Advances in Animal and Veterinary Sciences
Research Article
Avian Influenza H5N1 Infection in Poultry and Their Handlers in Egypt: Risk Factors and Zoonotic Potential
Eman Abdel-Raouf Taha1*, Nahed Hamed Ghoneim2, Eman Hamza2, Abdelsatar Arafa3
1Department of Epidemiology, General Organization for Veterinary Services, Giza, Egypt; 2Department of Zoonoses, Faculty of Veterinary Medicine, Cairo University, Cairo, Egypt; 3National Laboratory for Veterinary quality control on Poultry production, Animal Health Research Institute, Giza, Egypt.
Abstract | Highly pathogenic avian influenza (HPAI) H5N1 subtype introduction into Egypt was documented to be of wild bird origin in 2005, the first description of AI in commercial poultry was in 2006. The period between 2006 and 2017 showed huge outbreaks of H5N1 with a high loss in poultry population, as well as 359 human cases. This study aimed to investigate the possible risk factors associated with H5N1 infection in poultry as well as human infection in poultry handlers. A total number of 824 tracheal swabs were collected from three poultry species, chickens (n=345), ducks (n=246), and turkeys (n=233) during the period from March 2016 to March 2017. Data were collected from farms and household sectors located in the Upper region (Assyut and Menia), the Central region (Cairo and Giza), and the Lower region (Sharqia and Qaliobia). The poultry samples were tested using Real-Time RT-PCR for detection of the HPAI H5N1. Serum samples were collected from 53 humans in contact with poultry and examined for antibodies against HPAI H5N1 using hemagglutination inhibition test. The risk factors for infection with HPAI H5N1 were investigated using two questionnaires for the collection of epidemiological data from poultry and humans, which was analyzed using Biostatistics software. The prevalence of HPAI H5N1 infection in poultry was 4.7 % (39 / 824). The temporal distribution, spring (7.9%) and winter (5.9%) compared to that in summer (2.3%) and autumn (1.7%). The prevalence was in the household sector (7.6%) higher than in the farm sector (1.9%). All tested human serum samples were negative to H5N1 antibodies. In conclusion, the risk factors include season (spring), bird species (duck), the household products sector, as well as frequent transmission of birds along governorates. Furthermore, the Upper Egypt region is a high risk for HPAI H5N1 infection, indicating the necessity for regular examination of possible risk factors. This can help in the control of H5N1 infection in poultry and minimize its impact on the public health.
Keywords | HPAI, H5N1, Poultry, Egypt, Avian influenza
Received | March 27, 2021; Accepted | June 05, 2021; Published | August 15, 2021
*Correspondence | Taha EA-R, Ghoneim NH, Hamza E, Arafa A (2021). Avian influenza H5N1 infection in poultry and their handlers in Egypt: Risk factors and zoonotic potential. Email: [email protected]
Citation | Eman Abdel-Raouf Taha, Department of Epidemiology, General Organization for Veterinary Services, 1 Nadi El-Sejd Street, 12618 Giza, Egypt; Adv. Anim. Vet. Sci. 9(10): 1517-1524.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.10.1517.1524
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Taha et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Avian influenza (AI) is a contagious disease caused by avian influenza viruses (AIVs) which occurs naturally in wild aquatic birds and can infect domestic poultry, pet birds, and other animal species (CDC, 2017). In domestic poultry including chickens, ducks, goose, and turkeys, the AIVs typically cause subclinical infection, while some strains can lead to a high mortality rate (Abou El-Amaiem et al., 2013). Cases of human infection with AI have been reported and human-to-human transmission occurred rarely (CDC, 2017).
AIVs belong to type A influenza genus, family Orthomyxoviridae. Influenza A viruses are RNA with a segmented genome that constantly change either by going through antigenic drift or shift. The antigenic drift represents the small changes that happen continually over time as the virus replicates, and usually result in new strain variants that are closely related. However, the antigenic shift denotes either major changes in the virus, or re-assortment between avian and human influenza strains, or between avian and other animal influenza strains which results in a new influenza A subtype (Wimmer et al., 2009), representing a major public health concern (Fournié et al., 2012).
