Whey Neutralization with Different Concentration of Sodium Hydroxide and Sodium Bicarbonate
Whey Neutralization with Different Concentration of Sodium Hydroxide and Sodium Bicarbonate
Ali Muhammad*, Yasser Durrani, Majid Suhail Hashmi, Ihsan Mabood Qazi, Muhammad Ayub and Saifullah
Department of Food Science and Technology, The University of Agriculture Peshawar, Khyber Pakhtunkhwa, Pakistan.
Abstract | Whey proteins have many nutritional and beneficial therapeutic properties, which are indispensable for the functional and nutritional properties of foods. The aim of the study was to neutralize whey by addition of NaOH (0.1 and 0.01N) and NaHCO3 (0.1 and 0.01N) for fortification and enrichment of foods. However, addition of these solutions results in an increase in total solids and ash content of the whey sample The samples were normal whey (not treated), neutralized whey (neutralized with NaOH) and neutralized whey (neutralized with NaHCO3). Whey was pasteurized at 720C for 15 second followed by neutralization. All the samples were analyzed physico chemically (pH, %acidity, %moisture, crude protein) and organoleptically (color, taste, texture and overall acceptability) with an interval of one week for storage period of one month. The treatments were T0 (not treated), T1 (neutralized with NaOH) and T2 (neutralized with NaHCO3). Statistical analysis showed that storage intervals and treatments had a significant (p<0.05) effect on physicochemical and sensory quality of neutralized whey.
Received | March 18, 2018; Accepted | November 06, 2018; Published | November 26, 2018
*Correspondence | Ali Muhammad, Department of Food Science and Technology, The University of Agriculture Peshawar, Khyber Pakhtunkhwa, Pakistan; Eamil: [email protected]
Citation | Muhammad, A., Y. Durrani, M.S. Hashmi, I.M. Qazi, M. Ayub and Saifullah. 2018. Whey neutralization with different concentration of sodium hydroxide and sodium bicarbonate. Sarhad Journal of Agriculture, 34(4): 910-916.
DOI | http://dx.doi.org/10.17582/journal.sja/2018/34.4.910.916
Keywords | Whey, Neutralization, Sodium hydroxide, Sodium bicarbonate
Introduction
Whey is byproduct of the cheese industry which contains mainly water soluble proteins, lactose and most of the salts. Whey can be obtained during cheese making process in which fat and casein are separated as curds are broken. The composition of whey varies with formation of different types of cheese, heat treatment and handling (Hargrove and Alford, 1974. Cheese production is increasing day by day around the globe, the problem related to this is the proper utilization of surplus whey. (USDA, 1981). Physically active Individuals and athletes are widely consuming protein supplements in their diet (Phillips, 2012).
Whey protein has high nutritional value when compared to other proteins sources which are commercialized in the sports nutrition market. To manufacture this type of product ingredients such as wheat protein, egg, caseinates, soy and whey are used (Ha and Zemel, 2003; Khanam et al., 2013).
In recent years, the malnourished segments of human population can be overcome by utilization of whey because it contains available nutrients for feeding. Due to the presence of important constituents (such as lactose, fat content, protein with different frictions and minerals) having excellent functional and nutritional characteristics whey and whey constituents in the food industry can enhances opportunities for a wide-range application for food and food products. To utilize whey various technologies has been developed for the manufacture of a variety of new food products as well as for the replacement of comparatively costly food ingredients (Mathur and Shahani, 1979).
The composition of whey per 100g contain 26kcal food energy, 93.1g water, 0.3g fat, 5.1g lactose, 0.9gm protein, 0.6gm ash, 53mg phosphorus, 51mg calcium, 0.1mg iron, 10 IU (International Unit) vitamin A, 0.14mg riboflavin, 0.1mg niacin and 0.03mg thiamine (Watt and Merrill, 1963).
As an ingredient for human food or animal feed, most of the whey is converted to whey solids by traditional processes such as roller drying, spray drying, production of sweetened condensed whey and concentration to semisolids feed blocks (Hargrove and Alford, 1974). In the development of new products or a number of traditional products whey and its products can be used as a substitute (Goldenko, 1993). In ice cream and soft cheese production, micro-particulated whey proteins are utilized. Selective thermal or high-pressure-based fractionation of whey proteins can also use to produce selectively enriched fractions that can be used in products such as infant formulae (Kulozik, 2000).
