Urbanization Favors Some Over Others: Bird Diversity Pattern in the Suburban-Urban Gradient of Butwal City, Central Lowland of Nepal
Urbanization Favors Some Over Others: Bird Diversity Pattern in the Suburban-Urban Gradient of Butwal City, Central Lowland of Nepal
Bibek Aryal, Naresh Pandey, Laxman Khanal*
Central Department of Zoology, Institute of Science and Technology, Tribhuvan University, Kathmandu, Nepal.
Abstract | Urbanization results in habitat modification causing alteration in the assemblage of biological diversity. Bird diversity serves as a good ecological indicator; hence, this study examined the effects of urbanization on bird species richness and diversity in Butwal Sub-Metropolitan City, one of the rapidly expanding cities in the central lowland Nepal. Bird surveys were conducted during the winter and post-monsoon seasons of 2020 along eight transects each of 2 km length by positioning the point count stations at every 200 m interval. The associations of bird richness and abundance with NDVI as the proxy of productivity and human footprint data as the proxy of human disturbance were established. A total of 3,297 birds belonging to 69 species from 33 families in 14 orders were observed. The order Passeriformes was the most dominant order, while insectivore was the most dominant feeding guild. The suburban sites had higher bird diversity and richness than urban sites; whereas, the abundance of urban exploiter birds was higher in the urban area. Vegetation cover showed a positive association with the bird species richness. Conversely, human disturbance showed negative effects. In an urban setting, green spaces and parks shelter more diverse species. Thus, cities should focus on developing green city concept simultaneously with other developmental projects.
Novelty Statement | This is a comprehensive study using rigorous bird surveys, and analyzing the bird richness and abundance with high-resolution data on vegetation and anthropogenic pressure. Our findings could be instrumental in the proper management of urban biodiversity.
Article History
Received: September 14, 2021
Revised: November 18, 2021
Accepted: December 3, 2021
Published: December 20, 2021
Authors’ Contributions
BA and LK conceptualized the study. BA and NP performed the fieldworks, analyzed data and prepared the manuscript. LK supervised the research and finalized the manuscript. All authors approved the manuscript for submission.
Keywords
Abundance, Bird diversity, Human footprint, Species richness, Urban exploiters
Corresponding author: Laxman Khanal
lkhanal@cdztu.edu.np
To cite this article: Aryal, B., Pandey, N. and Khanal, L., 2021. Urbanization favors some over others: Bird diversity pattern in the suburban-urban gradient of Butwal City, Central Lowland of Nepal. Punjab Univ. J. Zool., 36(2): 175-184. https://dx.doi.org/10.17582/journal.pujz/2021.36.2.175.184
Introduction
Global human population shift from rural to urban areas is increasing (Anderies et al., 2007) possessing threats to biodiversity conservation (Goddard et al., 2010). This leads to increased intensity of urbanization resulting in habitat fragmentation or alteration (Marzluff and Ewing, 2008) and causing loss of biodiversity (Callaghan et al., 2018). Hence, such areas require specific attention to biodiversity conservation (Blair and Johnson, 2008). Urbanization ranks as the most common factor causing species endangerment and extinction second to interactions with invasive species (Czech et al., 2000). However, the impact of urbanization is not the same to all the taxa as it favors abundance of some urban exploiter species but reduces the richness and diversity (Marzluff, 2001).
Birds serve as good ecological indicators showing sensitiveness to environmental degradation (Clergeau et al., 2001; Lin et al., 2008). Habitat degradation is a detrimental consequence of urbanization affecting bird communities (McKinney, 2006). Bird community structure and composition changes with urbanization gradients as species richness decreases with urbanization, while abundance and density of some of the species increases (Chace and Walsh, 2006; Sandström et al., 2006). Urban adapted species, commonly known as urban exploiters, tend to utilize a wide range of resources found in urban sites because of their generalist behavior and can thrive well even in altered habitat (Menon and Rangaswamy, 2016; Rodrigues et al., 2018; Palacio, 2020). The suburban areas with moderate levels of disturbances provide heterogeneity in habitats and support higher bird diversity (Chace and Walsh, 2006). Habitat heterogeneity beyond the threshold also results in habitat fragmentation and in turn supports less biodiversity. Therefore, it is important to know compositional and configurational heterogeneity of a habitat which affects the diversity that it can support.