Sixteen haemagglutinin HA (H1-H18) and nine neuraminidase NA (N1-N9) subtypes are well-known for influenza viruses A (ICTV, 2017). According to, the World Organization for Animal Health (OIE), AI is caused by any influenza A virus with high pathogenicity (H1-H16 HPAI) and by H5 and H7 subtypes with low pathogenicity (H5/H7 LPAI). Low pathogenicity non-H5 and non-H7 influenza A viruses (i.e. H1-4, H6 and H8-16) are not defined as AI and are not notifiable (OIE, 2018). Among the HP AIVs, H5N1 subtype represents a great concern particularly in south-east Asia and in Egypt (Fasanmi et al., 2017). This is attributed to the massive mortality caused by H5N1 in the domestic poultry, the unusual high virulence in wild birds, and the ability to infect humans (Haider et al., 2017), few cases of human-to-human transmission of H5N1 have been reported (CDC, 2018). In 2006, the occurrence of HP H5N1 of clade 2.2.1 has been reported in domestic poultry in Egypt. In 2008, the country was declared to be H5N1-enzootic (Kaoud et al., 2014; Abdelwahab et al., 2015). Despite the implementation of different control and preventive strategies including vaccination, yet, it failed to prevent outbreaks of the H5N1 in poultry (Kayali et al., 2016). Moreover, approximately 359 human cases of H5N1 were recorded in Egypt during the period from 2006 to 2017, 120 of them were fatal (case fatality rate: 33%) (WHO, 2017). Almost all human cases had close contact with infected live or dead birds, or virus-contaminated environments (Lai et al., 2016).
Poultry production systems in Egypt are ranging from rural very small-scale, extensive poultry production to highly intensive systems with over 70,000 birds per house in industrial commercial systems. Commercial poultry farms of various sizes provide about 90 % of chicken produced in Egypt, with the remaining 10 % provided by the small-scale household poultry farms that are abundant in villages and cities (Shatokhin et al., 2017). The household type of breeding is essential as a source of high-quality animal protein as well as a financial resource for low-income people. According to the Egyptian Poultry Association, in 2017 the number of broiler parents has reached 10 million heads, 88% of them are located in the Delta region. The live bird markets (LBMs) and poultry shops are the important traders of the commercial poultry industry, as well as the household producers (Shatokhin et al., 2017).
Many factors can affect the spread of the HPAI H5N1 virus between birds and the transmission to humans (Abou El-Amaiem et al., 2013), like high poultry density, geo-ecological settings as the higher percentage of surface water which would support higher densities of domestic and wild water birds compared with other adjacent regions. Additionally, demographic characteristics of the human population leading to increased contact with poultry, poor biosecurity in smallholder units and culturally determined food market habits linked to poor poultry hygiene (Ly et al., 2016). Therefore, the objectives of the current study were to determine the high-risk areas for infection with HPAI H5N1 in poultry along with different governorates in Egypt, investigate the risk factors associated with the high prevalence of infection, and assess the level of exposure of poultry handlers to the virus.
MATERIALS AND METHODS
Ethics approval and consent to participate
The study was conducted and performed according to ethical guidelines approved by the faculty of veterinary Medicine, Cairo University, Cairo, Egypt (Number: ReVet CU 28/04/2021/32). There were no experiments on human participants, oral consent was taken from humans for agreement on the blood samples.
Sampling
Poultry samples and data collection
A total number of 824 tracheal swabs were taken from poultry during the period between March 2016 and March 2017, taking into consideration the four seasons, spring, summer, autumn, and winter. The selection of the samples was based on species of poultry, production units (household and farms), and the geographical distribution in Egypt, stratified sampling and simple random sample models were applied. Three species of poultry were included, chickens (n=345), ducks (n=246), and turkeys (n=233). The samples were collected from 6 governorates throughout the 3 main regions in Egypt, represented as follow; the Upper Egypt region: Assyut (EG-AST, n=116) and Menia (EG-MN, n = 178); the Central region: Cairo (EG-C, n = 131) and Giza (EG-GZ, n =127) and the Lower region: Sharqia (EG-SHR, n = 150) and Qaliobia (EG-KB, n = 122). Selection of governorates was performed according to the presence of an intensive number of birds and confirmed poultry cases of H5N1 infection recorded in the previous years.
