-Eosin stain for histopathological analysis. For PCR, after DNA extraction, a 578-bp fragment of avian pox virus from 4b core protein gene was amplified. The data were analyzed descriptively. Both chickens and pigeon samples were positive for avian pox virus on propagation, histopathological, and PCR examination, respectively. The conclusion of this report there is potential transmission of avian poxvirus between chickens and pigeon, so other birds should not be kept inside the farm.
Keywords | Avian pox virus, Chicken, Pigeon, PCR, Histopathology
Received | April 03, 2020; Accepted | June 11, 2021; Published | August 15, 2021
*Correspondence | Alfarisa Nururrozi, Departement of Internal Medicine, Faculty of Veterinary Medicine, Universitas Gadjah Mada, Yogyakarta, Indonesia; Email: [email protected], [email protected]
Citation | Hartati S, Untari T, Nuraini AL, Nururrozi A (2021). A case report of outbreak avian pox virus from layer chickens and a pigeon in Yogyakarta, Indonesia. Adv. Anim. Vet. Sci. 9(10): 1559-1563.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.10.1559.1563
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Hartati et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Avian pox is a contagious disease in domestic and wild birds of all ages, sexes, and breeds (Alehegn et al., 2014). Avian pox is caused by the avian pox virus (APV) (Gilhare et al., 2015). Avian poxvirus belongs to the Poxviridae family (Manarolla et al., 2010). The Poxviridae family is subdivided into the subfamily Entomopoxvirinae and Chordopoxvirinae, which infect insects and chordates, respectively. Avipoxvirus (APV) in the subfamily Chordopoxvirinae is the only genus that can infect non-mammalian hosts including more than 230 of 9,000 known avian species with worldwide distribution. Avian pox virus can infect various species of birds such as chicken, pigeon, canary, psittacine, quail, sparrows, starlings, turkeys, crows, penguins, peacocks, and condors (Tripathy and Reed, 2013).
Avian pox is a slow-spreading disease and has a worldwide distribution (Tripathy and Reed, 2013; OIE, 2018). Clinically, birds that infected with avian pox show three forms of the disease, cutaneous, diphtheritic, and systemic forms. The birds show nodular lesions on unfeather parts in cutaneous form. The characteristic of the diphtheritic form is fibro-necrotic lesions in the mucosal layer of the oropharyngeal route, and the systemic form showed change on multiple internal tissues (Gilhare et al., 2015).
Avian pox is an economically important disease of commercial poultry because it can cause drop in egg production, slow growth and mortality. Mortality in flocks showing a mild cutaneous form of the disease is usually low (Tripathy and Reed, 2013). However, the mortality rate increases up to 50% when the diphtheritic form is accompanied by a secondary bacterial infection (Gilhare et al., 2015). The disease lasts for about 3-4 weeks, but if complications occur with other diseases it can last a very long time. Avian pox is re-emerging disease and a variant of APV has been widely reported, however, treatment for avian pox disease is not available (Gilhare et al., 2015; Singh et al., 2000). Prevention of this disease can be done mainly by vaccination (Garcia et al., 2003)
The racing pigeon is one of the most popular birds as pets in Indonesia. Pigeons are kept in a poultry farm location, allowing for transmission between species of birds. The article reported the outbreaks of APV based on isolation and identification from chicken and pigeon in a layer farm in Yogyakarta Province, Indonesia. In recent years, a problem of skin lesions with suspected avian pox was observed and it interferes with the productivity of the farm. This case report aims to provide information on confirmed APV case which have not received much attention from farmers so the awareness of APV will be increased.
CASE REPORT
Materials
Three layer chickens was collected from farm located in Bantul district, Yogyakarta Province, Indonesia, 15 months old, kept in a battery cage system, and had a history of vaccination against fowl pox. A unvaccinated pigeon from the same farm 20 months old was also sampled. Both chickens and pigeon examined showing nodular and/or fibro-necrotic lesions of the pox infections (Figure 1). All dead birds were necropsied following standard procedures. This research was carried out after procuring the necessary approval from the Ethical Clearance Commission for Research, Faculty of Veterinary Medicine, Universitas Gadjah Mada, Indonesia (No. 0078/EC-FKH/Int/2020).
Methods
APV isolation and identification included the preparation of viral suspensions, propagation on embryonic chicken eggs (ECE), histopathological preparations, and polymerase chain reaction (PCR) tests.
