Advances in Animal and Veterinary Sciences
Research Article
Impact of Environmental Impurities on Performance, Immunity and Tissue Architecture in Broiler Chickens
Omnia F. Mohamed, Mahmoud M. Hussein, Essam S. Soliman*
Animal, Poultry, and Environmental Hygiene Division, Department of Animal Hygiene, Zoonosis, and Animal Behavior, Faculty of Veterinary Medicine, Suez Canal University, Ismailia 41522, Egypt.
Abstract | Air and water represented essential and critical elements of life in broiler chickens farms. The influence of some air and water impurities on performance, biochemical profile, cortisol levels, intestinal bacterial load, immunoglobulin concentrations, and tissue architecture in broiler chicken farms was studied. A total of 210 one-day-old classic Hubbard broilers were divided into six groups and housed on deep litters into six independent rooms. Broilers of G1, G2, G3, G4, and G5 were challenged with ammonia (75 ppm), lead nitrate (0.1 mg.L-1), E. coli O157: H7 (1.5 x 108 CFU.ml-1), magnesium sulfate (0.075 mg.L-1), and ammonium chloride (0.005 mg.L-1) respectively, and G6 were kept as a control group. Challenges were applied for 2 hr/week for three successive weeks starting from 14th days old. A total of 1368 samples were collected including 228 air samples, 190 sera, 190 intestinal swabs, and 760 organ samples including liver, heart, spleen, and bursa of Fabricius. Weight gains, feed intakes, and performance indices revealed highly significant improvement (P < 0.01) in most challenged broiler groups. Total protein, triglycerides, total cholesterol, glucose, cortisol sera concentrations, and Total bacterial and Enterobacteriaceae counts revealed highly significant increases (P < 0.01) in all challenged broiler groups. Meanwhile, alanine aminotransferase, creatinine, IgG, and IgM revealed highly significant declines (P < 0.01) in all challenged broilers compared to control. Histopathological photomicrographs confirmed the disturbances in tissue architecture in all challenged broilers compared to the normal histologic microscopic appearance in the control group. The study concluded that environmental impurities impaired most of the physiological functions and histological appearance even after relieving the challenges and for a while. Some impurities were able to act as growth promoters after the relief of the overwhelming challenge conditions.
Keywords | Ammonia, Broiler chickens, Immunity, Performance, Water Impurities
Received | July 19, 2020; Accepted | August 29, 2020; Published | November 15, 2020
*Correspondence | Essam S. Soliman, Department of Animal Hygiene, Zoonosis and Animal Behavior, Faculty of Veterinary Medicine, Suez Canal University, Ismailia 41522, Egypt; Email: [email protected]
Citation | Mohamed OF, Hussein MM, Soliman ES (2020). Impact of environmental impurities on performance, immunity and tissue architecture in broiler chickens. Adv. Anim. Vet. Sci. 8(12): 1266-1277.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.12.1266.1277
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Mohamed et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Air is one of the most important keystones for life and prosperity in broiler chicken farms (Menegali et al., 2012). There are a variety of air pollutants that gain access to the indoor microclimatic air inside broiler farms and alter its quality, producing a variety of negative impacts on the broiler’s production, physiology, and tissue architecture (Nääs et al., 2007). Ammonia is an indoor air pollutant that is frequently produced from various activities inside the broiler’s houses like fermentation processes, degradation of litter materials, and breathing activities (Skóra et al., 2016). Ammonia production inside broiler chicken houses is controlled by many factors like ambient temperature and humidity, air movement and ventilation rate, as well as, pH, moisture content, and temperature of the litter (Marín et al., 2015). The presence of high ammonia concentration can negatively influence growth traits, and immunity levels (Cemek et al., 2016).
Water takes over an important role in many physiological functions like consumption, digestion, metabolism, transportation, and utilization of nutritive substances, chemical and enzymatic reactions, lubrication of joints, and regulation of body temperature (Batal et al., 2005). Water is also necessary inside broiler farms for cleaning and sanitation issues and the regulation of microclimatic temperature (Bell, 2002). Water consumption depends on many factors like age, environmental temperature, water temperature, electrolytes, lighting program, water quality, and geographical area (Fairchild and Ritz, 2015). Water can be used as a measure for broiler performance based on its consumption and based on water intake to feed intake ratios (Dozier et al., 2002).
Broiler chickens should be supplied with water of high quality to eliminate the chances for the development of negative effects on broilers health, these quality criteria were set by many agencies, but many breeders failed to sustain these standards and thus carry negative influences on broiler’s health and performance (Jafari et al., 2006). Also providing water of poor quality might reduce the effectiveness of the medications and vaccination provided to the broiler chickens during the fattening cycle (Scandurra, 2013). Many types of researches reported that the use of water additives was recommended to improve water quality as ionized alkaline water (Jassim and Aqeel, 2017), water soaking of sweet orange (Orayaga et al., 2016), potassium chloride (Yosi et al., 2016), silicon (Sgavioli et al., 2016), and amino acid-chelated trace mineral (Baxter et al., 2020)
The study aimed for studying the influence of some air gas impurities like ammonia gas (75 ppm) and some water impurities like metals (lead nitrate by 0.1 mg.L-1), microbial contaminants (E. coli O157: H7 1.5 x 108 CFU/ml), Hardness salts (magnesium sulfate by 0.075 mg.L-1), and nitrogenous source (ammonium chloride by 0.005 mg.L-1) on performance traits, biochemical profile, stress markers (serum cortisol levels), intestinal bacterial load, immunoglobulin concentrations (IgG and IgM to determine the efficiency of vaccination act in broiler chicken farms), and tissue architecture. Broilers were subjected to these challenges for two hours weekly for three successive weeks starting from 14th days old.
MATERIALS AND METHODS
Ethical approval
The procedures and protocols in the current study were approved by the Scientific Research Ethics Committee, Faculty of Veterinary Medicine, Suez Canal University, Ismailia, Egypt with approval number (2020033).
Experimental birds’ microclimate and management
A total of 210 one-day-old classic Hubbard broilers were purchased from El-Helal Co-Ismailia. Broilers were divided into six groups, 35 each (five replicates of seven birds), and housed on a deep litter system (Hay) according to Soliman and Hassan (2020) into sex independent rooms. Rooms were provided with strict hygienic, preventive, and biosecurity measures represented as follow: feet dip at the entrance of each room, proper disinfection protocol before brooding broilers, fly and rodent-proof nets, secured entrance for rations, clean feeders, and waterers, circumcised passage for serving broilers, and prevention of entrance of flying and/or wild birds as recommended by Soliman and Abdallah (2020). Rooms’ floors were covered with a thin layer of superphosphate by a rate of 0.5 g/m2 before applying hay litters as recommended by Soliman et al. (2018) to maintain optimum abiotic conditions. Rooms were ventilated using V-shaped windows located on each side of the wall-ceiling junction and ceiling fans to stimulate air convection. Rooms were supplied with blue and white LED lights serving a continuous lighting regimen for 18 hours of light and 6 hours of darkness (18L: 6D) as recommended by Soliman and Hassan, (2019). The experiment lasted for 38 days during which microclimatic temperature and relative humidity, as well as crude mortalities, were monitored daily.