A questionnaire was designed for the collection of the following epidemiological data of poultry: Species of poultry, health status (whether the birds show signs of respiratory diseases), vaccination status (influenza or other diseases), production unit, source of poultry in terms of location in governorates, and density of poultry population/governorates.
The tracheas of the live birds were swabbed using sterile cotton swabs by gently moving against the tracheal wall. The swabs were directly placed in viral transport media (Helal et al., 2017) and transported in an icebox to the laboratory. The samples were kept at -80°C until tested.
Human samples and data collection
A total of 53 blood samples were collected from humans (n=53) working in contact with poultry, including poultry shop sellers, poultry housekeepers, and veterinarians.
A questionnaire was designed for the collection of the following epidemiological data from humans: Profession, age, gender, health status, location in governorates, human population density/ governorates and season of sample collection.
Blood samples from humans were left to clot at room temperature and serum was separated by centrifugation and kept at -80°C until tested.
Direct detection of HPAI H5N1 virus in tracheal swab samples from poultry using real-time reverse transcription polymerase chain reaction (RT-PCR)
Viral RNAs were extracted from tracheal swab samples using QIAamp Viral RNA Mini Kit (Qiagen, Germany), according to the company’s instruction. The extracted viral RNAs were amplified for influenza A virus using one-step RT-PCR kit (Qiagen), according to (Spackman et al., 2002). RNAs that were positive to influenza A were further amplified for detection of H5N1 by RT-PCR using the following primers and probe; H5-LH: 5-ACATATGACTACCCACARTATTCA-3; H5-RH1: 5-AGACCAGCTAYCATGATTGC-3; Probe: 5 FAM TCWACAGTGGCGTTCCCTAGCA–Tamra-3; (Slomka et al., 2007). NA subtyping was carried out using RT-qPCR primers: IAV-N1-3-F: AGR CCT TGY TTC TGG GTT GA, IAV-N1-3-R: ACC GTC TGG CCA AGA CCA and probe IAV-N1-3-FAM: FAM-ATY TGG ACY AGT GGG AGC AGC AT-BHQ1 as described by (Hoffmann et al., 2016).
Positive and negative controls were run in parallel with the viral RNA extracted from the tracheal samples. RNA was extracted from a positive sample of local H5N1 Egyptian virus, whereas negative control was no template RNA control. The negative control should have no Ct (threshold cycle) value. The positive control should have a Ct value of less than 35 cycles with a typical amplification curve. Samples with Ct values of less than 35 cycles were considered positive. Samples with no amplification curves were considered negative.
Serological detection of antibodies against HPAI H5N1 in human serum samples using hemagglutination inhibition (HI) test
The presence of antibodies against H5N1 in the prepared serum samples was tested using the HI test according to (OIE, 2015). The H5N1 virus used was the local Egyptian H5N1 virus. The virus was used in a standardized working solution of 4 hemagglutination units (HAU) per 25 ul PBS. The chicken RBCs were used in a 1% solution of PBS. For each run of the test, a positive control serum (SPF chickens immunized with the same H5N1 antigen used in the HI test) and negative control sera (uninfected SPF chickens) were used. The HI titer was expressed as the reciprocal value of the highest dilution of serum causing complete agglutination inhibition of antigen of 4 HAU. The minimal detectable titer given by the HI tests was 4 (2 log2). To differentiate positive from negative results, HI titers of 16 (4 log2) or greater were considered positive for avian influenza antibody, according to OIE guidelines (OIE, 2015).
Statistical analysis
Analysis was performed by SPSS software Version 19.0.IBM (SPSS Inc., Chicago, IL, USA). Correlation between possible risk factors and infection with AI was assessed using Chi-square of Test of Independence, Fisher’s Exact Test (FET), as well as the odd ratios. A Graphic illustration chart for each risk factor was performed. The Arc GIS program (ArcMap version 10.1 software) was used to draw a Map with foci of HPAI H5N1 infection of the examined poultry along with the different governorates.