Built area and building density is one of the main factors altering natural habitats and negatively affecting bird richness in urban areas. Other anthropogenic and ecological factors like noise, human disturbances, predators, etc. escalate the effects (MacGregor-Fors and Schondube, 2011; Menon and Rangaswamy, 2016; Rodrigues et al., 2018). Conversely, vegetative components, mainly trees, positively influence bird diversity and richness (Fontana et al., 2011; Menon et al., 2015). With increasing urbanization, the natural habitat is degraded, so, urban green spaces in cities are major factors for determining diversity and richness (Dale, 2018) and a good indicator for bird heterogeneity (Callaghan et al., 2018). Urban surroundings alter the natural flow of food (Fuller et al., 2012), influence feeding guilds, favor habitat generalists like omnivores, rather than food and habitat specialists (Evans et al., 2011; Silva et al., 2016; Altaf et al., 2018). All bird species are not equally and negatively influenced by the urbanization as it favors abundance of some selected species which can exploit the urban environment. For example, bird species that nest in cavities/buildings and have wider dietary adaptation may have an advantage in living and settling in urban areas (Jokimaki et al., 2016).
Butwal City, a rapidly expanding metropolis in central lowland Nepal, is the current capital of Lumbini Province. It is pressurized by population surge and anthropogenic development increasing the intensity of urbanization. Most importantly, Butwal City lies along the north-south migration route of trans-Himalayan birds along the Kaligandaki River Valley (Prins and Namgail, 2017; Neupane et al., 2020). Hence, it is important to know the bird species composition and effects of that rampant urbanization poses to the bird in Butwal City. Environmental variables like vegetation cover, human footprint index, and land use land cover data could be used to analyze the habitat heterogeneity and assess their influence on the bird diversity. Additionally, climatic variables like temperature and precipitation and their seasonal variations may also have their effects in bird community structure. The intermediate disturbance hypothesis posits that moderate level of habitat heterogeneity supports higher diversity. Hence, we aimed to examine bird community structure at different level of human disturbance in a rapidly urbanizing city. This study assessed the pattern of bird species richness and abundance along the urbanization gradient (measured in terms of vegetation cover and human footprint index) in Butwal City, central lowland Nepal during winter and post-monsoon seasons of 2020.
Materials and Methods
Study area
Butwal Sub-Metropolitan City lies in Rupandehi District of Lumbini Province at the central lowland of Nepal. It lies between 83.36° to 83.50°E and 27.61° to 27.74°N (Figure 1) and covers an area of 101.61 square km (Thapa and Poudel, 2018). This city stands beside the bank of the Tinau River at the northern edge of the Terai plain below the Siwalik Hills. The elevation of the city ranges from around 100 m in the south-western lowland to 1,229 m in the northern hills (Thapa and Poudel, 2018). The climate of this region is tropical type. The summer is hotter and dry with temperature rising up to 45°C whereas the winter is cold with temperature declining below to 8°C. Major precipitation takes place during the monsoon that usually begins in June. About 65% of the surface area of Butwal Metropolitan City is covered by the forest (Thapa and Poudel, 2018). Dominant vegetation in urban and suburban areas of Butwal City include Ficus religiosa, Ficus benghalensis, Azadirachta indica, Bombax ceiba, Aegle marmelos, Dalbergia sissoo, Garuga pinnata, Saraca asoca, Neolamarckia cadamba, Eucalyptus camaldulensis, Melia azedarach, Ficus benjamina, Premna barbata, Tamarindus indica, Ficus racemose, Morus alba, etc.
Study design and bird survey
Urban area was defined as the area with high population densities and higher built surfaces (Pickett et al., 2011). Considering this, Butwal City was categorized into two levels of urbanization i.e. urban and suburban areas. Urban and suburban sites were illustrated through supervised classification Lansat-8 image of the city on the basis of land cover (Figure 1c) combining band images (2, 3, 4). Landsat-8 imageries were layer stacked and Butwal City area was extracted. Next, the images were then classified by object-based image classification by defining training areas or samples such as built-up area, farmland, etc. that led to development of signature file. At last, interactive supervised and maximum likelihood classification was applied and post-classification processing was done. Urban and suburban demarcation was done on the basis of Normalized Difference Vegetation Index (NDVI) data. A median value of the NDVI data (0.143) was calculated after processing Landsat-8 OLI/TIRS image downloaded from the US Geological Survey (https://espa.cr.usgs.gov) and through visual inspection. An area with greater NDVI than the median value was regarded as the suburban area and that with lower NDVI was regarded as urban area.