Broilers were brooded at 35°C with a latter comfort zone of 21-24 °C (brooding temperature was decreased by a rate of 3°C weekly until obtaining thermo-neutral zone by the end of the 3rd week). Broilers were supplemented with corn-soybean ration contained 23% protein, 5.60% fat, 3.8% crude fiber, and 2990 Kcal/kg energy in the starter ration (provided for 13-14 days) and 21.5% protein, 3.95% fat, 3.55% crude fiber, and 3100 Kcal/kg energy in the grower ration (provided from 15th day until the end of the cycle) as recommended by NRC (1994) and Applegate and Angel (2014). Broilers in each room were given ad libitum access to drinking water. Broilers were vaccinated using the most common vaccination programs based on protection against infectious bronchitis by live attenuated virus of IB-H120 ≥ 103.5 at 6th day, against infectious bursal disease by live attenuated virus of VMG91 ≥ 103.0 at 14th and 21st days, and against Newcastle disease virus by live lentogenic ND virus of Lasota ≥ 106.0 at 18th and 28th days.
Impurities challenge
The impurities were prepared in-stock solutions of 1000 ppm of the element according to the following formula:

Broilers’ groups were challenged with some environmental impurities as follow: broilers in the 1st room (G1) were challenged with ammonia 75 ppm, broilers in the 2nd room (G2) were challenged with lead nitrate by a rate of 0.1 mg.L-1, broilers in the 3rd room (G3) were challenged with E. coli O157: H7 1.5 x 108 CFU/ml, broilers in the 4th room (G4) were challenged with magnesium sulfate by a rate of 0.075 mg.L-1, broilers in the 5th room (G5) were challenged with ammonium chloride by a rate of 0.005 mg.L-1, and broilers in the 6th room (G6) were kept as a control untreated group.
Ammonia gas in the 1st room was delivered using vaporizers supplied with 100ml ammonium hydroxide 35%, while impurities in the other rooms (G2, G3, G4, and G5) were added to one-liter drinking water. Broilers groups were subjected to the environmental challenges in air and water for two hours weekly for three successive weeks starting from 14th days old.
Performance indices
Live body weights (LBW.g-1) were measured by weighing a representative number of birds in each room (28 birds per group were weighed weekly to obtain average live body weights), these numbers were calculated via simple random sampling procedures with an expected error 5% (Thrusfield, 2005) using the following formula:
n =1.962 Pexp (1 - Pexp) / d2
Where; n = required sample size, Pexp = expected prevalence, d = desired absolute precision. Feed intakes (FI.g-1) were calculated by diving the total amount consumed in each room by the number of live birds occupying this room. Performance indices like weight gains (WG.g-1), feed conversion ratios (FCR), and performance indices (PI) were calculated as recommended by Soliman and Hassan (2017).
Sampling
A total of 1368 samples including 228 air samples (6 samples daily/ 38 days), 190 sera, 190 intestinal swabs, and 760 organ samples including liver, heart, spleen, and bursa of Fabricius were collected by the end of the study period. Air samples were collected using a suction pump and passed on 9 ml buffered peptone water for the laboratory examinations. Blood samples were collected via sacrificing the birds at the end of the fattening cycle, held in a water bath at 37°C for 30 minutes, and centrifuged at 3500 rpm for 15 minutes (Centrifuge Centurion Scientific C2006, 13010-4, 230V, 50-60hr, T6-3A). Clear sera were transferred by automatic pipette into Eppendorf tubes and stored at -20 °C for the biochemical, hormonal, and immunological analysis (Soliman et al., 2017). The liver, spleen, heart, and bursa of Fabricius were removed from the sacrificed birds and placed on buffered formalin for the histopathological examination. Intestinal swabs were collected and added to 9 ml buffered peptone water and transferred to the laboratory for bacteriological assessment. Sacrificed birds were finally disposed of using burial techniques.
Aerial ammonia examination
Air samples (228 samples were collected by a rate of 6 samples daily for 38 days) were examined for levels of ammonia using 0.05M sulfuric acid solution and potentiometric titration against ammonium hydroxide in the presence of methyl orange as a pH color indicator (APHA, 2017). The calculated results were compared to the ammonia meter (Digital Portable NH3 Meter Ammonia Gas Detector 0: 100 ppm).
Biochemical and hormonal profile
Sera (190 sera samples optioned by sacrificing the birds by the end of the fattening period, approximately 38 days) were examined for total protein (TP, g/dl), alanine aminotransferase (ALT, IU/L), creatinine (CREAT, mg/dl), triglycerides (TG, mg/dl), total cholesterol (TC, mg/dl), and glucose (GLUCO, mg/dl) calorimetrically using Roche Integra 400 Plus chemical analyzer. Immunoglobulin concentrations like IgG and IgM (mg/dl) and cortisol levels (µg/dL) were measured using ROCHE ELECSYS 1010 Immunoassay Analyzer (Wu et al., 2017).
Bacteriological examination
Intestinal swabs were prepared by running tenfold serial dilutions up to 10-10 to cover the theoretically expected range of contamination (APHA, 2012). Total bacterial count (TBC) onto standard plate count agar (OXOID CM0325, Tryptone Glucose Yeast Agar, 500g) and total Enterobacteriaceae count (TEC) onto eosin methylene blue agar (CM0069, Eosin Methylene Blue Agar, Levine, 500g) at 37ºC for 24-48 hours were performed using drop plate technique as recommended by Kim and Lee (2016) and Soliman et al. (2016). Plates were counted as recommended Murray et al. (2015) using the Dark-field colony counter (Quebec, Reichert-Jung).
Histopathological examination
Liver, heart, spleen, and bursa of Fabricius fixed in 10% buffered formalin solution were cut into 5-mm thickness sections, put into tissue cassettes, dehydrated by transferring through increasing concentrations of alcohols, cleared into two changes of xylene, infiltrated into different grades of melted paraffin in the oven, embedded in paraffin and the sections were cut in 5 μm thick using rotator microtome.
