Virulence Genes and Immunological Biomarkers for Brucellosis in Sheep & Goat
Research Article
Virulence Genes and Immunological Biomarkers for Brucellosis in Sheep & Goat
Asmaa A. Darwish*, Adel M. El-Kattan, Mona A. Mahmoud, Mohamed T. Ragab, Amani A. Hafez
Animal and Poultry Health Department, Animal and Poultry Division, Desert Research Center, Cairo, Egypt.
Abstract | Brucella melitensis is an annoying problem for animal breeders. It attacks different farm animals causing infertility, abortion, and stillbirth. This study aimed to figure out the common genes related to B. melitensis field strains virulence and assess the immunological, hormonal, and iron profile alterations related to B. melitensis infection in sheep and goat with special reference to their value as biomarkers for the disease. Vaginal swabs were collected from 110 ewes and 150 does for bacteriological examination. Blood samples were collected for immunological, hormonal, and iron profile estimation. The total recovery of B. melitensis was 13.50% with nearly equal percentages in sheep (13.60%), and goat (13.33%). B. melitensis isolates had bvfA gene, virB gene, and ure gene with percentages of 62.8%, 77%, and 37% respectively. B. melitensis infection in sheep and goat resulted in a significant (P˂0.05) increase in the pro-inflammatory cytokines, acute phase proteins (APPs), free radicals, cortisol, growth hormone, and TSH concentrations and a significant (P˂0.05) decline in the anti-inflammatory cytokine, anti-oxidants, insulin, T3, and T4 levels. The iron profile of infected animals was characterized by a significant (P˂0.05) hypoferremia, hyperferritinemia, and hypotransferrinemia. The estimated pro-inflammatory cytokines and APPs yielded high sensitivity and specificity values in both infected species, but IL-1β, IL-6, and Fb had the highest likelihood ratios in infected sheep and IL-1β and IL-1α had the highest likelihood ratios in infected goat while Hp and IL-1β scored the highest percentages of increase among these markers. We concluded that bvfA, virB, and ure are the main virulence genes in B. melitensis isolates obtained from vaginal swabs of infected sheep and goat. B. melitensis infection elicits a prominent innate immune response in sheep and goat, resulting in several hormonal and iron profile alterations. IL-1β, IL-6, Fb, and Hp are good markers for sheep brucellosis, and IL-1β, IL-1α, and Hp are good indicators for goat brucellosis.
Keywords | Brucella melitensis; Virulence genes; Immunological changes; Hormonal changes; Iron profile.
Received | March 03, 2023; Accepted | April 05, 2023; Published | May 01, 2023
*Correspondence | Asmaa A Darwish, Animal and Poultry Health Department, Animal and Poultry Division, Desert Research Center, Cairo, Egypt; Email: [email protected]
Citation | Darwish AA, M. El-Kattan A, Mahmoud MA, Ragab MT, Hafez AA (2023). Virulence genes and immunological biomarkers for brucellosis in sheep & goat. J. Anim. Health Prod. 11(2): 165-175.
DOI | http://dx.doi.org/10.17582/journal.jahp/2023/11.2.165.175
ISSN | 2308-2801

Copyright: 2023 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Livestock breeding, particularly sheep and goat farming, is a significant source of income for people residing on the Egyptian north coast. However, Brucellosis remains a major challenge for sheep and goat breeders in Egypt and other producing countries. The disease is known to cause infertility, abortion, and stillbirth in various animal species, and it also poses a significant health risk to humans in developing countries like Egypt (Nayel et al., 2020; Akhtar et al., 2021). Controlling Brucellosis is a challenging task, as the bacterium has developed several mechanisms to evade the host’s immune system, particularly macrophages. Therefore, analyzing the molecular pathways that Brucella uses to survive within host cells is critical in developing effective strategies for controlling and preventing Brucellosis (Mohamed et al., 2008).
Brucella is divided into six species based on their animal hosts. In Egypt, B. melitensis is the most common species in humans and animals followed by B. abortus and B. suis (Abdel-Hamid et al., 2020). All Brucella members are closely related to each other, and their pathogenicity depends on the virulence factors (Gandara et al., 2001). The most common virulence-related gene elements in Brucella are Brucella virulence factor A (bvfA), VirB, and urease gene (ure). BvfA is concerned with Brucella’s ability to stay alive in the host. It is a small 11 kDa periplasmic protein that is unique to the genus Brucella (Lavigne et al., 2005). It may help in setting up the intracellular niche (Lavigne et al., 2005; Mohamed et al., 2008). VirB proteins are another virulence factor of Brucella. They are 12 proteins responsible for making up the type IV secretion system (T4SS) thus facilitating Brucella intracellular replication and escaping from the host immune system (Delrue et al., 2004). Urease gene (ure) is the virulence factor that helps Brucella to survive in-vivo and in-vitro at low pH. Although Brucella has two different groups of urease genes (ure1 and ure2), ure1 only codes for an active urease and no one knows the role of ure2 in Brucella biology (Felix et al., 2010).
The immune response is the body’s reaction against pathogens’ attacks. Many protein molecules are involved in the immune response, but the domination is for the inflammatory cytokines. They are the main organizers of the immune response. They are the initiators, magnifiers, and terminators of the immune response (Demirdag et al., 2003; Hashem et al., 2020). They are classified into: pro-inflammatory cytokines which stimulate other immune cells to start their functions and coordinate between them and anti-inflammatory immune cytokines which inhibit and prevent inflammation exacerbation. Under their stimuli, the hepatocytes synthesized a group of α and β globulins, called acute phase proteins (APPs) (Shalby et al., 2020). During infection, they try to restrict and limit microbial growth and keep the host body homeostasis till the antibodies form. Free radicals, complement systems, and immunoglobulin are also activated under the effect of the pro-inflammatory cytokines. The pro-inflammatory cytokines and APPs were introduced as herd health indicators and sensitive markers for different pathological conditions and malignancy (El-Boshy et al., 2009).
Keeping in mind these facts, the present study was carried out to find and figure out common genes related to virulence in B. melitensis field strains taken from sheep and goats in the Northwest coast of Egypt and assess some immunological, hormonal, and iron profile alterations related to B. melitensis infection with especial reference to the pro-inflammatory cytokines and APPs validity as diagnostic markers and predictors for the disease in the two species.
Materials and methods
Animals
After the ethical approval of the Animal and Poultry Health Department, Animal and Poultry Health Division, DRC, Cairo, Egypt, this study was carried out on 260 animals (110 ewes (3-5 years) and 150 does (3-5 years)) with a history of recent abortion, randomly collected from different areas of the Egyptian north coast.