Results and Discussion
Prevalence of HPAI H5N1 in poultry
During the period from March 2016 to March 2017, 824 tracheal swab samples were collected from poultry raised in farms (n=418) and households (n=406) along six governorates in Egypt. Table 1, Figure 1A revealed that HPAI H5N1 virus infection occurred in 9.5% of examined poultry from EG-AST, 6.1% from EG-C, 5.7% from EG-KB, 3.9% from EG-GZ, 3.4% from EG-MN, and 1.3% from EG-SHR. The relation between the governorates and the rate of AI infections was significant, χ2 (5, N = 824) = 11.38, P = 0.044. HPAI H5N1 infections are more prevalent in EG-AST than in other governorates. H5N1 AI virus infections occurred at a rate of 1.9% in the commercial farm sector and 7.6% in the household sector (Table 1). The relation between the production sector and the rate of AI infections in poultry was significant, χ2 (1, N = 824) = 14.95, P < 0.0001. The household sector is more likely to spread HPAI H5N1 infection than the commercial farm sector. The poultry samples were obtained during summer, autumn, winter, and spring, rate of infection was compared between the four seasons. HPAI H5N1 virus infections occurred at a rate of 1.7% in summer, 2.3% in autumn, 5.9% in winter, and 7.9% in spring (Table 1, Figure 1B). In spring, birds are more likely to get infected by the virus four times more than in summer. The relation between the season and the rate of HPAI H5N1 infections in poultry was significant, χ2 (3, N = 824) = 12.14, P = 0.007. Spring is more likely to spread HPAI H5N1 infection than other seasons.
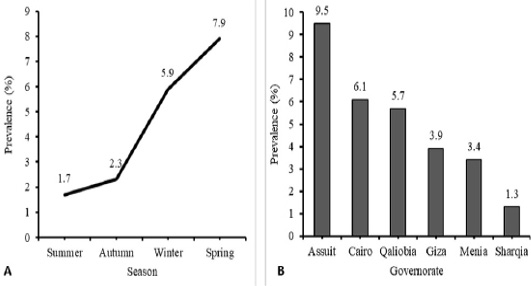
Figure 1: Temporal (A) and Spatial (B) prevalence of Avian influenza (H5N1) virus in different season and different tested governorate in Egypt. Analysis and figures were performed by SPSS software Version 19.0.IBM (SPSS Inc., Chicago, IL, USA).
Table 1: Prevalence of HPAI H5N1 virus in farms and household units, during the period from March 2016 to March 2017 (n = 824 units).
| Governorates | Production unit no. (%) | Season no. (%) | Total no. (%) | ||||
| Farms | Household | Summer | Autumn | Winter | Spring | ||
| EG-AST | 5/53 (9.4) | 6/63 (9.5) | 0/41 (0.0) | 0/23 (0.0) | 0/20 (0.0) | 11/32 (34.4) |
11/116 (9.5)a |
| EG-MN | 1/101 (0.9) | 5/77 (6.5) | 0/31 (0.0) | 0/21 (0.0) | 4/70 (5.7) | 2/56 (3.6) |
6/178 (3.4)bc |
| EG-C | 0/60 (0.0) | 8/71 (11.3) | 0/38 (0.0) | 3/18 (16.6) | 1/19 (5.3) | 4/56 (7.1) |
8/131 (6.1)ab |
| EG-GZ | 1/60 (1.7) | 4/67 (5.9) | 1/36 (2.8) | 0/24 (0.0) | 4/24 (16.7) | 0/43 (0.0) |
5/127 (3.9)abc |
| EG-KB | 0/56 (0.0) | 7/66 (10.6) | 2/38 (5.3) | 0/24 (0.0) | 2/22 (9.1) | 3/38 (7.9) |
7/122 (5.7)ab |
| EG-SHR | 1/88 (1.1) | 1/62 (1.6) | 1/50 (2) | 0/20 (0.0) | 0/29 (0.0) | 1/51 (1.9) |
2/150 (1.3)c |
| Total | 8/418 (1.9) | 31/406 (7.6) |
4/234 (1.7)C |
3/130 (2.3)BC |
11/184 (5.9)AB |
21/276 (7.9)A |
39/824 (4.7) |
a,b,c Different superscripts in the same column and A,B,C Different superscripts in the same row indicate significance at P < 0.05.