The sections were floated on a warm water bath at 38°C for stretching and mounted on clean slides using egg albumin and dried on a slide warmer at 38°C (Luna, 1968; Darboux, 1994). The sections were stained using Hematoxylin and Eosin (H and E). The histological structures of the examined organs were observed using a light microscope (Olympus DP-73 microscope digital camera) under (x10) and (x20) magnification.
Table 1: Performance indices (Mean ±SE) in broilers challenged with air and water impurities.
| Groups | Age/weeks | BWG / g | FI / g | FCR % | PI | WI |
| Overall means in concern with treatments | ||||||
| G1 |
319.5c±6.12 |
538.2e±3.23 |
2.00a±0.12 |
4.9b±0.30 |
353a±9.54 |
|
| G2 |
391.7a±1.24 |
603.8b±4.91 |
1.99a±0.27 |
6.5a±0.48 |
172cd±6.87 |
|
| G3 |
366.7ab±2.12 |
569.8c±2.45 |
1.70a±0.06 |
6.2a±0.53 |
175c±6.93 |
|
| G4 |
346.7bc±4.08 |
614.6a±4.82 |
1.75a±0.04 |
5.0b±0.72 |
180b±8.38 |
|
| G5 |
335.8bc±4.61 |
560.7d±1.68 |
1.76a±0.06 |
5.3b±0.32 |
168d±6.84 |
|
| Gc |
317.1c±4.90 |
533.4f±1.84 |
1.83a±0.09 |
4.9b±0.29 |
176bc±8.94 |
|
| P-Value | 0.001 | 0.000 | 0.422 | 0.000 | 0.000 | |
| Overall means in concern with broiler’s age | ||||||
|
1st week |
104.0d±1.17 |
121.0e±0.53 |
1.20c±0.02 |
1.3d±0.02 |
58e±0.65 |
|
|
2nd week |
238.5c±3.49 |
393.5d±2.55 |
1.70b±0.02 |
2.4c±0.08 |
96d±0.68 |
|
|
3rd week |
440.6b±1.56 |
656.0c±6.21 |
1.68b±0.05 |
5.7b±0.18 |
325a±0.46 |
|
|
4th week |
509.4a±2.08 |
783.2b±1.99 |
1.72b±0.05 |
8.9a±0.27 |
261c±0.82 |
|
|
5th week |
438.9b±7.95 |
896.7a±4.91 |
2.88a±0.24 |
9.1a±0.47 |
281b±3.65 |
|
| P-Value | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |
| Treatments by broiler’s age interactions | ||||||
| G1 |
1st |
91.6d±4.75 |
122.4e±0.34 |
1.52bc±0.13 |
1.0d±0.08 |
69d±0.64 |
|
2nd |
254.3c±6.59 |
323.2d±1.43 |
1.39c±0.06 |
3.3c±0.04 |
111c±0.75 |
|
|
3rd |
342.3b±2.64 |
566.3c±1.98 |
1.99b±0.19 |
4.5c±0.37 |
156b±3.57 |
|
|
4th |
585.6a±5.72 |
823.8b±0.95 |
1.49c±0.06 |
9.5a±0.58 |
261a±1.43 |
|
|
5th |
323.7b±8.41 |
855.4a±1.24 |
3.59a±0.46 |
6.3b±0.59 |
266a±0.94 |
|
| G2 |
1st |
104.8e±1.28 |
124.3e±1.04 |
1.19c±0.01 |
1.2d±0.2 |
57d±0.68 |
|
2nd |
223.3d±4.91 |
422.5d±0.89 |
1.91b±0.04 |
1.9d±0.06 |
97c±0.78 |
|
|
3rd |
551.1b±6.82 |
674.7c±0.62 |
1.25c±0.03 |
7.6c±0.39 |
184b±0.45 |
|
|
4th |
604.0a±5.65 |
789.2b±0.84 |
1.46bc±0.08 |
12.0a±0.97 |
258a±0.45 |
|
|
5th |
475.5c±9.37 |
999.7a±0.69 |
4.12a±1.31 |
9.9b±1.30 |
266a±0.51 |
|
| G3 |
1st |
106.5d±1.87 |
124.2e±0.57 |
1.17c±0.02 |
1.3d±0.04 |
58d±0.46 |
|
2nd |
230.1c±6.11 |
398.0d±0.51 |
1.76b±0.04 |
2.2c±0.09 |
96c±0.47 |
|
|
3rd |
520.0a±2.21 |
674.2c±0.59 |
1.37b±0.06 |
7.1b±0.43 |
197b±0.53 |
|
|
4th |
406.1b±9.35 |
778.1b±1.43 |
2.10a±0.14 |
6.9b±0.40 |
258a±0.42 |
|
|
5th |
570.8a±4.95 |
874.8a±0.57 |
2.12a±0.20 |
13.3a±1.96 |
266a±0.48 |
|
| G4 |
1st |
104.6c±1.33 |
123.6e±0.73 |
1.18c±0.01 |
1.2e±0.03 |
64e±0.48 |
|
2nd |
229.0b±1.90 |
425.6d±0.64 |
1.86b±0.01 |
2.0d±0.03 |
97d±0.92 |
|
|
3rd |
499.1a±6.51 |
798.5b±1.01 |
1.64b±0.05 |
5.5c±0.28 |
185c±0.54 |
|
|
4th |
475.5a±9.02 |
750.6c±0.81 |
1.66b±0.07 |
8.6a±0.38 |
254b±0.63 |
|
|
5th |
424.5a±8.06 |
974.8a±0.69 |
2.43a±0.11 |
7.8b±0.39 |
302a±1.97 |
|
| G5 |
1st |
116.6c±2.22 |
124.5e±0.52 |
1.07c±0.02 |
1.5e±0.05 |
56d±0.44 |
|
2nd |
258.5b±8.14 |
399.8d±0.55 |
1.59b±0.04 |
2.7d±0.14 |
94c±0.52 |
|
|
3rd |
401.0a±5.14 |
675.5c±0.71 |
1.95a±0.16 |
5.1c±0.43 |
175b±0.51 |
|
|
4th |
436.8a±7.75 |
751.8b±0.79 |
2.08a±0.20 |
7.4b±0.52 |
254a±0.51 |
|
|
5th |
466.0a±2.64 |
852.0a±0.72 |
2.10a±0.14 |
9.7a±0.84 |
264a±0.57 |
|
| Gc |
1st |
99.6d±2.24 |
107.1e±0.43 |
1.09c±0.02 |
1.3e±0.04 |
42e±0.43 |
|
2nd |
235.6c±3.09 |
392.1d±0.53 |
1.67b±0.02 |
2.3d±0.05 |
82d±0.45 |
|
|
3rd |
330.3b±9.28 |
547.2c±1.25 |
1.89b±0.15 |
4.4c±0.37 |
154c±0.47 |
|
|
4th |
547.3a±8.60 |
796.7b±0.51 |
1.56b±0.07 |
8.7a±0.55 |
284b±0.47 |
|
|
5th |
372.8b±6.55 |
823.7a±0.51 |
2.94a±0.03 |
7.6b±0.62 |
320a±0.37 |
|
| P-Value | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | |
Means carrying different superscripts in the same column are significantly different at (P ≤ 0.05) or highly significantly different at (P < 0.01). Means carrying the same superscripts in the same column are non-significantly different at (P < 0.05). G1: Broilers challenged with ammonia 75 ppm; G2: Broiler challenged with lead nitrate 0.1 mg.L-1; G3: Broiler challenged with E. coli O157: H7 1.5 x 108 CFU.ml-1; G4: Broilers challenged with magnesium sulfate 0.075 mg.L-1; G5: Broilers challenged with ammonium chloride 0.005 mg.L-1; Gc: Control untreated group; BWG: Body weight gain; FI: Feed intake; FCR: Feed conversion ratio; PI: Performance index; WI: Water intake; SE: Standard error.