Point count method has been described to be more efficient method for urban habitat, requiring less effort per count, having less potential for error than transect count (Mortimer and Clark, 2013) and is better method for population with high density like that of urban exploiter birds (Gibbons and Gregory, 2006). Eight transects (four in urban areas and four in suburban areas), each of 2 km length with point station at every 200 m (Filloy et al., 2019) were deployed representing the study area. It resulted a total of 88 point-count stations. The urban transects run through major residential areas, fewer trees and green spaces and creeks while suburban transects run through dispersed residential areas, farmlands and rivers.
The bird survey was conducted on 01–18 February and 06–23 October 2020, from 7 AM to 11 AM. Birds observed in the radius of 50 m from the count station were noted for 10 minutes. Distance between the observer and the birds was measured using the range-finder (Leica Range master CRF 2400-R). The birds were identified using binoculars and field guide book (Grimmett et al., 2016). Birds were categorized to their feeding guilds following Katuwal et al. (2018) and Pandey et al. (2021).
Habitat characteristics and environmental variables
The NDVI data obtained from processing Landsat-8 OLI/TIRS images for both the study seasons were used as the proxy of vegetation cover or the productivity. The human footprint index (Venter et al., 2018) was also used as the proxy of human disturbance. Both the NDVI values and footprint index were extracted for each point stations. The average monthly temperature and average monthly precipitation for respective seasons were also extracted for point count stations from the WorldClim database (https://www.worldclim.org/bioclim). Extraction and processing of images and environmental variables were done on ArcGIS 10.2.
Data analysis
Diversity indices (Shannon-Weiner index and Simpson’s index) were calculated for the bird diversity in both the urban and suburban areas as follows:
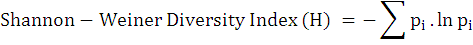
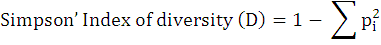
Where, pi is the proportion (n/N) of a species obtained as individuals of one particular species (n) divided by the total number of individuals of all species (N).
The relative abundance (RA) of bird species was calculated by dividing the number of records of each species by the total number of records of all species in both urban and suburban areas.
Difference in species richness between the urban and suburban areas was tested for significance by the t-test. Regression analysis was performed to establish relationship of species richness with NDVI and human footprint index for each season. The effect of NDVI and human footprint on species richness and abundance were modelled singularly. General Linear Model (GLM) was used to assess association of bird richness/abundance with environmental variables (NDVI, temperature and precipitation). These statistical analyses were done on R program (R Core Team, 2013) using vegan package (Oksanen et al., 2019).
Results and Discussion
A total of 3,297 birds of 69 species were observed from 33 families under 14 orders. Among the 69 species, 60 were residents and 09 were migrants (Table 1). The species count was higher in winter (n=56) than in post-monsoon (n=52). In both seasons, suburban area recorded higher species richness than the urban area (Table 2). The species accumulation curve revealed a linear relationship with the possibility of finding more species with increasing sampling efforts (Figure 2).
Passeriformes was the most dominant order represented by 38 species followed by Pelecaniformes (n=6) and Psittaciformes (n=4) (Table 1). House sparrow (Passer domesticus) was the most abundant species followed by House crow (Corvus splendens) and Common pigeon (Columba livia) (Table 1). Insectivore was the most
Table 1: Checklist of birds recorded in Butwal Sub-Metropolitan City, Nepal with their residential status, feeding guild, relative abundance and IUCN Red List status.