Whey protein represents 20-30% of the proteins present in bovine milk; it is a complex mixture of globular protein molecules consisting mostly of α-lactalbumin (α-La), β-lactoglobulin (β-Lg) (Urista et al, 2011). The protein fractions α-La and β-Lg represent almost 70% of the proteins present in whey (Walstra, et al, 2006). Differences in the physicalechemical composition of whey protein supplements potentially influence its nutritional effect on the human body (Manninen, 2009). The nutritional quality of whey protein supplement depends on amino acid composition, bioavailability of essential amino acids, protein digestibility, and physiological utilization of specific amino acids after digestion and absorption (Lemon, Berardi and Noreen, 2002).
Whey protein contains the proteins that remain in the liquid part of milk after it has coagulated (curdled). The main proteins left are beta-lacto globulins (BLG), alpha-lactalbumins, bovine serum albumin (BSA) and immunoglobulin (Ig). These proteins contain large amounts of the branched chain amino acids that are important for maintaining muscles. (Byron and Whittier, 1970). Whey Proteins are used as nutritional supplement for people who may be protein-deficient. Whey Protein is a popular dietary supplement among weightlifters and body builders, who require a high dietary protein intake to increase their muscle bulk (Health Point Technologies, 2004). A particular Whey Protein, called bovine lactoferrin (BLF) may help to prevent bowel cancer and other types of cancers. Certain Whey Proteins also have the ability to fight bacteria and other disease-causing particles and promote wound healing. Whey Proteins help to reduce the ‘stickiness’ of platelets and help to stop blood clots forming (Arrick and Nathan, 1984; Bounous and Pold, 1991).
The whey protein concentrates are used in the manufacture of dietetic foods or as a substitute for dried skim milk in ice cream, confectionery, bakery products and sauces. Mineral-rich residue remaining after removal of proteins and lactose is used in the manufacture of animal feeds (Rolland, 1993). Principal users of whey products for human foods are dairies and bakeries. Lactose, which is derived primarily from whey, is used mainly in infant foods and pharmaceuticals (Whey Products Institute 1980).
Objective of the study was to obtain neutralize whey by adjusting its pH by treatment of NaOH and NaHCO3 with various concentration such as 0.1N and 0.01N. By keeping in view the above mentioned facts the present research work will be undertaken to produce whey supplemented bakery products.
Materials and Methods
Whey was collected from dairy shops of the local market in Peshawar. Then it was transferred to the laboratory of Food Science and Technology Department, The University of Agriculture, Peshawar. Then the whey was pasteurized at 72o C for 15 seconds. After that the whey were neutralized with sodium hydroxide NaOH and sodium bicarbonate (NaHCO3).
Preparation of samples
T0 = Normal whey (not treated).
T1 = Neutralized whey (neutralized with NaOH).
T2 = Neutralized whey (neutralized with NaHCO3).
Physiochemical analysis
Total soluble solids: The total soluble solids were recorded by the recommended method of AOAC (1984) using the hand refractometer at room temperature. A Well-mixed drop from the sample was taken by a capillary tube and a drop was placed on the dry prism of hand refrectometer to get instant reading.
pH determination: The pH of the samples was measured by the recommended method of AOAC (1984). pH of was determined by an electronic digital pH meter. The electrodes of the pH meter were standardized in buffer solution of pH 4.0 and 7.0 before taking reading of the samples.
Percent acidity: The total titratable acidity was determined by standard method of AOAC (1984) by titrating against standard alkali solution.
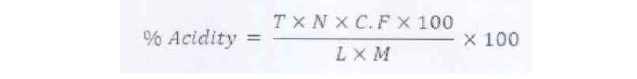
Where; T= titration reading; C.F= conversion factor; N= normality of NaOH; L= ml of sample taken for titration; M= ml of sample taken for dilution.
Protein determination: The protein was determined by standard method of AOAC (1984).