Statistical analysis
Statistical analysis was carried out using statistical package for social sciences SPSS version 24 (Green and Salkind, 2016; IBM, 2016). The recorded results were analyzed using multifactorial two-tailed Analysis of Variance (ANOVA) to measure the influence of different air and water impurities (ammonia, lead nitrate, E. coli, magnesium sulfate, and ammonium chloride) on broiler’s age and their interactions. The overall means and the interactions were displayed in the illustrated tables. The statistical model was summarized as follow:
Yijk=µ + αi + βj + (αβ)ij + Ɛijk
Where Yijk was the measurement of the dependent variables; µ was overall mean; αi was the fixed effect of the environmental impurities; βj was the fixed effect of the age; (αβ)ij was the interaction effect of the environmental impurities by age; Ɛijk was the random error. The bacterial counts were transferred into a logarithmic number (Log10) using Microsoft Excel 2016. Results were expressed for high significance at (p<0.01), significant at (p≤0.05), and non-significant at (p>0.05).
RESULTS and Discussion
Performance indices
Bodyweight gains (WG, Table 1) revealed superior recordings with highly significant differences (P < 0.01) in G2 (lead nitrate 0.1 mg.L-1) and an inferior recording with highly significant differences (P < 0.01) in G1 (ammonia 75 ppm) compared to all other challenged groups and to control unchallenged group with no significant differences between G1 (ammonia 75 ppm) and G6 (control unchallenged) groups. On an age basis, Table 1 showed highly significant increases (P <0.01) in WG at the 4th, 5th, 3rd, 2nd, and 1st week respectively with no significant differences between WG recording at the 5th and 3rd weeks.
Feed intakes (FI) in Table 1 revealed the superior recordings with highly significant differences (P < 0.01) in G4 (magnesium sulfate 0.075 mg.L-1) and the lowest in G1 (ammonia 75 ppm) compared to all other challenged groups and to control unchallenged group. Meanwhile, highly significant increases (P < 0.01) were recorded in FI as age increased in broilers understudy. Feed conversion ratio (FCR) recorded in Table 1 no significant differences between challenged groups and control unchallenged group.
Performance index (PI) revealed in Table 1 superior calculations with highly significant differences (P < 0.01) in G2 (lead nitrate 0.1 mg.L-1) and G3 (E. coli O157: H7 1.5 x 108 CFU.ml-1) with no significant differences between the latter groups. All of G1 (ammonia 75 ppm), G4 (magnesium sulfate 0.075 mg.L-1), G5 (ammonium chloride 0.005 mg.L-1), and G6 (control unchallenged) revealed no significant differences between them. Highly significant increases (P < 0.01) were recorded in PI as age increased in broilers understudy with no significant differences in the 4th and 5th weeks.
Water intake (WI, Table 1) revealed the superior recordings with highly significant differences (P < 0.01) in G1 (ammonia 75 ppm) and the lowest in G5 (ammonium chloride 0.005 mg.L-1) compared to all other challenged groups and to control unchallenged group. Water intake recorded highly significant increases at the 3rd, 5th, 4th, 2nd, and 1st week respectively.
Biochemical profile
Total protein (Table 2) revealed highly significant increases (P < 0.01) in G2 (lead nitrate 0.1 mg.L-1), G1 (ammonia 75 ppm), G5 (ammonium chloride 0.005 mg.L-1), G4 (magnesium sulfate 0.075 mg.L-1), G3 (E. coli O157:H7 1.5 x 108 CFU.ml-1), G6 (unchallenged broilers) respectively. Alanine aminotransferase in Table 2 revealed a highly significant decline (P < 0.01) in all challenged broilers compared to control. Creatinine (Table 2) revealed a highly significant decline (P < 0.01) in G1, G3, G4, and G5 challenged broilers compared to G2 (lead nitrate 0.1 mg.L-1) and G6 control. Triglycerides, total cholesterol, and glucose revealed highly significant increases (P <0.01) in all challenged broilers compared to the control unchallenged broilers (Table 2).
Stress markers and immunoglobulin
Cortisol sera concentrations revealed in Table 3 highly significant increases in all challenged broiler groups as G4 (magnesium sulfate 0.075 mg.L-1), G5 (ammonium chloride 0.005 mg.L-1), G3 (E. coli O157: H7 1.5 x 108 CFU.ml-1), G2 (lead nitrate 0.1 mg.L-1), and G1 (ammonia 75 ppm) respectively compared to control unchallenged group.
Immunoglobulin G (IgG) and Immunoglobulin M (IgM) in a synchronized pattern revealed highly significant declines (P < 0.01) in G4 (magnesium sulfate 0.075 mg.L-1), G2 (lead nitrate 0.1 mg.L-1), G5 (ammonium chloride 0.005 mg.L-1), G3 (E. coli O157:H7 1.5 x 108 CFU.ml-1), and G1 (ammonia 75 ppm) respectively.
Intestinal microbial load
The total bacterial count revealed a superior highly significant count (P < 0.01) in G3 (E. coli O157: H7 1.5 x 108 CFU.ml-1) compared to all other challenged and control broiler groups. Total Enterobacteriaceae count revealed highly significant increases (P<0.01) in G4 (magnesium sulfate 0.075 mg.L-1), G3 (E. coli O157:H7 1.5x108 CFU.ml-1), and G5 (ammonium chloride 0.005 mg.L-1) respectively with no significant differences between the groups.