Bacteriological examination
Samples: Vaginal swabs were collected from all animals on Stuart transport medium for bacterial isolation and molecular detection. Swabs were transported in cool conditions to the desert research center’s bacteriology laboratory and stored at - 20 °C until processed.
Isolation of Brucella: All bacteriological samples were processed under Biosafety level two (BSL2) with high personal protections as described (OIE, 2009).
About 1 ml of the contents, were grown on a tryptose agar medium with a selective antibiotic supplement (Ewalt et al., 1983) (Oxoid). According to Alton et al (1988), plates were put in an incubator at 37°C with 10% CO2 and checked for growth every day for 15 days. Isolates were identified as Brucella.
Brucella-suspected colonies exhibiting a characteristic spherical, shiny, with intact borders, pinpoint, and have a honey
color under transmitted light (Alton et al., 1988; OIE, 2016).
Identification of the isolates: The isolates were preliminarily identified by morphology, urease, and oxidase tests (OIE 2018; Saavedra et al., 2019). Typical isolates smooth colony, Gram-negative coccobacilli, urease-, and oxidase-positive were identified as Brucella spp, and they were stored at –18 °C in a Tryptone Soy Broth (Oxoid CM0129) supplemented with 10% glycerine.
DNA preparation: DNA extraction from samples was performed using the QIAamp DNA Mini kit (Qiagen, Germany, GmbH) with modifications from the manufacturer’s recommendations. Briefly, 200 µl of the sample suspension was incubated with 10 µl of proteinase K and 200 µl of lysis buffer at 56OC for 10 min. After incubation, 200 µl of 100% ethanol was added to the lysate. The sample was then washed and centrifuged following the manufacturer’s recommendations. Nucleic acid was eluted with 100 µl of elution buffer provided in the kit.
Table 1: Primers sequences, target genes, amplicon sizes, and cycling conditions for conventional PCR.
| Target gene |
Primers sequences |
Amp lified segment (bp) |
Primary dena turation |
Amplification (35 cycles) |
Final extension |
Reference |
||
| Secon dary denatu ration |
Annealing |
Extension |
||||||
|
B. melitensis- IS711 |
AAATCGCGTCCTT GCTGGTCTGA TGCCGATCACTT AAGGGCCTTCAT |
731 |
94˚C 5 min.
|
94˚C 30 sec. |
54˚C 40 sec. |
72˚C 45 sec.
|
72˚C 10 min |
Gupta et al. (2014) Akhtar, , et al 2021 |
| BvfA | ACCCTTCGTC GATGTGCTGA |
1282 |
94˚C 5 min.
|
94˚C 1 min. |
65˚C 1 min. |
72˚C 1.3 min.
|
72˚C 10 min. |
Derakh shandeh et al., 2013
|
CCGCGCTGAT TTCATCGCTG |
||||||||
| virB | CGCTGATCTAT AATTAAGGCTA |
881 |
94˚C 5 min.
|
94˚C 30 sec. |
54˚C 40 sec. |
72˚C 45 sec.
|
72˚C 10 min. |
|
|
TGCGACTGCCTCC TATCGTC |
||||||||
| Ure | GCTTGCCCTTG AATTCCTTTGTGG |
2100 |
94˚C 5 min.
|
94˚C 1 min. |
65˚C 1 min. |
72˚C 1.3 min.
|
72˚C 10 min. |
|
ATCTGCGAAT TTGCCGGACTCTAT |
||||||||
Polymerase Chain Reaction (PCR)
Traditional PCR was used to amplify virulence genes of Brucella spp. BvfA, VirB, and Ure were amplified with the help of specific oligonucleotide primers, Metabion (Germany) (Table 1).
Primers were used in a 25-µl reaction that had 12.5 µl of Emerald Amp Max PCR Master Mix (Takara, Japan), 1 µl of each primer at a concentration of 20 pmol, 5.5 µl of water, and 5 µl of DNA template, the reaction was performed in an applied biosystem 2720 thermal cycler.
Analysis of the PCR Products
Electrophoresis on 1.5% agarose gel (Applichem, Germany, GmbH) in 1x TBE buffer at room temperature with 5V/cm gradients was used to separate the PCR products In each gel slot, 20 µl of the multiplex PCR products were put for gel analysis The sizes of the fragments were figured out by using a gene ruler 100 bp ladder (Fermentas, Germany), a 100 bp DNA ladder H3 RTU (Genedirex), and a gel pilot 100 bp plus ladder (Qiagen, Gmbh, Germany) A gel documentation system (Alpha Innotech or Biometra) took pictures of the gel, and computer software was used to look at the data.
Immunological, hormonal, and iron profile parameters estimation
A 5 ml blood was collected from the Brucella-infected animals (15 ewes and 20 does which were positive for the bacteriological examination and were considered the diseased groups (DG)) and from apparently-healthy animals (Control group (CG); 15 ewes and 20 does with normal ranges of body temperature, respiratory and heart rate, no abortion and were negative for bacteriological examination), and separated into two tubes. The first tube contained heparin (5000 I.U.) to stop the coagulation process while the second tube was plain and the blood was allowed to coagulate. Both samples were centrifuged at 3000 r.p.m for 15 min to obtain heparinized plasma and serum respectively. The heparinized plasma and serum were kept in clean Eppendorf at -80 and used later to determine immunological, hormonal, and iron profile parameters.
Serum pro-inflammatory cytokines (IL-1α, IL-1β, IL-6, TNF-α) and anti-inflammatory cytokine (IL-10) were estimated using ELISA kits (MyBioSource Company, San Diego, USA). The plasma fibrinogen (Fb), serum amyloid A (SAA) and serum haptoglobin (Hp) were measured using commercial ELISA kits of IBL International Crop (Canda). The serum caeruloplasmin (Cp) and serum transferrin (Tf) were detected by turbidimetric method using kits purchased from commercial company (Elab Science USA), and serum ferritin was evaluated by Chemiluminescence immunoassay (CLIA) method using the kit procured from Abnova (Taipei, Taiwan).
Serum concentrations of free radicals (malondialdehyde (MDA), nitric oxide (NO)), antioxidants (catalase (CAT), glutathione peroxidase (GPx), glutathione reductase (GR)), serum iron (SI), and total iron binding capacity (TIBC) were determined spectrophotometrically using commercial kits of Biodiagnostic company (Giza, Egypt).
Serum hormonal assays (cortisol, insulin, TSH, T3, T4, and growth hormone (GH)) were estimated by CLIA method using kits supplied by DiaSorin (Saluggia Italy).