Calculation:
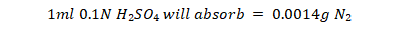
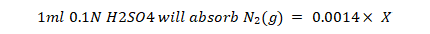



Percent moisture: The moisture content of the samples was determined by standard method of AOAC (1984). Samples were heated for 24 hours at 60 0C instead of 110 0C. After cooling the weight was taken again. This process was repeated until constant weight, % Moisture was determined by the following formula.

Organoleptic evaluation
All the samples were evaluated orgnaoleptically for colour, texture, flavor and overall acceptability by the method as describe by Larmond (1977). Samples were presented to trained judges to compare them and assign them scores between 1-9, where 1 represent extremely disliked and 9 represented extremely liked. (9 Points Hedonic Scale).
Statistical analysis
The results of various treatments were analyzed statistically by analysis of variance using Complete Randomized Design (CRD) and the means were separated by LSD test as recommended by Steel and Torrie, 1980.
Results and Discussion
The neutralization of whey was carried out with different concentration of NaOH (0.1 and 0.01N) and NaHCO3. Whey was subjected to various analysis and storage stability for a duration of four weak.
Proximate composition of liquid whey
Liquid whey used in present research has pH about 4.0 at 250C. The data regarding proximate composition of liquid whey is presented in Table 1. The data showed that liquid whey contained about 92.50 % water while dry matter is about 7.5 %. Dry matter contained 0.9 % protein, 4 % lactose (milk sugar), 0.4 % fat, 0.5 % ash contents and 0.6 % fiber. These results are fully in conformity with the findings of Watt and Merrill (1963).
Table 1: Proximate composition of liquid whey.
| Nutrients | % amount |
| Water | 92.50% |
| Protein | 0.90% |
| Fat | 0.40% |
| Ash | 0.50% |
Neutralization of whey
Acid whey has remained the least utilized form of whey due to its poor flavor and difficult handling characteristics. As whey has the pH round about 4. With such low pH, utilization of whey to the food products is not good, as it will leave bitter final taste and flavor of the product. One of the solutions that have always been desired is to convert acid whey into a condition which can be better utilized in the production of other food products. Before acid whey can be widely utilized, it must be substantially deacidified. There are two technical approaches with are generally considered to offer viable means to this end. The first is the routine neutralization of acid whey with an alkali solution and the other is electrodialysis or membrane ion exchange (Eun et al., 1998). During present research work first way of deacidification of whey is used i.e. neutralization of whey (Table 2). Del et al. (1995) also used whey which was neutralized with 25% NaOH solution and use this neutralized whey in the manufacture of a Cuban dessert (Table 3).
Table 2: Neutralization of 100ml whey with different concentrations of NaOH and NaHCO3 solutions.
| Reagents | Conc. | Volume used | pH adjusted |
| NaOH | 0.1 | 76ml | 7 |
| 0.01 | 800ml | 7 | |
|
NaHCO3 |
0.1 | 166.8ml | 7 |
| 0.01 | 1500ml | 7 |
Table 3: Neutralization of 100ml whey with NaOH and NaHCO3.
| Reagents | gram used | pH adjusted |
| NaOH | 0.3g | 7 |
|
NaHCO3 |
1.4g | 7 |
During this research work 0.3g NaOH and 1.4g NaHCO3 neutralized whey was used in different treatments.
Storage effects on the quality of normal and neutralized whey
The results of various physico-chemical characteristics of normal and neutralized whey after four weeks of storage period at room conditions are summarized in Table 5.
% Moisture
Table 4 shows the results pertaining to the effects of various treatments and storage intervals on percent moisture of normal and neutralized whey stored at room temperature. The mean values for (T0) normal whey, (T1) NaOH-neutralized whey and (T3) NaHCO3-neutralized whey were 91.60, 91.24 and 91.24 respectively. The differences were statistically significant (P<0.05). Normal whey (T0) had high percentage of moisture; this is due to its natural composition i.e. no addition of any reagent as other two treatments had. The analysis of variance for storage interval means showed that there were high significant (P<0.05) differences among the % moisture of normal and neutralized whey at different storage intervals. Table 4 shows a gradual and significant decrease in the moisture as storage period increases. Evaporation is the main reason of this decrease; other miner factors are production of some acids and growth of mold and yeast with the passage of time in liquid whey. These all factors are same in liquid whey as in other milk products. These results are closely resembled with the finding of Mehta and Basette (1978) and Sinha et al. (1968), who investigated the keeping quality of milk during storage at room temperature.