Table 2: Biochemical profile (Mean ±SE) in broilers challenged with air and water impurities.
| Groups | TP g/dl | ALT IU/L | CREAT mg/dl | TG mg/dl | TC mg/dl | GLUCO mg/dl |
| Overall means in concern with treatments | ||||||
| G1 |
15.6b±0.86 |
23.9bc±0.51 |
1.5b±0.35 |
831a±5.48 |
209b±8.17 |
282b±2.02 |
| G2 |
18.9a±0.68 |
15.8d±0.29 |
4.4a±0.75 |
646ab±4.70 |
113c±1.52 |
297b±1.92 |
| G3 |
11.7c±0.52 |
19.3cd±0.04 |
1.1b±0.28 |
582b±5.31 |
70de±7.86 |
359a±2.30 |
| G4 |
12.2c±0.42 |
20.4bc±0.43 |
2.7ab±0.32 |
590b±1.05 |
66e±5.50 |
162d±6.20 |
| G5 |
14.5b±0.04 |
24.8ab±0.35 |
2.6ab±0.34 |
764ab±6.72 |
255a±1.75 |
237c±9.70 |
| Gc |
3.1d±0.42 |
28.7a±0.46 |
4.3a±0.22 |
163c±3.01 |
94cd±9.02 |
78e±7.01 |
| P-Value | 0.000 | 0.000 | 0.001 | 0.000 | 0.000 | 0.001 |
Means carrying different superscripts in the same column are significantly different at (P ≤ 0.05) or highly significantly different at (P < 0.01). Means carrying the same superscripts in the same column are non-significantly different at (P < 0.05). G1: Broilers challenged with ammonia 75 ppm; G2: Broiler challenged with lead nitrate 0.1 mg.L-1; G3: Broiler challenged with E. coli O157: H7 1.5 x 108 CFU.ml-1; G4: Broilers challenged with magnesium sulfate 0.075 mg.L-1; G5: Broilers challenged with ammonium chloride 0.005 mg.L-1; Gc: Control untreated group; TP: Total protein; ALT: Alanine aminotransferase; CREAT: Creatinine; TG: Triglycerides; TC: Total cholesterol; GLUCO: Glucose; SE: Standard error.
Table 3: Stress markers and immunoglobulin concentrations (Mean ±SE) in broilers challenged with air and water impurities.
| Groups | Stress markers | Immunoglobulin concentrations | |
| CORT mcg/dl | IgG mg/dl | IgM mg/dl | |
| Overall means in concern with treatments | |||
| G1 |
35.7b±0.81 |
968d±3.85 |
210e±1.23 |
| G2 |
36.0b±0.83 |
1071bc±17.43 |
238c±3.04 |
| G3 |
37.5ab±1.04 |
1033c±13.00 |
215d±0.93 |
| G4 |
39.3a±0.92 |
1100b±10.95 |
261b±1.73 |
| G5 |
37.8ab±1.25 |
1049c±23.59 |
235c±0.71 |
| Gc |
9.5c±0.30 |
1334a±2.49 |
307a±1.24 |
| P-Value | 0.000 | 0.000 | 0.000 |
Means carrying different superscripts in the same column are significantly different at (P ≤ 0.05) or highly significantly different at (P < 0.01). Means carrying the same superscripts in the same column are non-significantly different at (P < 0.05). G1: Broilers challenged with ammonia 75 ppm; G2: Broiler challenged with lead nitrate 0.1 mg.L-1; G3: Broiler challenged with E. coli O157: H7 1.5 x 108 CFU.ml-1; G4: Broilers challenged with magnesium sulfate 0.075 mg.L-1; G5: Broilers challenged with ammonium chloride 0.005 mg.L-1; Gc: Control untreated group; CORT: Cortisol; IgG: Immunoglobulin G; IgM: Immunoglobulin M; SE: Standard error.
Histopathological examination
The liver of G1 (ammonia 75 ppm) in Figure 1a showed severe congestion of central vein, with moderate fibrous connective tissue proliferation around the central vein. Hepatic cells showed degeneration with severe vaculation of cytoplasm, mild hemorrhage, and mononuclear cell infiltration as compared to normal control views in Figure 1f. Broilers’ liver of G2 (lead nitrate 0.1 mg.L-1) revealed in Figure 1b severe degeneration of hepatic cells with severe vaculation of cytoplasm, degeneration of portal area with severe mononuclear cell infiltration. Liver of G3 (E. coli O157: H7 1.5 x 108 CFU.ml-1) showed in Figure 1c mild mononuclear cell infiltration. Broilers of G4 (magnesium sulfate 0.075 mg.L-1) revealed in Figure 1d that the liver developed congestion of central vein with hemosiderosis, hepatocytes showed mild degeneration with mild cytoplasmic vaculation and mononuclear cell infiltration. Figure 1e showed that the liver of G5 (ammonium chloride 0.005 mg.L-1) developed severe congestion of central vein, degeneration of hepatic cells with cytoplasmic vaculation mononuclear cell infiltration compared to the typical normal picture of G6 in Figure 1f.
Table 4: Logarithmic bacterial counts (Mean ±SE) in broilers challenged with air and water impurities.
| Groups | TBC CFU/ml | TEC CFU/ml |
| Overall means in concern with treatments | ||
| G1 |
3.16ab±0.32 |
1.85b±0.26 |
| G2 |
1.73c±0.37 |
1.69b±0.29 |
| G3 |
3.91a±0.58 |
3.45a±0.60 |
| G4 |
1.47c±0.39 |
3.87a±0.44 |
| G5 |
1.27c±0.31 |
3.24a±0.37 |
| Gc |
1.20c±0.31 |
1.64b±0.29 |
| P-Value | 0.000 | 0.000 |
Means carrying different superscripts in the same column are significantly different at (P ≤ 0.05) or highly significantly different at (P < 0.01). Means carrying the same superscripts in the same column are non-significantly different at (P < 0.05). G1: Broilers challenged with ammonia 75 ppm; G2: Broiler challenged with lead nitrate 0.1 mg.L-1; G3: Broiler challenged with E. coli O157: H7 1.5 x 108 CFU.ml-1; G4: Broilers challenged with magnesium sulfate 0.075 mg.L-1; G5: Broilers challenged with ammonium chloride 0.005 mg.L-1; Gc: Control untreated group; TBC: Total bacterial count; TEC: Total Enterobacteriaceae count; SE: Standard error.