All manual instructions were carefully followed.
- Transferrin saturation percent (TF sat. %) = SI/TIBC*100.
-Unsaturated iron binding capacity (UIBC) = TIBC-SI.
Statistical analysis
Mean values of CG and DG of the same species were compared by independent-samples T test using SPSS® program version 23. A difference was considered significant at P< 0.05.
Graph pad prism version 8 program was used to evaluate the area under the curve (AUC), cut off points, sensitivity, specificity, and likelihood ratio (LR) for the measured cytokines and APPs between DG and CG in the same species.
The positive predictive value (PPV), negative predictive value (NPV), and accuracy rate for them were calculated according to the next equations:
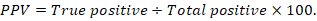
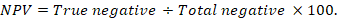
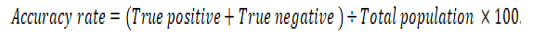

Results
Isolation and identification of B. melitensis
Brucella organisms were obtained from 35 out of 260 vaginal swabs (13.50%) with nearly equal percentages in sheep and goat (15/110 sheep (13.60%), and 20/150 goat (13.33%)). Brucella cultures showed typical characteristics for the genus Brucella. Colonies were spherical, shiny, smooth pinpoint, with intact borders, brilliant surfaces, and have a honey color under transmitted light, Gram-negative coccobacilli, with positive oxidase and urease tests.
Molecular characterization
PCR Assays: Universal PCR in this study confirmed the presence of genetic material of genus Brucella in all 35 (100%) DNA extracts from which Brucella melitensis has been isolated from their cultures. The assay has amplified the target gene (B. melitensis- IS711) with amplification of the fragment of 731 bp (Fig. 1) characteristic for the Genus Brucella melitensis, the virulence genes bvfA, virB, and ure genes assays produced at amplicons of 1282, 881, and 2100 bp respectively (Fig. 2,3,4).
PCR for virulence gene
62.8% (22/35) of the isolates carried bvfA gene (Fig. 2), virB gene was recorded in 77% (27/35) of the isolates (Fig. 3), ure gene was detected in 37% (13/35) of the isolates (Fig. 4).
Immunological, hormonal, and iron profile results
Table (2) cleared that B. melitensis infection in sheep and goat is associated with a strong innate immune response
Table 2: Comparison between the cytokines, acute phase proteins, oxidant-antioxidant status, hormonal assays and iron profile in diseased and control groups of both species. value= mean ±SD.
| Parameter |
Sheep |
Goat |
||
| CG |
DG |
CG |
DG |
|
|
IL-1α (Pg/ml) |
31.59±4.10 | 89.60±6.86* | 31.37±2.62 | 84.90±4.33* |
|
IL-1β (Pg/ml) |
28.24±4.34 | 132.10±9.74* | 24.92±2.01 | 130.20±7.82* |
| IL-6 (Pg/ml) | 29.88±1.98 | 65.56±2.78* | 26.91±2.24 | 64.98±2.05* |
|
TNF-α (Pg/ml) |
27.22±2.97 | 75.90±4.58* | 26.60±1.62 | 72.86±2.42* |
| IL-10 (Pg/ml) | 102.50±4.04 | 75.76±8.81* | 100.57±2.70 | 71.63±6.18* |
| Fb (mg/dl) | 127.75±15.33 | 248.15±14.46* | 103.70±3.96 | 194.90±10.69* |
| Cp (mg/dl) | 3.39±0.83 | 7.48±0.35* | 3.40±0.84 | 7.48±0.35* |
| Hp (g/dl) | 0.15±0.02 | 3.12±0.53* | 0.13±0.01 | 2.68±0.29* |
| SAA (mg/L) | 2.76±0.26 | 6.76±0.64* | 2.34±0.32 | 6.39±0.48* |
| MDA (nmol/ml) | 13.27±0.99 | 20.94±2.11* | 12.50±0.22 | 19.70±2.09* |
| NO (μmol/L) | 25.66±1.26 | 40.40±2.11* | 22.08±1.60 | 39.14±2.42* |
|
CAT (U/L) |
464.80±30.22 | 292.20±7.92* | 414.01±27.80 | 288.35±5.60* |
| GPx (mU/L) | 1065±22.42 | 792.80±15.71* | 955.50±30.32 | 760.60±39.36* |
| GR (ng/ml) | 21.90±0.91 | 12.36±0.58* | 21.35±1.04 | 12.30±0.55* |
| Cortisol (μg/dl) | 1.76±0.13 | 7.01±0.41* | 1.30±0.06 | 6.90±0.25* |
| Insulin (μIU/ml) | 8.69±0.28 | 7.18±0.24* | 8.10±0.28 | 7.06±0.18* |
| T3(ng/ml) | 1.82±0.12 | 1.03±0.04* | 1.72±0.06 | 1.01±0.04* |
| T4 (µg/ml) | 0.81±0.06 | 0.70±0.05* | 0.78±0.08 | 0.65±0.03* |
| TSH (µIU/ml) | 0.010±0.002 | 0.028±0.007* | 0.011±0.002 | 0.028±0.007* |
| GH (ng/dl) | 12.28±1.41 | 17.26±0.80* | 11.82±0.88 | 17.18±0.70* |
| SI (μg/dl) | 104.89±2.07 | 94.71±1.78* | 103.14±2.02 | 92.56±1.93* |
| TIBC (μg/dl) | 263.17±21.48 | 378.04±11.75* | 213.17±21.49 | 328.04±11.75* |
| UIBC (μg/dl) | 158.28±21.26 | 283.34±11.76* | 110.03±21.76 | 235.48±12.62* |
| Transferrin(mg/dl) | 126.30±2.75 | 85.60±2.39* | 124.45±3.25 | 81.60±3.28* |
| Tf sat. % | 40.11±3.35 | 25.07±0.92* | 48.87±5.21 | 28.26±1.43* |
| Ferritin (ng/ml) | 15.85±1.34 | 23.60±1.54* | 15.25±1.12 | 22.40±1.54* |
Significant differences in the values between the diseased groups and the control groups of the same species were indicated by (*) at P< 0.05.
IL-1α: interleukin 1 alpha, IL-1β: interleukin 1 beta, IL-6: interleukin 6, TNF-α: tumor necrosis alpha, IL-10: interleukin 10, Fb: fibrinogen, Cp: caeruloplasmin, Hp: haptoglobin, SAA: serum amyloid A, MDA: malondialdehyde, NO: nitric oxide, CAT: catalase, GPx: glutathione peroxidase, GR: glutathione reductase, TSH: thyroid stimulating hormone, GH: growth hormone, SI: serum iron, TIBC: total iron capacity, UIBC: unsaturated iron binding capacity, Tf sat. %: transferrin saturation %.