Table 4: Moisture (%) of whey treated with different concentrations of NaOH and NaHCO3 during storage.
| Treatments |
Storage intervals |
Means | ||||
| 0 | 1 | 2 | 3 | 4 | ||
|
T0 |
92.7 | 91.9 | 91.3 | 91.10 | 91 | 91.60a |
|
T1 |
92.4 | 91.5 | 90.9 | 90.70 | 90.7 | 91.24b |
|
T2 |
92.3 | 91.5 | 90.85 | 90.80 | 90.75 | 91.24b |
| Means | 92.46a | 91.63b | 91.01c | 90.86d | 90.81d | |
Values followed by different letters are significantly (P < 0.05) difference from each other.
% Acidity
The data regarding to the %acidity of normal and neutralized whey stored at room temperature are shown in Table 5. The mean values for (T0) normal whey, (T1) NaOH-neutralized whey and (T3) NaHCO3-neutralized whey were 1.02, 0.28 and 0.30 respectively. The differences were highly significant (P<0.05). Normal whey (T0) had high %acidity as compare to other two treatments. T2 and T3 had very low values of %acidity, infect these two treatments were deacidified by adding NaOH and NaHCO3 respectively. Storage intervals also caused significant (P<0.05) change in %acidity of all samples. Table 5 shows a gradual and significant increase in the %acidity as storage period increases. The reason for this is the production of some acid i.e. lactic acid and formic acid during storage at room temperature. The formation of formic acid and lactic acid were the same in all milk products with some variation. These results are in agreement with Gould et al. (1946), who investigated that titratable acidity increases in milk during storage.
Table 5: Acidity (%) of whey treated with different concentrations of NaOH and NaHCO3 during storage.
| Treatments |
Storage intervals |
Means | ||||
| 0 | 1 | 2 | 3 | 4 | ||
|
T0 |
0.9 | 0.95 | 0.98 | 1.0 | 1.3 | 1.02a |
|
T1 |
0.31 | 0.30 | 0.28 | 0.27 | 0.25 | 0.28b |
|
T2 |
0.35 | 0.33 | 0.30 | 0.28 | 0.26 | 0.30b |
| Means | 0.52a | 0.52a | 0.52a | 0.51a | 0.60a | |
Values followed by different letters are significantly (P < 0.05) difference from each other.
pH
Table 6 shows the results pertaining to the effects of various treatments and storage intervals on pH of normal and neutralized whey stored at room temperature. The mean values for (T0) normal whey, (T1) NaOH-neutralized whey and (T3) NaHCO3-neutralized whey were 3.52, 7.18 and 7.14 respectively. The differences were statistically significant (P<0.05). Normal whey (T0) had low pH as compared with other two treatments. T1 and T3 had pH near 7, as the pH of these two treatments was shifted to 7 (neutralization) by adding NaOH and NaHCO3. The analysis of variance for storage interval means showed that there were high significant (P<0.05) differences among the pH values of normal and neutralized whey at different storage intervals. Table 6 shows a significant decrease in the pH as storage period increases. The reason for this is the gradual increase in the % acidity due to the production of some acid i.e. lactic acid and formic acid during storage at room temperature. These changes in liquid whey are same as in other milk products. These results are in resemblance with the finding of the Gould et al. (1946), who investigated that pH decreases in milk during storage.
Table 6: pH of whey treated with different concentrations of NaOH and NaHCO3 during storage.
| Treatments |
Storage intervals |
Means | ||||
| 0 | 1 | 2 | 3 | 4 | ||
|
T0 |
4 | 3.80 | 3.6 | 3.2 | 3.0 | 3.52b |
|
T1 |
6.8 | 7.0 | 7.2 | 7.4 | 7.5 | 7.18a |
|
T2 |
6.6 | 6.9 | 7.1 | 7.5 | 7.6 | 7.14a |
| Means | 5.80a | 5.90a | 5.96a | 6.03a | 6.03a | |
Values followed by different letters are significantly (P < 0.05) difference from each other.