Virus isolation
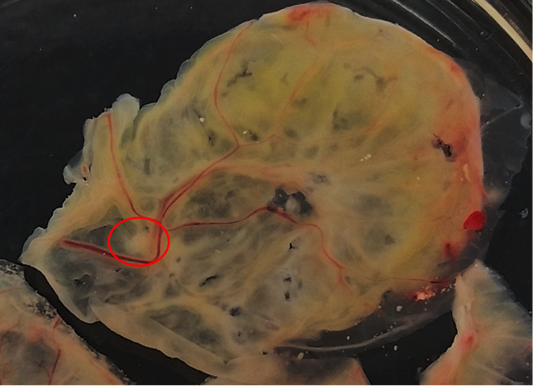
Nodular and/or fibro-necrotic lesions collected from chickens and pigeon that showing clinical symptoms of avian pox disease were grinded separately in a sterilized mortar and pestle then suspended in a sterile phosphate buffered saline (PBS) solution to make 10% suspension. The suspension was cleared by centrifugation at 7000 rpm for 10 minutes. The suspension then added with liquid gentamycin (5.5 mg/ml suspension) then homogenized with vortex for 30 seconds and incubated in biosafety cabinet for 60 minutes at 37°C. Specific-pathogen-free (SPF) ECE aged 10 days were candled. The location of the air sacs and the location of the virus inoculation are marked with a pencil. The marked areas were sterilized with alcohol 70%. The eggshell in the center of the air sac and the location of the virus inoculation are pierced by the egg perforator. The air sac is sucked with suction rubber to form a cavity at the site of inoculation of the viral suspension. The puncture hole were closed with scotch tape and then ECE incubated at 37°C before viral inoculation. After 24 hours ECE incubation, 0,2 mL of viral suspension was injected into the chorioallantoic membrane of ECE. The puncture hole was closed with nail polish and then incubated at 37°C in an incubator which has a coaster tray to keep moisture for 5-7 days. Chorioallantoic membranes were harvested and examined for pock lesions.
Histopathology
Nodular lesions from eyelids of pigeon and pock lesions on CAM from virus isolation were put into 10% formalin solution, embedded in paraffin, and cut into section 4 μm thick. After deparaffinization, the sections were stained with the hematoxylin-eosin (H and E) method.
Extraction of viral DNA
The infected CAMs, lyophilized live pox vaccine (Medivac Pox Medion, Indonesia) as positive control, and ddH20 as negative control were examined by APV-specific polymerase chain reaction (PCR). The DNA was extracted by the Pure Link™ Genomic DNA Mini Kit Invitrogen. Extraction was performed according to the protocol supplied by the manufacturer.
Avian pox-specific PCR
PCR was performed using the primer described by Lee and Lee (1997) based on the FPV 4b core protein (P4b) gene sequence. The sequences of the primer sets are as follows: forward primer, 5′-CAGCAGGTGCTAAACAACAA-3′ and reverse primer, 5′- CGGTAGCTTAACGCCGAATA-3′. The size of amplified DNA fragment using these primers was expected to be 578 bp long (8). The protocol used as described by Prukner-Radovčić et al. (2006) with modification. Amplification was performed after initial denaturation of 5 minutes at 94°C for 35 cycles and consisted of 1 minute of denaturation at 94°C, 30 seconds of annealing at 54°C, and 30 seconds of extension at 72°C. The final extension step was carried out for 5 minutes at a temperature of 72°C. PCR products amplified by electrophoresis with 1% agarose gel and visualized by SYBR® Safe DNA Gel Stain and ultraviolet trans illumination (9).
RESULTS AND dISCUSSION
| Samples | Clinical signs | Propagation on ECE | Histopathology | PCR |
| chicken 1 | cutaneous | pock lesions, thickening of the CAM | epithelial hyperplasia, Bollinger bodies | positive |
| chicken 2 | cutaneous | pock lesions, thickening of the CAM | epithelial hyperplasia, Bollinger bodies | positive |
| chicken 3 | cutaneous | pock lesions, thickening of the CAM | epithelial hyperplasia, Bollinger bodies | positive |
| pigeon 1 | cutaneous, oral cavity | NA | epithelial hyperplasia, Bollinger bodies | positive |
Based on clinical examination, both chickens and pigeon showed clinical signs on cutaneous form APV. The clinical signs seen in chickens and pigeon found in Yogyakarta are nodular lesions on the comb, wattle, and around the eyes and fibro-necrotic lesions in pigeon in the mucosa membrane of the oral cavity (Figure 1). Based on Tripathy and Reed (2013), the cutaneous form of APV is characterized by the appearance of nodular lesions on the unfeather areas of the body. Symptoms are various depending on host sensitivity, viral virulence, distribution of the lesion, and other complicating factors (Alehegn et al., 2014). The farmers reported a low mortality rate, but the productivity of laying hens has decreased.