CG: control group, DG: diseased group.
Table 3: Cut off points, sensitivity%, specificity%, likelihood ratio (LR), PPV%, NPV%, accuracy rate (AR) and percentages of increase (+) or decrease (-) of the estimated pro-inflammatory cytokines (IL-1α, IL-1β, IL-6, TNF-α) and acute phase proteins (Fb, Cp, Hp, SAA, transferrin, ferritin) in DG compared to CG (of the same species).
| Parameter |
Species |
Cut off |
Sensitivity |
Specificity |
LR |
PPV |
NPV |
AR |
% of (+,-) |
|
IL-1α (Pg/ml) |
Sheep | 37.11 | 100% | 90% | 10 | 90.91% | 100% | 95% | 183.63% |
| Goat | 30.35 | 100% | 95% | 20 | 95.24% | 100% | 97.50% | 170.64% | |
|
IL-1β (Pg/ml) |
Sheep | 37.63 | 100% | 95% | 20 | 95.24% | 100% | 97.50% | 367.77% |
| Goat | 29.44 | 100% | 95% | 20 | 95.24% | 100% | 97.50% | 422.47% | |
| IL-6 (Pg/ml) | Sheep | 32.66 | 100% | 95% | 20 | 95.24% | 100% | 97.50% | 119.41% |
| Goat | 30.11 | 100% | 90% | 10 | 90.91% | 100% | 95% |
141.47% |
|
|
TNF-α (Pg/ml) |
Sheep | 31.00 | 100% | 85% | 6.67 | 86.96% | 100% | 92.50% | 178.84% |
| Goat | 28.73 | 100% | 90% | 10 | 90.91% | 100% | 95% | 173.91% | |
| Fb (mg/dl) | Sheep | 144.50 | 100% | 95% | 20 | 95.24% | 100% | 97.50% | 94.25% |
| Goat | 109 | 100% | 90% | 10 | 90.91% | 100% | 95% | 87.95% | |
| Cp (mg/dl) | Sheep | 4.40 | 100% | 90% | 10 | 90.91% | 100% | 95% |
120.64% |
| Goat | 4.40 | 100% | 90% | 10 | 90.91% | 100% | 95% | 120% | |
| Hp (g/dl) | Sheep | 0.1850 | 100% | 90% | 10 | 90.91% | 100% | 95% | 1980% |
| Goat | 0.1450 | 100% | 80% | 5 | 83.33% | 100% | 90% | 1961.54% | |
| SAA (mg/L) | Sheep | 2.96 | 100% | 80% | 5 | 83.33% | 100% | 90% | 144.93% |
| Goat | 2.84 | 100% | 90% | 10 | 90.91% | 100% |
95% |
173.08% | |
| Transferrin(mg/dl) | Sheep | 123.00 | 100% | 90% | 10 | 90.91% | 100% | 95% | -32.22% |
| Goat | 121.00 | 100% | 75% | 4 | 80% | 100% | 87.50% | -34.43% | |
| Ferritin (ng/ml) | Sheep | 17.50 | 100% | 85% | 6.67 | 86.96% | 100% | 92.50% | 48.90% |
| Goat | 16.50 | 100% | 85% | 6.67 | 86.96% | 100% | 92.50% | 46.89% |
LR= 0.5-5: low; LR=5-10: moderate; LR>10: high.
IL-1α: interleukin 1 alpha, IL-1β: interleukin 1 beta, IL-6: interleukin 6, TNF-α: tumor necrosis alpha, IL-10: interleukin 10, Fb: fibrinogen, Cp: caeruloplasmin, Hp: haptoglobin, SAA: serum amyloid A.
indicated by the significant (P˂0.05) increase in the concentrations of the pro-inflammatory cytokines (IL-1α, IL-1β, IL-6, TNF-α), APPs (Fb, Cp, Hp, SAA), free radicals (MDA, NO), and the significant (P˂0.05) decline in the levels of the anti-inflammatory cytokine (IL-10) and anti-oxidants (CAT, GPx, GR) in the DGs compared to CGs. In parallel, a significant (P˂0.05) elevation in cortisol, GH, and TSH concentrations while, insulin, T3, and T4 levels significantly (P˂0.05) decreased in DGs compared to CGs. The iron profile of both species, showed a significant (P˂0.05) hypoferremia, hyperferritinemia, hypotransferrinemia, increased TIBC, UIBC, and decreased Tf sat.% in DGs when compared to CGs.
Concerning the diagnostic value of the estimated pro-inflammatory cytokines and APPs, Table (3) clarified that all of the yielded high values of sensitivity, specificity, PPV, NPV, and AR and moderate to high LRs in both species, (except SAA in sheep and Hp and Tf in goat). Ordering them according to LR, IL-1β, IL-6, Fb were the best markers for sheep brucellosis (LR=20) followed by IL-1α, Cp, Hp, Tf (LR=10), then ferritin, TNF-α, SAA with LR as 6.67, 6.67 and 5 respectively. On the other hand, IL-1β, IL-1α were the best indicators for caprine brucellosis (LR=20) followed by IL-6, TNF-α, Fb, Cp, SAA (LR=10) then ferritin, Hp, Tf with LRs as 6.67, 5, 4 respectively. While, the percentage of increase strongly recommended Hp as the best marker for brucellosis in both species followed by IL-1β.
Discussion
Brucella is a gram-negative facultative intracellular coccobacillus, it has a unique ability to adapt to the conditions it finds inside the host cells. It changes over time to avoid and trick the host immune system by interfering with intracellular trafficking, resisting the respiratory burst, and adapting to the oxygen deprivation inside macrophages (Mohamed et al., 2008; El-Boshy et al., 2009). The total recovery rate of B. melitensis in our study was 13.5% (35/260) with nearly equal percentages in both species as 13.60% (15/110) in sheep and 13.33% (20/150) in goat. Similar recovery rates of B. melitensis were obtained before from milk samples and vaginal swabs collected from goats in Jordan (Samadi et al., 2010), vaginal exudate of goats in Mexico (Herrera et al., 2011), and vaginal swabs, spleen, and uterine fluid from goats in Peninsula Malaysia (Bamaiyi et al., 2012). Higher percentages of B. melitensis were recovered from cattle-milk samples in Iraq by Esmaeel (2019) as 18%, milk samples in Egypt by Aman et al. (2020) as 21.5%, goat and sheep milk as 35% and vaginal swabs as 62.5% in Iran by Shakerian et al. (2016), and lower percentage was recovered from aborted goat in Ethiopia as 7.8% by Tekle et al. (2019). Variations in the incidence of infection are related to, the fastidious nature of Brucella species, the stage of the disease, the number of shed bacteria through discharge, the course of the diseases, locality, rate of exposure, reproductive status, the improvements in diagnostic techniques, vaccination strategies and the enforcement of a national eradication policy (Nada et al., 1992; Luna-Martinez and Mejia-Teran, 2002).