Total soluble solids
Table 7 shows the results related to the effects of various treatments and storage intervals on total soluble solids (TSS) of normal and neutralized whey stored at room temperature. The mean values for (T0) normal whey, (T1) NaOH-neutralized whey and (T3) NaHCO3-neutralized whey were 7.44, 7.76 and 7.830Brix (degree Brix) respectively. The differences were statistically significant (P<0.05). Normal whey (T0) had high TSS; this is due to its natural composition i.e. no addition of any reagent as other two treatments had. Storage intervals also caused high significant (P<0.05) change in TSS of all samples. Table 7 shows a gradual and significant increase in the TSS as storage period increases. In fact, this is due to loss of moisture during storage, the TSS of liquid whey increased automatically. These results regarding to T.S.S. are in agreement with the finding of Siddiqi and Chughtai (1976), who found gradual increase in T.S.S. in flavored milk during storage.
Table 7: TSS (0Brix) of whey treated with different concentrations of NaOH and NaHCO3 during storage.
| Treatments |
Storage intervals |
Means |
||||
| 0 | 1 | 2 | 3 | 4 | ||
|
T0 |
7.32 | 7.41 | 7.49 | 7.5 | 7.52 | 7.44b |
|
T1 |
7.7 | 7.75 | 7.78 | 7.8 | 7.81 | 7.76a |
|
T2 |
7.8 | 7.82 | 7.85 | 7.86 | 7.86 | 7.83a |
| Means | 7.60c | 7.66b | 7.70ab | 7.72a | 7.73a | |
Values followed by different letters are significantly (P < 0.05) difference from each other.
% Crude protein
Table 8 shows the results pertaining to the effects of various treatments and storage intervals on %crude protein of normal and neutralized whey stored at room temperature. The mean values for (T0) normal whey, (T1) NaOH-neutralized whey and (T3) NaHCO3-neutralized whey were 0.87, 0.80 and 0.78 respectively. The differences were statistically significant (P<0.05). Normal whey (T0) had little high percentage of %crude protein as compare with other two treatments. As T1 and T2 had little less %crude protein, this is infact due to addition of NaOH and NaHCO3 for the purpose of neutralization, these reagents precipitate out the protein and some of this protein may be denatured. The analysis of variance for storage interval means showed that there were statistically significant (P<0.05) differences among the %crude protein of normal and neutralized whey at different storage intervals. Table 8 shows a gradual and significant decrease in the %crude protein as storage period increases. Infact the total nitrogen gradually decreased with the increasing storage intervals. Proteolytic enzymes originate from the growth of psychotropic vegetative organisms in liquid whey stored at room conditions (highly resistant against heat) destabilize the protein during subsequent storage leading to gel formation. These changes in % crude protein are similar in all milk products with some variation. These results are in agreement with Manji and Kakuda (1988), who observed that % crude protein decreases due to proteolytic enzymes and protein denaturation due to some chemical and physical factors in milk during storage. Results are also in agreement with Dannenberg and Kessler (1988), they reported that degree of denaturation of beta-lactglobulin in skim milk increased from 10 to 99%.
Table 8: Crude Protein of whey treated with different concentrations of NaOH and NaHCO3 during storage.
| Treatments |
Storage intervals |
Means | ||||
| 0 | 1 | 2 | 3 | 4 | ||
|
T0 |
0.9 | 0.89 | 0.87 | 0.87 | 0.85 | 0.87a |
|
T1 |
0.81 | 0.8 | 0.78 | 0.77 | 0.74 | 0.80ab |
|
T2 |
0.82 | 0.8 | 0.79 | 0.78 | 0.75 | 0.78b |
| Means | 0.84a | 0.83ab | 0.84a | 0.80ab | 0.80ab | |
Values followed by different letters are significantly (P < 0.05) difference from each other.
Organoleptic evaluation
All samples of normal and neutralized whey stored under room condition were presented to panel of trained judges for evaluation of color, taste, texture and overall acceptability. This was repeated after one week interval up to four weeks.