Table 2: Factors affecting the prevalence of HPAI H5N1 virus in poultry population in Egypt (n = 824 units).
| Positive AI (%) | Odds Ratio (OR) | 95% CI | P- value | |
| Source of poultry | 1.2 | 0.40-3.87 | 0.713 | |
| Another governorate | 14/335 (4.1) | |||
| Same governorate | 11/174 (6.3) | |||
| Same district | 10/237 (4.2) | |||
| Same village | 4/78 (5.1) | |||
| Region | 1.8 | 0.79-4.09 | 0.369 | |
| Lower Egypt | 9/272 (3.3) | |||
| Central Egypt | 13/258 (5.0) | |||
| Upper Egypt | 17/294 (5.8) | |||
| Species of poultry | 1.6 | 0.80-3.34 | 0.120 | |
| Chickens | 15/345 (4.3) | |||
| Ducks | 17/246 (6.9) | |||
| Turkeys | 7/233 (3.0) | |||
| Vaccination status | 4.3 | 1.96-9.53 | < 0.0001 | |
| Unvaccinated |
31/402 (7.7) a |
|||
| Vaccinated |
8/422 (1.9) b |
|||
| Total | 39/824 (4.7) |
a,b Different superscripts in the same column indicate significance at P < 0.05.
Risk factors influencing infection of poultry with HPAI H5N1
The effect of the following risk factors on infection of poultry with HPAI H5N1was examined. This includes the source of poultry in terms of location in governorates and geographical region (Upper, Central, and Lower Egypt), species of poultry (chickens, ducks, and turkeys), and vaccination status. As demonstrated in Table 2, prevalence of avian influenza (H5N1) infections among sources of poultry reported 6.3% among poultry transmitted from the same governorate, 5.1% among poultry transmitted from the same village, 4.2% among poultry transmitted from the same district, and 4.1% among poultry transmitted from another governorate. Poultry transmitted from same governorate has 1.5 times more chance to be infected by HPAI than poultry transmitted from other governorates. Prevalence of avian influenza (H5N1) infections in different regions reported 5.8% in the Upper Egypt, but 3.3% in Lower Egypt. Poultry in the Upper region has the chance to be infected by HPAI 1.8 times more than poultry in the Lower Egypt. On the other hand, ducks are the most infected species with a high prevalence of HPAI 6.9%, as duck is a carrier of HPAI, followed by chicken 4.3%, and the lowest prevalence was reported for turkey 3.0%. Ducks have the chance to be infected by HPAI 1.6 times more than chickens. The prevalence of AI infections in unvaccinated birds was 7.7%, while in vaccinated poultry was 1.9%. Unvaccinated poultry has 4.3 times more chance to be infected by HPAI vaccinated poultry. There were no significant associations between AI infection rates in poultry and the sources of birds (χ2 (3) = 1.37, P = 0.713), the geographical regions (χ2 (2) = 1.99, P = 0.369), and bird species (χ2 (2) = 4.25, P = 0.120). However, the vaccination status of birds showed a significant association with the rate of AI infections among poultry (χ2 (1) = 15.44, P < 0.0001). Accordingly, the effect of governorate, regions, and species of poultry on the prevalence of AI was compared between vaccinated and unvaccinated poultry (Table 3). Unvaccinated birds are more likely to acquire AI infections than vaccinated birds. Spatial Density analysis at Egypt map (Figure 2) showed that EG-AST governorate in the Upper Egypt represents a high density of H5N1 poultry cases. Additionally, EG-C in the Central region and EG-KB in the Lower region represent high density.
Prevalence of HPAI H5N1 infection in Humans having contact with poultry
Serum samples from 53 humans having contact with poultry were examined for the presence of antibodies against the HPAI H5N1 virus and all samples were negative.