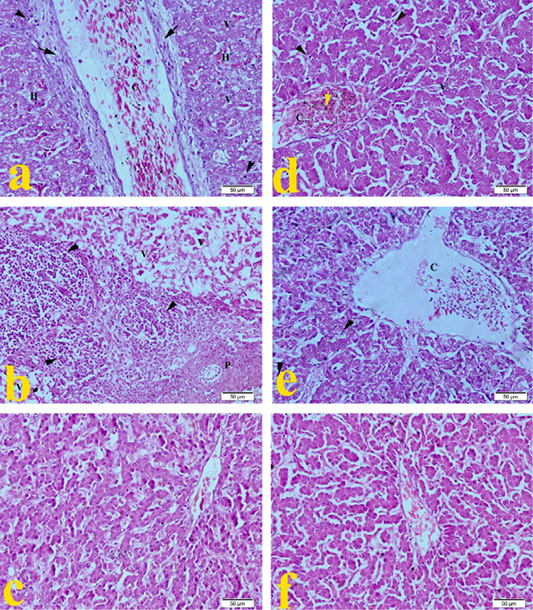
Figure 1: Representative photomicrographs of liver histopathology (20×): (a) Liver of Hubbard broilers challenged with ammonia 75 ppm showing congestion of central vein (C), mild fibrosis (arrow), mononuclear cell infiltration (arrowhead), vaculation of hepatocytes cytoplasm (V). (b) Liver of Hubbard broilers challenged with lead nitrate 0.1 mg.L-1 showing sever vaculation (V) and severe mononuclear cell infiltration (arrowhead) and degeneration of portal area (P). (c) Liver of Hubbard broilers challenged with E. coli O157: H7 1.5x108 CFU/ml. (d) Liver of Hubbard broilers challenged with magnesium sulfate 0.075 mg.L-1. (e) Liver of Hubbard broilers challenged with ammonium chloride 0.005 mg.L-1. (f) Liver of control Hubbard broilers. H and E. Bar 50 μm.
Heart of G1 broilers (ammonia 75 ppm) in Figure 2a showed mild pericarditis, cardiac muscle showed mild degeneration with mild hemorrhage and mononuclear cell infiltration as compared to the normal histopathological appearance in Figure 2f. Heart of G2 broilers (lead nitrate 0.1 mg.L-1) revealed in Figure 2b moderate fibrinous pericarditis, myocardial degeneration, cytoplasmic vaculation, mononuclear cell infiltration, mild congestion, and hemorrhage of cardiac muscle. Heart of G3 broilers (E. coli O157: H7 1.5 x 108 CFU.ml-1) showed in Figure 2c mild leukocytic cell infiltration. Heart of G4 Broilers (magnesium sulfate 0.075 mg.L-1) revealed in Figure 2d mild myocardial degeneration with mild hemorrhage, cytoplasmic vaculation, and mononuclear cell infiltration. Figure 2e showed that the heart of G5 (ammonium chloride 0.005 mg.L-1) developed mild pericarditis, degeneration of cardiac muscles with mild hemorrhage, cytoplasmic vaculation, and mononuclear cell infiltration compared to the normal histopathological microscopic picture of G6 in Figure 2f.
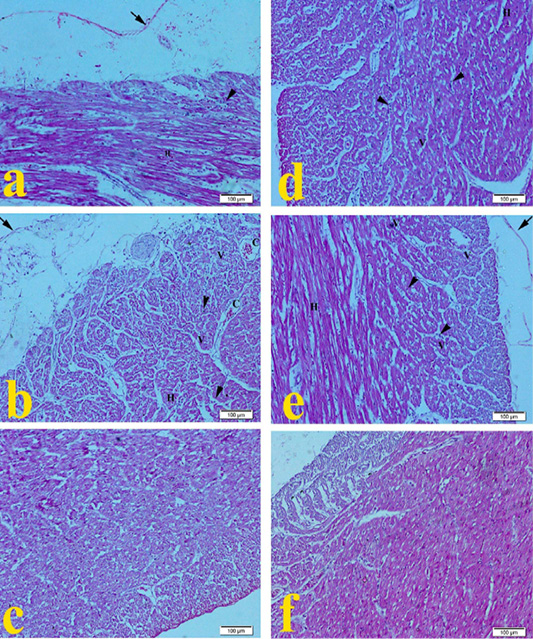
Figure 2: Representative photomicrographs of heart histopathology (10×): (a) Heart of Hubbard broilers challenged with ammonia 75 ppm showing mild hemorrhage (H), mild fibrinous pericarditis (arrow), mononuclear cell infiltration (arrowhead), vaculation of cytoplasm (V). (b) Heart of Hubbard broilers challenged with lead nitrate 0.1 mg.L-1. (c) Heart of Hubbard broilers challenged with E. coli O157: H7 1.5x108 CFU/ml. (d) Heart of Hubbard broilers challenged with magnesium sulfate 0.075 mg.L-1. (e) Heart of Hubbard broilers challenged with ammonium chloride 0.005 mg.L-1. (f) Heart of control Hubbard broilers. H and E. Bar 100 μm.
The spleen of G1 broilers (ammonia 75 ppm) in Figure 3a showed mild lymphoid depletion with mild hemorrhage and hemosiderosis compared to the normal histopathological architecture in Figure 3f. The spleen of G2 broilers (lead nitrate 0.1 mg.L-1) revealed in Figure 3b severe congestion of splenic sinus, mild hemorrhage with hemosiderosis. The spleen of G3 broilers (E. coli O157: H7 1.5 x 108 CFU.ml-1) showed in Figure 3c mild mononuclear cell infiltration. The spleen of G4 Broilers (magnesium sulfate 0.075 mg.L-1) revealed in Figure 3d normal histologic structure. Figure 3e showed that spleen of G5 broilers (ammonium chloride 0.005 mg.L-1) developed moderate hemorrhage with hemosiderosis compared to the normal histopathological microscopic picture of G6 in Figure 3f.
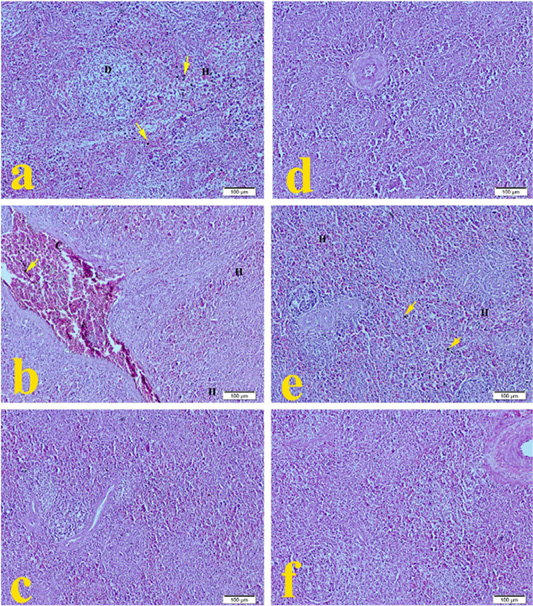
Figure 3: Representative photomicrographs of spleen histopathology (10×): (a) Spleen of Hubbard broilers challenged with ammonia 75 ppm showing depletion of lymphocytes (D), mild hemorrhage (H), and hemosiderosis (yellow arrow). (b) The spleen of Hubbard broilers challenged with lead nitrate 0.1 mg.L-1. (c) The spleen of Hubbard broilers challenged with E. coli O157: H7 1.5x108 CFU/ml. (d) The spleen of Hubbard broilers challenged with magnesium sulfate 0.075 mg.L-1. (e) The spleen of Hubbard broilers challenged with ammonium chloride 0.005 mg.L-1. (f) The spleen of control Hubbard broilers. H and E. Bar 100 μm.