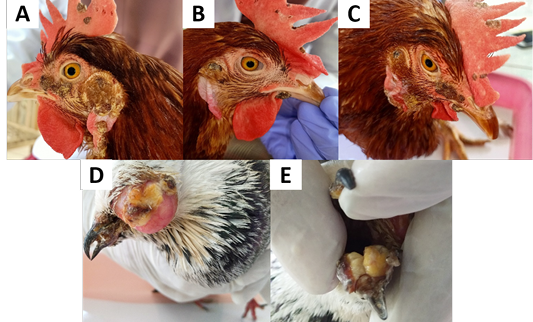
Figure 1: Clinical signs of avian pox in chickens and pigeon. (A, B, C) Nodular lesions of the comb, wattle, around the eyes and ears of the chickens. (D) Nodular lesions on the eyelids and beak of the pigeon. (E) Fibronecrotic lesion on oral mucosal membrane of pigeon.
Isolation of APV into Specific-pathogen-free (SPF) ECE aged 10 days showed pock lesions and thickening of the CAM after incubating for for 5-7 days (Figure 2). Identification of APV was performed by histopathological examination from cutaneous or diphtheritic lesions and pock lesions from CAM using HE staining. The results of histopathological examination, all samples are positive of APV infection. Histopathological lesions of fibro-necrotic lesions from pigeon and pock lesions from chickens showed epithelial hyperplasia, with enlargement and swelling of infected cells (Figure 3). Epithelial cells swell and separate from each other in the stratum spinosum layer. The cytoplasm of hyperplastic epithelial cells contains eosinophilic (Bollinger bodies) and vacuole inclusions. The inclusions bulge in the cell cytoplasm, producing cell necrosis (Figure 4).
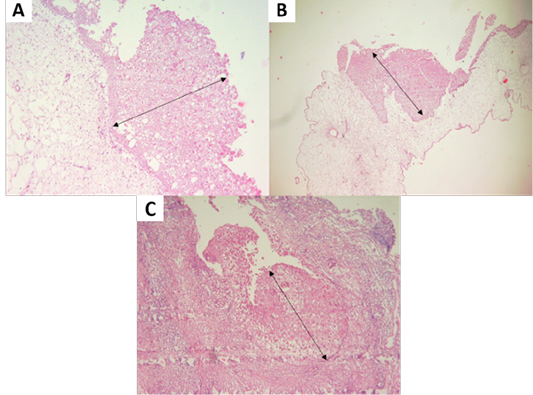
Figure 3: (A, B) Proliferation of chicken stratum spinosum cells (acanthosis) (oblique arrow). (C) Proliferation of pigeon stratum spinosum cells (acanthosis) (oblique arrow) (H and E staining, x100).
The sample for the polymerase chain reaction (PCR) test collected from the pock lesion on CAM harvested at the third passage. The primers were selected according to the 4b gene sequence from the FPV HP44 strain. Gen 4b is a core protein that has function as a structural protein (Lüschow et al., 2004; Afonso et al., 2000). The sequences of the primer are set as follows, forward primer, 5’-CAGCAGGTGCTAAACAACAA-3’ (identical to nucleotide 459-478), and reverse primer, 5’-CGGTAGCTTAACGCCGAATA-3’ (complementary to nucleotide 1016-1035). The size of the DNA fragment amplified using these two primers was 578 bp (Lee and Lee, 1997) The results of PCR examination on all samples of chickens and pigeon showed positive avian pox (Figure 5).
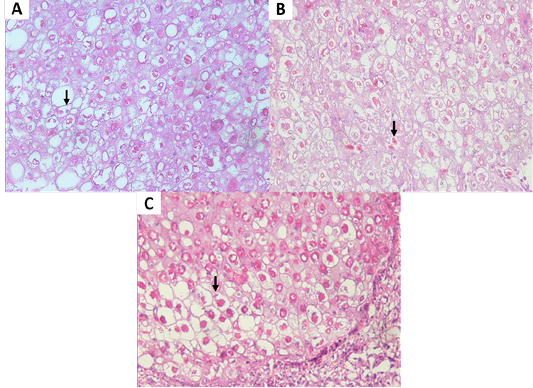
Figure 4: (A, B) Eosinophilic intracytoplasmic inclusion body (vertical arrow) of chicken. (C) Eosinophilic intracytoplasmic inclusion body (vertical arrow) of pigeon. (H and E staining, x400).