Avian influenza (AI) is a contagious disease caused by avian influenza viruses (AIVs) which occur naturally in wild aquatic birds and can infect domestic poultry, pet birds, and other animal species (CDC 2017). In the present study, it was of interest to examine the high-risk areas for infection with H5N1 in poultry along with different governorates in Egypt. Besides, assessing the level of exposure of poultry handlers to the virus, and investigating the risk factors associated with the high prevalence of HPAI. We found that the climatic season is one of the major risk factors. There is a high chance of poultry infection with HPAI H5N1 during spring (7.9%) and winter (5.9%) than during summer (1.7%) and autumn (2.3%). This is consistent with the temporal pattern of HPAI H5N1 in poultry in Egypt reported in previous studies, which demonstrated a peak prevalence between January and March or during August and September, as well as December and January (Arafa et al., 2016; El-Masry et al., 2015).
Table 3: Prevalence of HPAI H5N1 infections in unvaccinated and vaccinated poultry.
| Positive AI (%) | ||||
| Governorates | Unvaccinated | Vaccinated |
χ2 (1) |
P value |
| EG-AST | 6/61 (9.8) | 5/55 (9.1) | 0.019 | 0.891 |
| EG-C |
8/62 (12.9) a |
0/69 (0.0) b |
FET | 0.002 |
| EG-GZ | 4/64 (6.3) | 1/63 (1.6) | FET | 0.365 |
| EG-MN | 5/87 (5.7) | 1/91 (1.1) | FET | 0.112 |
| EG-KB |
7/66 (10.6) a |
0/56 (0.0) b |
FET | 0.015 |
| EG-SHR | 1/62 (1.6) | 1/88 (1.1) | FET | 1.000 |
| Region | ||||
| Lower Egypt |
8/128 (6.3) a |
1/144 (0.7)b |
FET | 0.014 |
| Central Egypt |
12/126 (9.5) a |
1/132 (0.8)b |
10.35 | 0.001 |
| Upper Egypt | 11/148 (7.4) | 6/146 (4.1) | 1.49 | 0.222 |
| Species of poultry | ||||
| Chickens | 11/169 (6.5) | 4/176 (2.3) | 3.72 | 0.054 |
| Ducks |
14/125 (11.2)a |
3/121 (2.5)b |
7.27 | 0.007 |
| Turkeys | 6/108 (5.6) | 1/125 (0.8) | FET | 0.051 |
| Total | 31/402 (7.7) | 8/422 (1.9) | ||
a, b Different superscripts in the same row indicate significance at P < 0.05; FET: Fisher’s Exact Test.
The current study showed that poultry from the Upper Egypt region has a 1.8 times chance to be infected with HPAI than poultry in the Lower Egypt, agreeing with previous findings (El-Masry et al., 2015). The uncontrolled bird transportation and traditions represent the main risk factor for disease spread among different governorates and even between districts and villages of the same governorate (Fasanmi et al., 2017).
The production unit is an additional risk factor that was examined in the present study. We found that the household sector represents a risk for infection with HPAI H5N1 with a percentage of (7.6%) as compared to rearing in commercial farms (1.9%). This finding is supported by previous work that described the role of household birds in the spread of H5N1 in poultry in Egypt with their significant role in human and poultry infections (Abdelwhab and Ahmed, 2015). The household sector is an important factor in sustaining the dynamic and spread of HPAI. This sector is common in rural areas of Egypt, that are lacking sufficient sanitary measures and are not regularly controlled (Kandeil et al., 2017). Moreover, the household reared poultry can be transported freely between districts and villages as well as between governments, which can spread the virus from rural to urban areas (Abdelwhab and Ahmed, 2015; Wang et al., 2018). Further epidemiological studies are needed to investigate the pattern of HPAI-H5N1 spread in the commercial sector and persistence, transmission pathways from and to the household sector (Abdelwhab and Ahmed 2015).