Bursa of Fabricius in G1 broilers (ammonia 75 ppm) in Figure 4a showed normal follicular epithelium with mild lymphocytic hypoplasia of lymphoid follicles compared to the normal histopathological architecture in Figure 4f. Bursa of G2 broilers (lead nitrate 0.1 mg.L-1) revealed in Figure 4b normal follicular epithelium with moderate lymphoid depletion of follicles. The spleen of G3 broilers (E. coli O157: H7 1.5 x 108 CFU.ml-1) showed in Figure 4c normal follicular epithelium. The spleen of G4 Broilers (magnesium sulfate 0.075 mg.L-1) revealed in Figure 4d normal follicular epithelium with hyperplasia of lymphoid follicles. Figure 4e showed that spleen of G5 broilers (ammonium chloride 0.005 mg.L-1) developed mild lymphoid depletion of lymphoid follicles compared to the normal histopathological microscopic picture of G6 in Figure 4f.
Microclimatic ammonia can be considered as a notice for the hygienic standards inside broiler farms. Microclimatic levels of ammonia are governed by numerous factors like building design, the house directions, ventilation rate, litter management, broiler’s age, stocking density, and drinkers’ types and position (Maliselo and Nkonde, 2015). Ammonia levels inside broiler farms should be optimized at no more than 10 ppm, higher ammonia concentrations can contribute to negative influences on growth and performance as recorded by Abouelenien et al. (2016) and Boggia et al. (2019).
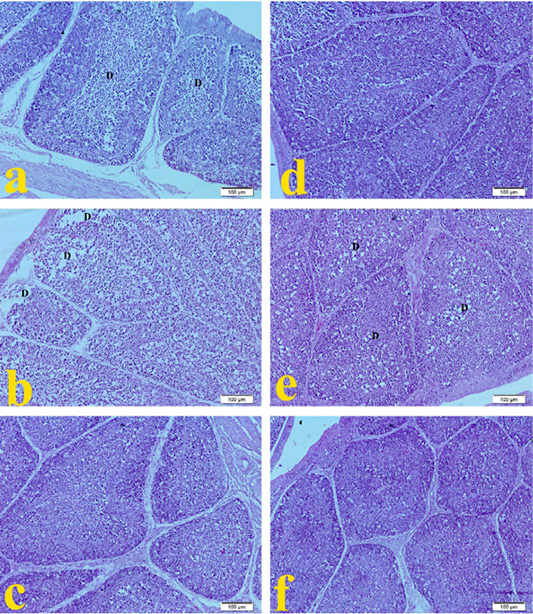
Figure 4: Representative photomicrographs of bursa histopathology (10×): (a) Bursa of Hubbard broilers challenged with ammonia 75 ppm showing depletion of lymphocytes (D). (b) Bursa of Hubbard broilers challenged with lead nitrate 0.1 mg.L-1. (c) Bursa of Hubbard broilers challenged with E. coli O157: H7 1.5x108 CFU/ml. (d) Bursa of Hubbard broilers challenged with magnesium sulfate 0.075 mg.L-1. (e) Bursa of Hubbard broilers challenged with ammonium chloride 0.005 mg.L-1. (f) Bursa of control Hubbard broilers. H and E. Bar 100 μm.
The current results revealed a significant reduction in performance and immunity, significant increases in biochemical parameters, cortisol levels, and bacterial counts, as well as, severe deterioration in tissue architecture in broilers exposed to 75 ppm of aerial ammonia. The results were consistent with these recorded by Yi et al. (2016) and Soliman et al. (2017) who evaluated the influence of aerial ammonia concentrations on two broiler breeds (Ross and Hubbard) during two successive seasons. They recorded severe negative influences of ammonia on live body weights, weight gains, feed conversion ratios, and performance index in both broiler breeds concerning the higher influence during the winter season.
Water as one of the essential elements of life has proper characteristics including polarity and the inclusion of hydrogen bonds that increase the solubility of many chemical compounds (WHO, 2007). Water can absorb many chemical contaminants from the surroundings that arise from the biological activities of both animals and humans (Mendie, 2005). Water intended for broiler’s consumption has to be safe and of high quality to obtain optimum health conditions and performance (Abbas et al., 2008), as well as to optimize the efficiency of vaccines and vaccination act during the fattening cycles (Hasselquist and Nilsson, 2009).
The current study revealed significant reductions of performance indices during the 2nd, 3rd, and 4th weeks in broilers challenged with ammonium chloride (0.005 mg.L-1), magnesium sulfate (0.075 mg.L-1), lead nitrate (0.1 mg.L-1), E. coli O157: H7 (1.5 x 108 CFU.ml-1), respectively. The results were consistent to those recorded by Momodu and Anyakora (2010), Orebiyi et al. (2010), Oyeku and Eludoyin (2010), Kanmani and Gandhimathi (2013) and Tukura et al. (2014) who all agreed and reported significant reductions of live body weights and weight gains in broilers exposed to water pollutants concerning lead when exceeding its maximum permissible limits in the water. Also, Rahman and Joshi (2009) evaluated the experimental influence of lead acetate from 2nd to 6th weeks at a rate of 250 and 400 ppm in drinking water on the growth and performance of broilers. They recorded lower body weights, weight gains, and feed conversion ratios concerning the 3rd weeks onward indicated the negative impact of lead.