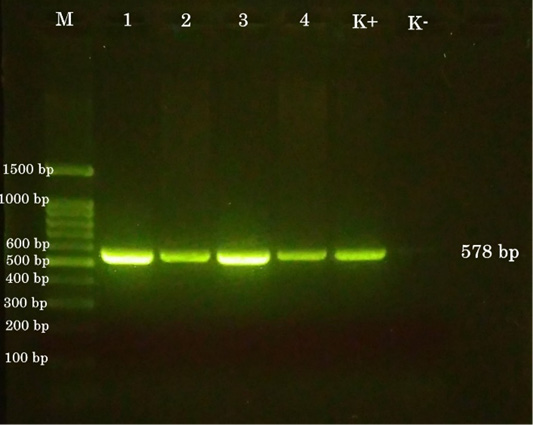
Figure 5: PCR results. (M) 100-bp DNA ladder. (1) chicken a. (2) chicken b. (3) chicken c. (4) pigeon. (K+) positive control. (K-) negative control.
The results of isolation and identification in this case report reconfirm the research conducted by Mohammed (2005), that there is a pathogenic relationship between the pox virus in chickens and pigeons. Furthermore, Mohammed (2005) stated that chickens can be infected with APV from 3 different virus strains including Fowl Pox, Canary Pox, and Pigeon Pox. This statement was previously stated by Jacob et al. (1998), which states that experimentally pigeon pox can infect pigeons, chickens, turkey, ducks, and gees.
Clinical features, histopathology, and PCR tests in chickens and pigeons have confirmed similar results. Vaccination in layer chickens using the Fowl Pox strain may not fully provide protection against challenges from the pigeon pox virus. Many factors can be related to the outbreak at the farm. The presence of mosquitoes, blood-sucking insects, and poor maintenance management have been identified as causes of outbreaks (Ferreira et al., 2018). According to Silva et al. (2009), APV is generally a self-limiting disease with mild manifestations. The clinical symptoms found in this case were nodular lesions on the comb, wattle, and around the eyes and fibro-necrotic lesions in the mucosa membrane of the oral cavity (Mohan and Fernandez, 2008). The clinical features were consistent with the cutaneous APV reported in chickens and pigeons by Alehegn et al. (2014). The lesions were characterized by nodular lesions on unfeathered skin and fibro-necrotic lesions on the mucosal oral cavity.
In this case, the mortality in chickens was not increase compared to before the outbreak. Mortality and mortality in this case are quite high in pigeon, with a morbidity of 16 birds (64%) and a mortality of 3 birds (12%) of the total population. Infection with pox virus does not result in primary mortality. Pigeons are sometimes kept by chicken farm workers as pets. Pigeons are allowed to freely fly around in the coop, which sometimes picks up feed that has fallen from the chicken coop. Pigeons that can fly freely between cages can transmit APV to layer chickens through scratch or direct contact.
Avian Pox Virus infections have been reported in more than 230 species of 900 bird species. Although the information was currently limited regarding host specificity and genome diversity (Gyuranecz et al., 2013), this case report informed that transmission between bird species is possible. Biosecurity and good management practices must be implemented in poultry production to prevent infection by various types of APV. Other bird species can be potential vectors for disease transmission. Therefore, pigeon should not be freely in the farm location because it allows APV transmission and causes an outbreak.
CONCLUSIONS AND RECOMMENDATIONS
Avian pox virus case was confirmed based on propagation, histopathological, and PCR examination in layer chickens and pigeon that showed the similar symptoms. A strict vaccination and biosecurity program is needed to prevent transmission of APV to a wide variety of birds. The other birds should not be kept inside the layer farm.
ACKNOWLEDGEMENTS
The authors are highly thankful to the Department of Internal Medicine and Department Microbiology, Faculty Veterinary Medicine, Universitas Gadjah Mada for support this research
Novelty Statement
This case report shows the potential transmission of avian poxvirus between chickens and pigeon at a farm in Yogyakarta, Indonesia.
AUTHOR’S CONTRIBUTION
All authors contributed equally and approved the final manuscript.
Conflict of interest
The authors have declared no conflict of interest.
REFERENCES