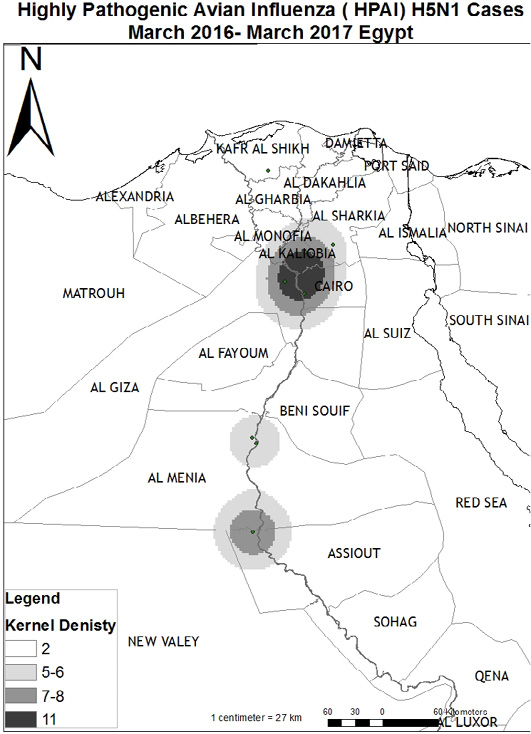
Figure 2: Map for Highly Pathogenic Avian Influenza (HPAI) H5N1 cases “ March 2016-March 2017, Egypt. The Arc GIS software (Geographic Information System) program (ArcMap version 10.1 software) was used to draw a Map with foci of AI infection of the examined poultry along the different governorates. Arc GIS is a computer system is produced by Environmental Systems Research Institute (ESRI) and are used for capturing, storing, checking, and displaying data related to positions on Earth’s surface.
Regarding the species of poultry, duck breeding is another risk factor for the spread of HPAI H5N1. Ducks have 1.6 times more chance to be infected with HPAI than chickens, while occurrence in turkeys is low (3.0%). Another study in Egypt reported that the infection rate in ducks is 5 times more than in chickens (El-Masry et al., 2015). This can be explained by the findings that ducks are considered one of the natural reservoirs of AIV. Infected ducks play a role in the maintenance of HPAI H5N1 viruses, as they carry the virus and can shed high amount without showing clinical signs (Wang et al., 2018).
The unvaccinated poultry has a 4.3 times chance to be infected with HPAI than vaccinated poultry, suggesting the importance of vaccination of birds. In this regard, (Yupiana et al., 2010) reported that vaccination is one of the most effective preventive measures to reduce the transmission and spread of HPAI H5N1. Additionally, (Kandeil et al., 2017) demonstrated that vaccinated chickens survived infection with AI without experiencing any symptoms; additionally these chickens were not shed the virus as examined in cloacal and oral swabs.
In this study, all human samples were negative for serological detection of antibodies against H5N1. However, there were three confirmed human H5N1 AI cases reported from EG-C, EG-GZ governorates in the first quarter of 2016 in Egypt (WHO, 2016). This indicates low human infection during that period. Further investigations are needed to examine the role of bird transmission as the main risk for human infection.
In conclusion, the most frequent risk factors found in the present study were the seasonal occurrence of HPAI- H5N1, particularly spring and winter. The Upper Egypt represents a high-risk area for infection with the HPAI H5N1 during the study period. Frequent transportation of birds from other governorates acts as an additional risk for the spread of the virus. The rearing of birds in farms appears to be of low risk than the household sector due to the controlled hygienic measures. Vaccination of birds can reduce the risk of infection as well as dissemination of the HPAI H5N1.
ACKNOWLEDGEMENTS
The authors acknowledge the support of work implemented by the General Organization for Veterinary Services (GOVS), jointly with Food and Agriculture Organization of the United Nations (FAO) . In particular, the Department of Epidemiology in data collection, the Department of Poultry in collect poultry samples. The cooperation of the owners of the chicken farms and the poultry backyards is highly appreciated. We are grateful to Dr. Elshaimaa Ismael, associate professor at Department of Veterinary Hygiene and Management, Faculty of Veterinary Medicine, Cairo University, Egypt for the guidance in the statistical analysis.
Novelty Statement
The present study investigated the possible risk factors associated with H5N1 infection in poultry as well as human infection in poultry handlers. The samples covered the four main regions in Egypt, in particular areas with high density of poultry and humans, as well as during the four seasons.
Author’s Contribution
All authors contributed equally to the work.
Conflict of interest
The authors have declared no conflict of interest.
REFERENCES