The results of this study showed that most B. melitensis isolates have virulence factor genes (bvfA, virB, and ure) in their genome. We found that 62.8% of B. melitensis isolates had bvfA genes, this percentage is similar to the percentage reported by Lavigne et al. (2005) and lower than the percentages recorded by Derakhshandeh et al. (2013), Hamdy and Zaki (2018), and Aman et al. (2020) who found bvfA genes in B. melitensis isolated from sheep, goats, and cattle in Egypt by percentages of 78.5%, 92.3%, and 79% respectively. They attributed this high prevalence of virulence-associated bvfA genes in the B. melitensis isolates to the potential virulence of this bacterium and the role of bvfA in the intracellular Brucella replication.
In this investigation, 77 % of B. melitensis isolates had virB gene. Derakhshandeh et al. (2013) and Aman et al. (2020) recorded percentages of virB gene close to ours as 73.8% and 74% respectively in B. melitensis recovered from aborted fetuses of sheep and goats in Iran and raw milk in Egypt. On the other hand, Hamdy and Zaki (2018) found virB genes in 96.2% of B. melitensis isolated from different samples of sheep, goats, and cattle in Egypt. While, Esmaeel (2019) obtained virB gene in only 37.5% of B. melitensis recovered from cattle milk in Iraq. VirB gene coded for Brucella’s T4SS, which is a major factor in Brucella virulence. It hides Brucella from the host immune system and keeps it alive inside cells and facilitates its intracellular replication (Delrue et al., 2004; den Hartigh et al., 2008; Saeedzadeh et al., 2012). 37% of B. melitensis isolates in the present study had ure gene, this percentage is much lower than Derakhshandeh et al. (2013), Aman et al. (2020), and Abdel-Hamid et al. (2020) who found ure gene in 88%, 88%, and 91.7% of B. melitensis isolates. This discrepancy in results of ure gene may be due to the difference of the sources, the number of samples, and the areas that were looked at. Ure gene has an important role in the microbes’ survival, it breaks down urea into carbon dioxide and ammonia to make the environment around it more alkaline and makes it easier for cells to live in acidic environments (Seleem et al., 2008; Bandara et al., 2007).
The interaction between the host and B. melitensis organism in the current work successfully evoked a prominent innate immune response in both infected species. It was indicated by the outstanding increase of the pro-inflammatory cytokines (IL-1α, IL-1β, IL-6, TNF-α) concentrations in the diseased ewes and does. These results coincided with Demirdag et al. (2003), and Hashem et al. (2020) who noted elevated activities of TNF-α, IL-1β, and IL-6 in humans, cow, and sheep brucellosis. The pro-inflammatory cytokines are the main triggers of the inflammatory immune response. They are defined as small glycoproteins, nonspecifically released from various immune cells during different types of infection, especially bacterial ones. They coordinate other immune cells functions to initiate and exacerbate an inflammatory immune response. IL-1β and TNF-α, are the most important pro-inflammatory cytokines during Brucella infection. As IL-1β influences the adhesion factors expression on endothelial cells to facilitate the leukocytes (pathogen killers) migration to sites of infection (Nicklin et al., 2000). While TNF-α is essential for optimal Brucella killing by macrophages. TNF-α also enhances IFN-γ production, which stimulates macrophages generation with strong intracellular Brucella-killing activity (Seder and Hill, 2000; Murphy et al., 2001). Additionally, IFN-γ-induced T-cell responses widely participate in the immunity against intracellular B. abortus (Saunders et al., 2000).
In contrast to our results, El-Boshy et al. (2009) reported diminished levels of TNF-α, IFN-γ, and IL-1α and non-significant changes in IL-6 concentrations in Brucella-infected camels. He attributed this result to the pronounced increased IL-10 concentrations, he obtained. As the enhanced lipid peroxidation usually recorded with brucellosis, encourages IL-10 transcription. IL-10 has anti-inflammatory characters and greatly downregulates the action of the pro-inflammatory cytokine. It also suppresses the antigen presentation capacity of antigen-presenting cells. Thus, IL-10 helped Brucella to escape from the host protective immune mechanisms. Furthermore, it was found that in-vivo IL-10 neutralization with an anti-IL-10 monoclonal antibody lead to a fewer bacteria count (up to 10 folds) in mice infected with a virulent B. abortus strain (Fernandes and Baldwin, 1995). Logically, the marked decline in IL-10 levels in the diseased groups of both species here, explained the augmentation of the inflammatory immune response in this work.
Subsequently, a marked increase in the acute phase proteins concentrations (Fb, Cp, Hp, SAA) was recorded in both infected species in the present study. APPs are another type of innate non-specific immunity. They are synthesized by hepatocytes and released in the peripheral blood, under the effect of the above-mentioned activated pro-inflammatory cytokines in different pathological conditions. They are also involved in some physiological conditions like pregnancy and parturition. Generally, they are charged with maintaining hemostasis and microbial growth limitation till antibodies production. According to their concentrations in the blood during infection, they are classified into positive APPs (usually increase during infection) and negative APPs (usually decrease during infection). They were suggested as reliable biomarkers for different diseases in animal and human as well. They are affected by species, sex and age. APR is a common finding in brucellosis in different species, it was indicated before in humans by elevated CRP and Fb levels (Uluğ et al., 2010), in cows by increased SAA and Hp concentrations (Sharifiyazdia et al., 2012), in does by raised C reactive protein values (Mahboub et al., 2013), in camels by hyperhaptoglobinemia (Hamdy et al., 2019) and in sheep by hyperfibrinogenemia (Shalby et al., 2020).