The current results also revealed extreme deviations of biochemical parameters including total protein, alanine aminotransferase, creatinine, total cholesterol, triglycerides, and glucose. These deviations were synchronized with extreme elevation of cortisol levels that might be attributed to the stresses enforced on broilers during the 2nd, 3rd, and 4th weeks via the challenges with ammonium chloride (0.005 mg.L-1), magnesium sulfate (0.075 mg.L-1), lead nitrate (0.1 mg.L-1), E. coli O157: H7 (1.5 x 108 CFU.ml-1), respectively. The deviations and high levels of liver and kidney enzymes might be attributed to the toxic metabolites produced by the chemicals used in the challenges. The results were consistent with those reported by Morsy et al. (2012) who found a significant decrease in total protein and albumin in broilers consumed water with high total dissolved solids. Hussein et al. (2013) recorded elevations in the liver and kidney enzymes, as well as, antioxidants like superoxide dismutase, catalase, and malondialdehyde in broilers supplemented with water contained high levels of chemical pollutants concerning lead.
The current results recorded a significant reduction in total immunoglobulin IgG and IgM in all challenged broilers even after the cessation of the challenge by the end of the 4th week that increased the cortisol serum concentrations in all challenged broilers compared to control broilers. The results were consistent with those recorded by Ahmed (2013) who reported significant increases in Newcastle virus antibody titers in broilers supplied with water of 2610 ppm total dissolved solids. Khalil and Khalafalla (2011) explained that broilers vaccinated using water derived from shallow and artesian wells or surface water can result in the production of fewer immunoglobulin compared to broilers vaccinated using tab water. They attributed the variation of the immunoglobulin levels for the higher levels of total dissolved solids and microbial contamination in shallow wells, artesian wells, and surface water.
Bartlett and Smith (2003) and Park et al. (2004) recorded that the presence of high water hardness levels attributed to calcium salts can contribute to lower levels of cellular and humoral immunity. Gharaibeh and Mahmoud (2013), Kamal (2015), and Ayoub et al. (2018) recorded a significant negative impact of total dissolved solids content (1560 mg/L), hardness (960 mg/L), chloride (640 mg/L), nitrite (0.1269 mg/L), and sulfate (877mg /L) on Newcastle disease virus titers in 300 one-day-old Cobb 500 broiler chickens. Haggag et al. (2016) conducted an epidemiological survey on the chemical water quality used in broiler farms in Behera and Kafr El-Sheikh governorates and its influence on performance and immune response to Newcastle disease virus and infectious bursal disease virus vaccines. They recovered lead (0.23 mg/L), copper (0.67 mg/L), iron (2.15 mg/L), zinc (7.75 mg/L), and manganese (2.99 mg/L), they concluded from the immunological investigation using ELISA and haemoagglutinin inhibition test the negative influences of copper and lead on immunoglobulin concentrations and performance, and body weights.
Water usually acts as a good vehicle for the growth and multiplication of many pathogenic micro-organisms. The results revealed significant increases in total bacterial and Enterobacteriaceae counts in all challenged groups compared to control. The increase of bacterial counts might be contributed to the lowering of resistance following the challenges, causing the micro-organisms to flourish and multiply excessively. The results are consistent with the results reported by Jafari et al. (2006) who collected water samples from 40 broiler farms in the rural area of Ahvaz, Iran during the period May to July 2005. They found that coliform counts were over the maximum permissible limits (50 CFU.ml-1) in 20 farms. The colonies were exposed to serotyping and Salmonella serotypes Nienstedten, Tinda, Calvinia, Oysterbeds, and Sterrenbos were recovered. Soliman et al. (2009) conducted an epidemiological survey on bacterial and fungal contaminants in the drinking water in ten broilers farms in Ismailia and Zagazig governorates Egypt. They recovered bacterial microorganisms like Proteus vulgaris, Pseudomonas aureuginosa, Citrobacter spp., E. coli, Salmonella spp., Shigella spp., Klebsiella oxytoca, Staphylococcus aureus, and Streptococcus fecalis, and fungal organisms like Candida albicans, Aspergillus nidulans, Yeast spp., Aspergillus niger, Mucor, Penicillium spp., and Aspergillus flavus. Soliman et al. (2009) carried out an epidemiological survey on water samples collected on two stages, the first stage of sampling was collected from six broiler farms in Ismailia and Zagazig governorates, Egypt during the period January to July 2008, and the second stage of sampling was collected by Alabama State Veterinary Diagnostic Laboratory U.S.A. during the period September 2008 to January 2009. They recovered Proteus vulgaris, Pseudomonas aureuginosa, E. coli, Shigella spp., Salmonella spp., and Streptococcus fecalis.
Histopathological examination in the current study revealed a complete deviation in liver, heart, bursa of Fabricius, and spleen of broilers challenged with lead nitrate (0.1 mg.L-1), E. coli O157: H7 (1.5 x 108 CFU.ml-1), magnesium sulfate (0.075 mg.L-1), ammonium chloride (0.005 mg.L-1), respectively compared to control. These histopathological alterations were compatible with the biochemical deviations in some measured parameters. The results were consistent to Dobrzański et al. (2017), Karantika et al. (2016), and Hussein et al. (2013) who found extensive necrosis and lymphocyte infiltration of the liver and tubular epithelial damage with dilated Bowman’s capsule in broilers received well water contained high levels of cadmium, zinc, manganese, lead, chromium, nickel, and mercury. Also, Abdou et al. (2018) found that chemical pollutants that arise from drinking water in the poultry farms are partially stored in muscles, liver, and kidney, so transferred via the white meat products to the human consumers and affecting their health.
CONCLUSIONS AND RECOMMENDATIONS
The study revealed that environmental impurities like air (ammonia) and water impurities (lead nitrate, E. coli O157: H7, magnesium sulfate, and ammonium chloride) were able to induce some physiological disturbances like impaired performance traits, deviated biochemical profile, lower immunoglobulin concentrations, and abnormal tissue architecture when existing in broiler’s microclimate in levels exceed the permissible limits. Once the overwhelming challenges have been relieved, some of the used impurities were able to induce a growth-stimulating action (growth promoter).
Air resources inside broiler farms have to be filtered, as well as, litter has to be managed properly to avoid the volatilization of ammonia. Water has to be managed properly via conducting some regular and periodic examinations to ensure proper water quality, change water filter and maintain them regularly, flush water line with regular intervals, and the use of some elements that might improve water quality and enhance their consumption by broiler chickens.
ACKNOWLEDGMENTS
Sincere thanking should be provided to Prof. M.A.A. Sobieh for his tremendous directions during the experiment. Also, we would like to thank the community services and environmental development sector staff, Faculty of Veterinary Medicine, Suez Canal University, Ismailia, Egypt for their help.
AUTHOR’S CONTRIBUTION
ESS designed the experimental design, participated in the execution of the experiment, and writing of the manuscript. OMF participated in the execution of the experiment and writing of the manuscript. MMH supervised the execution of the experiment and writing of the manuscript.
CONFLICT OF INTEREST
The authors have declared no conflict of interest.
REFERENCES