Table 9 shows the results pertaining to the effects of various treatments and storage intervals on overall acceptability of normal and neutralized whey stored at room temperature. The score mean values for (T0) normal whey, (T1) NaOH-neutralized whey and (T3) NaHCO3-neutralized whey were 6.62, 7.58 and 7.46 respectively. The differences were statistically significant (P<0.05). Normal whey (T0) had fewer score as compared with other two treatments. As T0 was normal whey (i.e. unnaturalized whey) and it had fewer scores for color and taste, so the overall acceptability for the T1 and T2 was good then T0. Storage intervals also caused high significant (P<0.05) effects on overall acceptability. These results are similar to the results of Mehanna and Gonc (1988), they found that the organoleptic value of yoghurt decreases with increase of storage period particularly flavor and taste, texture and appearance.
Table 9: Mean score of color, flavor, texture and overall acceptability of whey treated with different concentrations of NaOH and NaHCO3 during storage.
| Sensory attributes | T0 | T1 | T2 |
| Color | 8.78a | 8.8a | 8.84b |
| Taste | 5.65c | 7.63a | 7.51b |
| Texture | 7.99a | 7.15c | 7.35b |
| Overall acceptability | 6.62b | 7.58a | 7.46a |
Conclusions and Recommendations
It is concluded that whey neutralized with 0.1N NaOH revealed better results as compared to normal and neutralized whey with NaHCO3 and has more shelf life during storage.
Acknowledgements
We are thankful to Department of Food Science and Technology, The University of Agriculture, Peshawar for providing opportunity to conducts this research work within the laboratories. All the authors carry equal contribution in conducting this research work and we have no conflict of interest.
Author’s Contribution
Ali Muhammad: Collected the whey, analysed the data, performed the experiments, wrote the article.
Yasser Durrani: Designed the research.
Majid Suhail Hashmi: Did statistical analysis, formatted and edited the article.
Ihsan Mabood Qazi: Layout of research article
Muhammad Ayub: Designed the experiments, formatted and edited the article.
Saifullah: Helped in reseach article, collection of samples and analysis.
References
AOAC. 1984. Official methods of analysis. Association of Official Analytical Chemists. 13th edi. Washington, D.C.
Arrick, B.A. and C.F. Nathan. 1984. Glutathione Metabolism as a Determinant of Therapeutic Efficacy. Cancer Res. 44: 4224-4232.
Bounous, G. and P. Pold. 1991. A whey protein introduction. J. Clin. Invest. Med. 14(40): 296-309.
Byron, H.W. and E.O. Whittier. 1970. Byproducts of milk. The AVI publish. Company. Inc.
Dannenberg, F., and H.G. Kessler .1988. Reaction kinetics of the denaturation of whey proteins in milk.J. Food Sci. 53: 258–263.
Del, C.C., M.G. Martinez, B. Espinosa, O. Ortega, E. Real, M. del and C. Cabrera. 1995. Use of acid whey in the manufacture of a concentrated dessert. Instituto de Investigaciones para la Industria Alimentaria, Carretera del Guatao, km 3,500, Punta Brava, La Lisa, Ciudad de la Habana 19200, Cuba. Aliment. 32: 260, 107-109.
Eun-Gyo, L., S.H.M, Y.K, K.C, Ik.K.Y. and H.C.N. 1998. Lactic acid recovery using two stage electrodialysis and its modeling. J. Member Sci. 145: 53-66. https://doi.org/10.1016/S0376-7388(98)00065-9
Goldenko, G.B. 1993. Use of whey in the manufacture of confectionery. Nauchno-Issledovatel ‘skii Institut Konditerskoi Promyshlennosti, Russia. Molochnaya-Promyshlennost’. 2: 16-18.
Gould, I.A. 1946. Lactic acid in milk. Effect of storage of evaporated milk on lactic and formic acid production on pH, acidity and formal titration. J. Dairy Sci. 29(1): p. 39. https://doi.org/10.3168/jds.S0022-0302(46)92440-X
Ha, E. and M.B. Zemel. 2003. Functional properties of whey, whey components, and essential amino acids: mechanisms underlying health benefits for active people (review). J. Nutr. Biochem. 14: 251-258. https://doi.org/10.1016/S0955-2863(03)00030-5
Hargrove, R.E. and J.A. Alford. 1974. Composition of milk products. In: fundamentals of Dairy Chemistry, 2nd ed. B.H. Webb, A.H. Johnson and J.A. Alford (Editors). AVI Publish. Co., Westport, Conn., pp. 58-86.