Another product for the activation of the pro-inflammatory cytokine in this work is free radicals generation (MDA and NO). Free radicals are non-stable atoms that steal electrons from their neighbors to achieve their chemical stability. Thus, they transform their neighbors into free radicals and a series of oxidative reactions begins. Basically, they have potent antimicrobial properties via pathogen component oxidation. They are produced from the host macrophage and neutrophils in response to the circulating pro-inflammatory cytokines as a part of their innate immunity. Under normal conditions, they are efficiently controlled by anti-oxidants. Unfortunately, the anti-oxidant depletion (CAT, GPx, GR) in the present report referred to free radicals massive production and subsequent anti-oxidant exhaustion. Hence, the free radicals start attacking different body cells causing severe organs damages and oxidative stress appears. In this aspect, many researchers confirmed the great contribution of oxidative stress in the brucellosis pathogenesis in different animal species (Kataria et al., 2010; Bozukluhan et al., 2017; Merhan et al., 2017; Shalby et al., 2020).
Not only are the immune components affected by the aforesaid pro-inflammatory cytokines activation, but also the endocrine system. Prior investigations threw light on the profound impact of the immune system on the endocrine system. As the invigorated pro-inflammatory cytokines, especially IL-1β and TNF-α, induce the anterior pituitary gland to secret more ACTH hormone which stimulates glucocorticoids secretion from the adrenal gland (Gentilini et al., 2015; Gentilini et al., 2019). In accordance with this theory, a marked hypercortisolemia was observed in the diseased ewes and goats in this study. Subsequently, decreased insulin, T3, and T4 levels and increased growth hormone concentrations were detected in the Brucella-infected animals. As cortisol inhibits insulin secretion by interfering with Zn metabolism and insulin signaling, causes partial thyroid dysfunction and increases growth hormone production and secretion (Stratakis et al., 2006; Morais et al., 2019; Cai et al., 2020). This action was mainly to reverse the hypoglycemia usually recorded with brucellosis (Hashem et al., 2020). While, the little elevation observed in TSH concentrations in the diseased animals, may be assigned to the pituitary response to the decreased T3 and T4 levels.
Interestingly, the iron profile of the diseased animals in the current study was also a reflection of the above-described pro-inflammatory cytokines activation. The pro-inflammatory cytokines restrict pathogens ability to obtain the iron required for their growth and multiplication through several mechanisms. Such as, induction of anorexia, inhibition of intestinal iron absorption, increasing ferritin formation, enhancing hepcidin synthesis, and decreasing transferrin (Arica et al., 2012; Ganz et al., 2015; Ganz, 2018; Salem et al., 2020). Therefore, considerable hypoferemia, hyperferritinemia, and hypotransferenemia were obtained in both diseased species here. In parallel to the hypoferemia, TIBC and UIBC markedly increased and Tf sat. % decreased. The acute phase response, commonly noticed with brucellosis, may be another cause for these changes as ferritin is a positive acute phase reactant and transferrin is a negative acute phase reactant (Arica et al., 2012; Salem et al., 2020).
Regarding the importance of the estimated pro-inflammatory cytokines and APPs as biomarkers for brucellosis, all of them achieved high sensitivity, specificity, PPV, NPV, and AR and moderate to high LR (except SAA in sheep and Hp and Tf in goats). The best markers among them were IL-1β, IL-6, and Fb for ovine brucellosis and IL-1β and IL-1α for caprine brucellosis. The calculation of these markers’ percentages of increase, strongly nominated Hp as the best indicator for the disease in both species and supported using IL-1β as an indicator for brucellosis in both species. These results were concomitant with previous opinions, suggested the pro-inflammatory cytokines and APPs as sensitive biomarkers for brucellosis in different species and disagreed with the markers` superiority in the disease diagnosis (Demirdag et al., 2003; El-Boshy et al., 2009; Uluğ et al., 2010; Sharifiyazdia et al., 2012; Mahboub et al., 2013; Hamdy et al., 2019; Hashem et al., 2020; Salem et al., 2020; Shalby et al., 2020). This variation may be attributed to the disease stage, Brucella species, host species, geographical distribution, and the feeding system.
Conclusion
The study confirmed the existence of Brucella melitensis in vaginal swabs of sheep and goat and attributed its virulence to the presence of the bvfA, virB, and ure virulence genes. B. melitensis elicits a prominent innate immune response in sheep and goat which deeply contributed to the hormonal and iron profile alterations associated with the disease. Knowing these alterations may be helpful in modifying disease-supportive treatment protocols. IL-1β, IL-6, Fb, and Hp are good markers for ovine brucellosis while, IL-1β, IL-1α, and Hp are good indicators for caprine brucellosis.
Acknowledgments
The authors thank members of the Animal and Poultry Health Department, Desert Research Center, Cairo, Egypt.
Conflict of interest
Authors have no conflict of interest.
Novelty statement
This research investigated the virulence genes in B. melitensis isolates obtained from vaginal swabs of sheep and goat and the most important immunological, hormonal, and iron profile alterations related to its infection. It also suggested new markers for brucellosis in both species.
Authors contribution
All authors are equally contributed.
References
Akhtar, Raheela, et al. (2021). Genotyping of Brucella strains isolated from humans and cattle of different geographical regions of Pakistan using MLVA‐15. Vet. Med. Sci. 7.5 (2021): 1688-1695.
Alton G.G., Jones L.M., Angus R.D., Verger J.M. (1988). Techniques for the brucellosis laboratory. 1st ed. INRA. Paris.
Aman I., AL-Hawary I., Helmy N., EL-Gushi A. (2020). Detection of Brucella organisms from Egyptian raw milk using cultural and molecular techniques. Kafrelsheikh Vet. Med. J., 18(2): 14-19.
Arica V, Şilfeler İ, Arica S, Tutanç M, Motor VK, İnci M (2012). Brucellosis with very high ferritin levels: report of five cases. Hum. Exp. Toxicol. 31(1):104-6. https://doi.org/ 10.1177/0960327111414281. Epub 2011 Jun 27. PMID: 21708883.
Abdel-Hamid NH, El-bauomy EM,Ghobashy HM, Shehata AA. (2020). Genetic variation of Brucella isolates at strain level in Egypt. Vet. Med. Sci., 6(3): 421– 432.
Bamaiyi PH, Hassan L, Khairani-Bejo S, Zainal Abidin M, Ramlan M, Krishnan N, Adzhar A, Abdullah N, Hamidah N HM, Norsuhanna MM, Hashim SN (2012). Isolation and molecular characterization of Brucella melitensis from seropositive goats in Peninsula Malaysia. Trop. Biomed. 29(4):513-8. PMID: 23202595.
Bandara A.B., Contreras A., Contreras-Rodriguez A., Martins A.M., Dobrean V., Poff-Reichow S., Rajasekaran P., Sriranganathan N., Schurig G.G., Boyle S.M. (2007). Brucella suis urease encoded by ure1 but not ure2 is necessary for intestinal infection of BALB/c mice. BMC Microbiol. 7:57. https://doi.org/10.1186/1471-2180-7-57.