|
English name |
Scientific name |
Order |
Family |
Status |
Feeding guild |
RA (%) |
IU-CN Sta-tus |
|
|
Urban |
Sub-urban |
|||||||
|
Black Kite |
Milvus migrans |
Accipitriformes |
Accipitridae |
R |
Carnivorous |
0.80 |
0.72 |
LC |
|
House Swift |
Apus affinis |
Apodiformes |
Apodidae |
R |
Insectivorous |
2.08 |
- |
LC |
|
Common Hoopoe |
Upupa epops |
Bucerotiformes |
Upupidae |
R |
Insectivorous |
0.06 |
0.06 |
LC |
|
Indian Grey Hornbill |
Ocyceros birostris |
Bucerotiformes |
Bucerotidae |
R |
Omnivorous |
0.31 |
0.24 |
LC |
|
Common Sandpiper |
Actitis hypoleucos |
Charadriiformes |
Scolopacidae |
WM |
Insectivorous |
0.06 |
0.44 |
LC |
|
Little-ringed Plover |
Charadrius dubius |
Charadriiformes |
Charadridae |
R |
Carnivorous |
- |
0.12 |
LC |
|
Red-wattled Lapwing |
Vanellus indicus |
Charadriiformes |
Charadridae |
R |
Carnivorous |
- |
0.12 |
LC |
|
Asian Openbill |
Anastomus oscitans |
Ciconiiformes |
Ciconiidae |
R |
Carnivorous |
- |
0.06 |
LC |
|
Asian Woolly-neck |
Ciconia episcopus |
Ciconiiformes |
Ciconiidae |
R |
Carnivorous |
- |
0.18 |
NT |
|
Lesser Adjutant |
Leptoptilos javanicus |
Ciconiiformes |
Ciconiidae |
R |
Carnivorous |
- |
0.12 |
VU |
|
Common Pigeon |
Columba livia |
Columbiformes |
Columbidae |
R |
Granivorous |
15.8 |
4.63 |
LC |
|
Spotted Dove |
Stigmatopelia chinensis |
Columbiformes |
Columbidae |
R |
Granivorous |
0.67 |
2.82 |
LC |
|
Yellow-footed pigeon |
Treron phoenicoptera |
Columbiformes |
Columbidae |
R |
Frugivorous |
0.73 |
- |
LC |
|
White-throated Kingfisher |
Halcyon smyrnensis |
Coraciiformes |
Alcedinidae |
R |
Carnivorous |
- |
0.36 |
LC |
|
Green Bee eater |
Merops orientalis |
Coraciiformes |
Meropidae |
R |
Insectivorous |
- |
0.12 |
LC |
|
Greater Coucal |
Centropus sinensis |
Cuculiformes |
Cuculidae |
R |
Carnivorous |
0.06 |
0.36 |
LC |
|
Asian Koel |
Eudynamys scolopaceus |
Cuculiformes |
Cuculidae |
SM |
Omnivorous |
- |
0.06 |
LC |
|
Common Hawk Cuckoo |
Hierococcyx varius |
Cuculiformes |
Cuculidae |
R |
Insectivorous |
0.06 |
0.06 |
LC |
|
Ashy Prinia |
Prinia socialis |
Passeriformes |
Cisticolidae |
R |
Insectivorous |
- |
0.66 |
LC |
|
Black Drongo |
Dicrurus macrocercus |
Passeriformes |
Dicruridae |
R |
Insectivorous |
0.37 |
3.13 |
LC |
|
Black-hooded Oriole |
Oriolus xanthornus |
Passeriformes |
Oriolidae |
R |
Omnivorous |
0.06 |
0.24 |
LC |
|
Brown Rockchat |
Cercomela fusca |
Passeriformes |
Muscicapidae |
R |
Insectivorous |
0.92 |
0.12 |
LC |
|
Oriental Magpie Robin |
Copsychus saularis |
Passeriformes |
Muscicapidae |
R |
Insectivorous |
0.43 |
0.90 |
LC |
|
Verditer Flycatcher |
Eumyias thalassinus |
Passeriformes |
Muscicapidae |
WM |
Insectivorous |
- |
0.06 |
LC |
|
Red-breasted Flycatcher |
Ficedula parva |
Passeriformes |
Muscicapidae |
WM |
Insectivorous |
- |
0.06 |
DD |
|
Black Redstart |
Phoenicurus ochruros |
Passeriformes |
Muscicapidae |
WM |
Insectivorous |
0.06 |
0.18 |
LC |
|
Common Stonechat |
Saxicola torquatus |
Passeriformes |
Muscicapidae |
R |
Insectivorous |
- |
0.18 |
LC |
|
Black-throated Thrush |
Turdus atrogularis |
Passeriformes |
Turdidae |
WM |
Omnivorous |
- |
0.42 |
LC |
|
Jungle Myna |
Acridotheres fuscus |
Passeriformes |