Health Point Technologies. 2004. Bio loop Pty Ltd, 33 Bank Street, South Melbourne, Vie 3205 Australia.
Khanam, A., Kumkum, R.C.G. and B. Swamylingappa. 2013. Functional and nutritional evaluation of supplementary food formulations. J. Food Sci. Technol. 50: 309-316. https://doi.org/10.1007/s13197-011-0344-x
Kulozik, U. 2000. Fractionation and functional properties of dairy proteins and their derivatives. Instt. Food Process. Engg. Dairy Tech. Univ. Munich. 65(3b): 495-501.
Larmond, E. 1977. Laboratory method of sensory evaluation of food: publication, 1673. Canada, Deptt. Agric. Ottawa.
Lemon, P.W., J.M. Berardi and E.E. Noreen. 2002. The role of protein and amino acid supplements in the athlete’s diet: does type or timing of ingestion matter? Curr. Sports Med. Rep. 4: 214-221. https://doi.org/10.1249/00149619-200208000-00005
Manji, B. and Y. Kakuda. 1988. The role of protein denaturation, extent of proteolysis and storage temperature on the mechanism of age gelation in amodel system. J. Dairy Sci. 71(6) pp: 1455-1463. https://doi.org/10.3168/jds.S0022-0302(88)79708-8
Manninen, A.H. 2009. Protein hydrolysates in sports nutrition. Nutr. Metab. 6: 1-5.
Mathur, B.N. and K.M. Shahani. 1979. Use of total whey constituents for human food. J. Dairy Sci. 62(1): 99-105. https://doi.org/10.3168/jds.S0022-0302(79)83209-9
Mehanna, N.M. and S. Gonc. 1988. Manufacture of yogurt from milk fortified with whey powder. Egypt. J. Dairy Sci. 16(2) pp: 234-248.
Mehta, R.S. and R. Basette. 1978. Organoleptic, Chemical and microbiological changes in ultra-high temperature sterilized milk stored at room temperature. J. Food Prot. 41 (10): pp. 806-810. https://doi.org/10.4315/0362-028X-41.10.806
Phillips, S.M. 2012. Dietary protein requirements and adaptive advantages in athletes. Br. J. Nutr. 12: S158-S167. https://doi.org/10.1017/S0007114512002516
Rolland, J.R. 1993. Whey - its uses. Les Fromages Saputo Ltee, Canada. Producteur-de-Lait-Quebecois. 13: 9, 34-36.
Siddiqi, S.Z. and M.D. Chughtai. 1976. Standardization of process for the production of mango flavored milk. Pak. J. Sci. Ind. Res. 19(1): 32p.
Sinha, R.N., J.P. Singh and V.K.N. Nambudripal. 1968. Studies on the keeping quality of pasteurized milk. India. J. Dairy Sci. 21(1): pp. 1-5. Biol. Abst. 1969, 50(12): pp. 66173.
Steel, G.R.D. and J.H. Torrie. 1980. Principles and Processes of Statistics, 2nd Ed. McGraw Hill Book Company Inc. New York. P. 507.
Urista, C.M., R.A. Fern-andez, F.R.A. Rodriguez, A.A. Cuenca and A.J. Tellez. 2011. Review: production and functionality of active peptides from milk. Food Sci. Technol. Int. 17: 293-317. https://doi.org/10.1177/1082013211398801
USDA. 1981C. Foreign Agric. Circular, FAS, FD9 - 81. U.S. Deptt. of Agric. Washington, D.C.
Walstra, P., J.T.M. Wouters and T.J. Geurts. 2006. Dairy science and technology (2nd ed.). Boca Raton: CRC/Taylor and Francis.
Watt, B.K. and A.L. Merrill. 1963. Composition of foods. Agr. Hand book 8. U.S. Dept. Agric. Washington, D.C.
Whey Products Instt. 1980. Whey Products – A Survey of Utilization and Production Trends 1980. Bullet. No. 25. Whey Products Instt. Chicago.
To share on other social networks, click on any share button. What are these?