Bozukluhan K, Merhan O, Celebi O, Buyuk F, Ogun M, Gokce G. (2018). Levels of certain biochemical and oxidative stress parameters in cattle with Brucellosis. Journal of the Hellenic Vet. Med. Societ., 68(3): 285–290. https://doi.org/10.12681/jhvms.15470
Cai R, Zhou W, Jiang L, Jiang Y, Su T, Zhang C, Zhou W, Ning G, Wang W (2020). Association between thyroid function and serum cortisol in cortisol-producing adenoma patients. Endocrine. 69(1):196-203. https://doi.org/10.1007/s12020-020-02278-5. Epub 2020 Apr 14. PMID: 32291738.
Delrue RS, Lestrate L, Tibor A, Letesson JJ, Bolle XD (2004). Brucella pathogenesis, genes identified from random large-scale screens. FEMS Microbiol. Lett. 231:1-12
Demirdağ, K, Ozden M, Kalkan A, Godekmerdan A, Kilic S (2003). Serum cytokine levels in patients with acute brucellosis and their relation to the traditional inflammatory markers. FEMS Immunol. Med. Microbiol. 39: 149-53.
den Hartigh A.B., Rolan H.G., de Jong M.F., Tsolis R.M. (2008). VirB3-VirB6 and VirB8- VirB11, but not VirB7, are essential for mediating persistence Brucella in the reticuloendothelial system. J. Bacteriol. 190: 4427-4436.
Derakhshandeh A., Firouzi R., Goudarztalejerd A. (2013). Detection of virulence genes (bvfA, virB and ure) in Brucella melitensis isolated from aborted fetuses of sheep and goats. Iranian J. Microbiol., 5(4): 402.
El-Boshy M., Abbas H, El-Khodery S, Osman S. (2009). Cytokine response and clinicopathological findings in Brucella infected camels (Camelus dromedarius). Vet. Med., 54: (1): 25–32.
Esmaeel J.R. (2019). Molecular identification of Brucella abortus and its virulence genes (bvfA, virB, and ure) in infected humans and cattle from Al-Diwaniyah province, Iraq. Ann. Trop. Med. Health., 22: 21-32.
Ewalt D. R., Packer R. A., Harris S. K. (1983). An improved selective medium for isolating Brucella sp. from bovine milk, p. 577–589 In Proceedings of the Third International Symposium of the World Association of Veterinary Laboratory Diagnosticians College of Veterinary Medicine, Iowa State University, Ames, IA [Google Scholar]
Felix JS, Ana MC, Asuncion S, Juan MG (2010). Brucella abortus ure2 region contains anacid-activated urea transporter and a nickel transport system. BMC Microbiol. 10:107.
Fernandes D.M., Baldwin C.L. (1995). Interleukin-10 downregulates protective immunity to Brucella abortus. Infect. Immun., 63: 1130–1133.
Gandara B., Merino A.L., Rogel M.A., Martinez-Romero E. (2001). Limited genetic diversity of Brucella spp. J. Clin. Microbiol. 39: 235-240.
Ganz T, Nemeth E (2015). Iron homeostasis in host defence and inflammation. Nat Rev Immunol. 2015 Aug;15(8):500-10. doi: 10.1038/nri3863. Epub 2015 Jul 10. PMID: 26160612; PMCID: PMC4801113.
Ganz T (2018). Iron and infection. Int. J. Hematol. 107:7–15 https://doi.org/10.1007/s12185-017-2366-2.
Gentilini MV, Velásquez LN, Barrionuevo P, Arriola Benitez PC, Giambartolomei GH, Delpino MV (2015). Adrenal steroids modulate the immune response during Brucella abortus infection by a mechanism that depends on the regulation of cytokine production. Infect Immun. 83(5):1973-82. doi: 10.1128/IAI.03090-14. Epub 2015 Mar 2. PMID: 25733519; PMCID: PMC4399066.
Gentilini MV, Giambartolomei GH, Delpino MV (2019). Adrenal Steroids Modulate Fibroblast-Like Synoviocytes Response During B. abortus Infection. Front. Endocrinol. 10:722. https://doi.org/10.3389/fendo.2019.00722.
Gupta V.K., Shivasharanappa N., Vijay Kumar, Ashok Kumar. (2014). Diagnostic evaluation of serological assays and different gene-based PCR for detection of Brucella melitensis in goat. Small Rumin. Res. 117(1):94-102.
Hamdy M, Khoudair RM, Ibrahim MA, Shalby NA, El-Shafei, AA, Abo El-Maaty AM, Abd El Hameed AR and, Mohamed RH. Acute Phase Proteins (APP) and Minerals Levels Associated with Brucellosis in CamelsAcute Phase Proteins (APP) and Minerals Levels Associated with Brucellosis in Camels. Anim. Health Res. J. Vol. 7, No. 4, November 2019 pp. 732-741.
Hashem MA, El-Mandrawy SA, El-Diasty MM, Zidan AZ (2020). Hematological, Biochemical and Immunological Studies on Brucellosis in Cows and Ewes in Dakahlia and Damietta Governorates, Egypt. Zag Vet. J., 48 (1): 23-35, March 2020.
Herrera E, Rivera A, Palomares EG, Hernández-Castro R, Díaz-Aparicio E (2011). Isolation of Brucella melitensis from a RB51-vaccinated seronegative goat. Trop. Anim. Health Prod. 43(6):1069-70. https://doi.org/10.1007/s11250-011-9822-4. Epub 2011 Apr 1. PMID: 21455694.
Kataria N, Kataria A, Maan R, Gahlot AK (2010). Evaluation of oxidative stress in brucella infected cows. J. Stress Physiol. Biochem., 6 (2): 19-25 ISSN 1997-0838.
Lavigne JP, Patey G, Sangari FJ, Bourg G, Ramuz M (2005). O’Callaghan D, et al. Identification of a new virulence factor, BvfA, in Brucella suis. Infect. Immun. 73:5524-5529.
Luna-Martinez J.E., C. Mejia-Teran, (2002). Brucellosis in Mexico: Current status and trends. Vet. Microbiol., 90: 19-30.
Mahboub HD, Helal MA, Abd Eldaim MA, Abd El-Razek EM, Elsify AM (2013). Seroprevalence of Abortion Causing Agents in Egyptian Sheep and Goat Breeds and Their Effects on the Animal’s Performance. J. Agricult. Sci. 5(9) ISSN 1916-9752 E-ISSN 1916-9760. Published by Canadian Center of Science and Education
Merhan O, Bozukluhan K, Kuru M, Büyük F, Özden Ö, Kükürt A (2017). Investigation of oxidative stress Index and lipid profile in cattle with Brucellosis. Kafkas Univ Vet Fak Derg, 23 (6): 933-937. https://dx.doi.org/10.9775/kvfd.2017.18004.
Mohamed NS, Stephen MB, Nammalwar S (2008). Brucella: Apathogen without classic virulence genes. Vet. Microbiol. 129: 1-14
Morais JBS, Severo JS, Beserra JB, de Oiveira ARS, Cruz KJC, de Sousa Melo SR, do Nascimento GVR, de Macedo GFS, do Nascimento Marreiro D (2019). Association Between Cortisol, Insulin Resistance and Zinc in Obesity: a Mini-Review. Biol. Trace Elem. Res. Oct;191(2):323-330. https://dx.doi.org/10.1007/s12011-018-1629-y. Epub 2019 Jan 7. PMID: 30617901.
Murphy E.A., Sathiyaseelan J., Parent M.A., Zou B., Baldwin C.L. (2001). Interferon-gamma is crucial for surviving a Brucella abortus infection in both resistant C57BL/6 and susceptible BALB/c mice. Immunology., 103: 511–518.
Nada A.R., S.I. Shalaby, W.M. Ahmed (1992). Minerals and trace elements blood profile of Brucella infected, pregnant and non-pregnant animals. Egypt J. Comp. Pathol. Clin. Pathol., 1.5: 2-2.
Nayel M, Ibrahim R, Zaghawa A, Abd El- Maksoud A, Elsify A, Salama A , Kamr A, Mousa W, Eldesoukey I, Shaker A (2020). Seroprevalence and Associated Risk Factors of Brucellosis among Sheep, Goats and Camels in North Western Coastal Area of Egypt. J. Curr. Vet. Res., 2(1): 25-34. https://dx.doi.org/10.21608/JCVR.2020.90220.
Nicklin M.J., Hughes D.E., Barton J.L., Ure J.M., Duff G.W. (2000). Arterial inflammation in mice lacking the interleukin 1 receptor antagonist gene. J. Experimen. Med., 191: 303–312.
(OIE). (2009) Office International des Epizooties Bovine Brucellosis; caprine and ovine brucellosis and porcine brucellosis. In: World assembly of delegates of the OIE chapter 2.4.3. Paris: OIE Terrestrial Manual; p. 1–35.
OIE. (2016) Brucellosis (Brucella abortus, B. melitensis and B. suis), Chapter 2.1.4. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. Office International des Epizooties, Paris.
OIE (2018). World Organisation for Animal Health. Brucellosis (infection with B. abortus, B. melitensis and B. suis). In: OIE. Manual of diagnostic tests and vaccines for terrestrial animals. Paris;. p. 355-98.
Saavedra MJ, Fernandes C, Queiroga C (2019). Laboratory diagnosis of brucellosis. In: Simomes JCC, Saavedra MJ,Hunter PA, editors. Brucellosis in goats and sheep: An endemic and re-emerging old zoonosis in the 21 st century. New York: Nova Science Publishers, Inc.; 2019. p. 15180.
Saeedzadeh A., Sharifiyazdi H., Firouzi R. (2012) Molecular. characterization of Brucella melitensis Rev.1 strain in aborted sheep and goats in Iran. Comp. Clin. Pathol. 22: 409-412.
Salem A, Ayady E , Amran Y , Alhasanat M (2020). High Serum Ferritin as a Sensitive Biomarker for Diagnosing, Follow up and Management Responsiveness for Brucellosis. J. Med. Sci. Clin. Res. 8(7): 99-102. https://dx.doi.org/10.18535/jmscr/v8i7.20
Samadi A, Ababneh MMK, Giadinis ND, Lafi SQ (2010). Ovine and caprine Brucellosis (B. melitensis) in aborted animals in Jordanian sheep and goat flocks. Vet. Med. Int. https://doi.org/10.4061/2010_458_695.
Saunders B.M., Liu Z., Zhan Y., Cheers C. (1993). Interleukin-6 production during chronic experimental infection. Immunol.Cell Biol., 71: 275–280.
Saunders B.M., Frank A.A., Orme I.M., Cooper A.M. (2000). Interleukin-6 induces early gamma interferon production in the infected lung but is not required for generation of specific immunity to Mycobacterium tuberculosis infection. Infect. Immun., 68, 3322–3326.
Seder R.A., Hill A.V. (2000). Vaccines against intracellular infections requiring cellular immunity. Nature, 17, 793–798.
Seleem, M.N., Stephen, M.B. and Nammalwar, S.2008. Brucella: A pathogen without classic virulence genes. Vet. Microbiol. 129, 1-14.
Shakerian A, Deo P, Rahimi E, Shahjavan A, Khamesipour F (2016). Molecular detection of Brucella melitensis in sheep and goat milk in Iran. Trop. J. Pharmaceut. Res. 15 (5): 913-918. http://www.tjpr.org http://dx.doi.org/10.4314/tjpr.v15i5.3.
Shalby NA, Abo El-maaty AM, Ali AH, El gioushy M (2020). Acute phase biomarkers, oxidants, and trace minerals of mobile sheep flocks naturally infected with brucellosis. Bulgarian J. Vet. Med., ISSN 1311-1477; https://dx.doi.org/10.15547/bjvm.2020-0002.
Sharifiyazdia H, Nazifi S, Nikseresht K, Shahriari R (2012). Evaluation of Serum Amyloid A and Haptoglobin in Dairy Cows Naturally Infected with Brucellosis. J. Bacteriol. Parasitol 3:157. https://dx.doi.org/10.4172/2155-9597.1000157.
Stratakis CA (2006). Cortisol and growth hormone: clinical implications of a complex, dynamic relationship. Pediatr Endocrinol. Rev. Apr;3 Suppl 2:333-8. PMID: 16675933.
Tekle M., Legesse M., Edao B.M. et al. (2019). Isolation and identification of Brucella melitensis using bacteriological and molecular tools from aborted goats in the Afar region of north-eastern Ethiopia. BMC Microbiol. 19: 108 (2019). https://doi.org/10.1186/s12866-019-1474-y
Uluğ M, Can-Uluğ N, Selek Ş (2010). Levels of Acute Phase Reactants in Patients with Acute Brucellosis. Klimik Dergisi 23(2): 48-50.
To share on other social networks, click on any share button. What are these?