Sturnidae |
R |
Omnivorous |
0.31 |
0.54 |
LC |
|
Common Myna |
Acridotheres tristis |
Passeriformes |
Sturnidae |
R |
Omnivorous |
5.14 |
4.81 |
LC |
|
Asian Pied Starling |
Gracupica contra |
Passeriformes |
Sturnidae |
R |
Omnivorous |
1.16 |
3.73 |
LC |
|
Brahminy Starling |
Sturnia pagodarum |
Passeriformes |
Sturnidae |
R |
Omnivirous |
- |
0.18 |
LC |
|
Brown Shrike |
Lanius cristatus |
Passeriformes |
Laniidae |
WM |
Insectivorous |
- |
0.36 |
LC |
|
Common Tailorbird |
Orthotomus sutorius |
Passeriformes |
Cisticolidae |
R |
Insectivorous |
0.55 |
2.88 |
LC |
|
Greater Racket-tailed Drongo |
Dicrurus paradiseus |
Passeriformes |
Dicruridae |
R |
Insectivorous |
- |
0.24 |
LC |
|
Grey Wagtail |
Motacilla cinerea |
Passeriformes |
Motacillidae |
WM |
Insectivorous |
0.37 |
0.24 |
LC |
|
Indian Jungle Crow |
Corvus culminatus |
Passeriformes |
Corvidae |
R |
Carnivorous |
0.18 |
1.98 |
LC |
|
House Crow |
Corvus splendens |
Passeriformes |
Corvidae |
R |
Omnivorous |
16.23 |
4.27 |
LC |
|
Rufous Treepie |
Dendrocitta vagabunda |
Passeriformes |
Corvidae |
R |
Frugivorous |
0.37 |
0.36 |
LC |
|
House Sparrow |
Passer domesticus |
Passeriformes |
Passeridae |
R |
Granivorous |
41.95 |
32.21 |
LC |
|
Jungle Babbler |
Turdoides striata |
Passeriformes |
Leio-thrichidae |
R |
Omnivorous |
0.61 |
0.84 |
LC |
|
Long-tailed Shrike |
Lanius schach |
Passeriformes |
Laniidae |
R |
Carnivorous |
0.67 |
1.32 |
LC |
|
Paddy-field Pipit |
Anthus rufulus |
Passeriformes |
Motacillidae |
R |
Insectivorous |
- |
1.08 |
LC |
|
Plain Prinia |
Prinia inornata |
Passeriformes |
Cisticolidae |
R |
Insectivorous |
- |
0.12 |
LC |
|
Table continue on next page................ |
||||||||
|
English name |
Scientific name |
Order |
Family |
Status |
Feeding guild |
RA (%) |
IU-CN Sta-tus |
|
|
Urban |
Sub-urban |
|||||||
|
Purple Sunbird |
Cinnyris asiaticus |
Passeriformes |
Nectariniidae |
R |
Nectarivorous |
0.06 |
0.12 |
LC |
|
Red Avadavat |
Amandava amandava |
Passeriformes |
Estrildidae |
R |
Granivorous |
- |
0.06 |
LC |
|
Red-vented Bulbul |
Pycnonotus cafer |
Passeriformes |
Pycnonotidae |
R |
Omnivorous |
2.45 |
3.49 |
LC |
|
Red-whiskered bulbul |
Pycnonotus jocosus |
Passeriformes |
Pycnonotidae |
R |
Omnivorous |
3.8 |
9.50 |
LC |
|
Scaly-breasted Munia |
Lonchura punctulata |
Passeriformes |
Estrildidae |
R |
Granivorous |
0.18 |
2.94 |
LC |
|
Scarlet Minivet |
Pericrocotus flammeus |
Passeriformes |
Campe-phagidae |
R |
Insectivorous |
0.37 |
- |
LC |
|
Hair-crested Drongo |
Dicrurus hottentottus |
Passeriformes |
Dicruridae |
R |
Insectivorous |
0.37 |
- |
LC |
|
Tickell's Leaf Warbler |
Phylloscopus affinis |
Passeriformes |
Phyllo-scopidae |
R |
Insectivorous |
- |
0.06 |
LC |
|
White-bellied Drongo |
Dicrurus caerulescens |
Passeriformes |
Dicruridae |
R |
Insectivorous |
0.06 |
0.36 |
LC |
|
White Wagtail |
Motacilla alba |
Passeriformes |
Motacillidae |
WM |
Insectivorous |
0.31 |
1.2 |
LC |
|
White-browed Wagtail |
Motacilla madera-spatensis |
Passeriformes |
Motacillidae |
R |
Insectivorous |
0.73 |
0.72 |
LC |
|
Intermediate Egret |
Ardea intermedia |
Pelecaniformes |
Ardeidae |
R |
Carnivorous |
- |
0.06 |
LC |
|
Indian Pond Heron |
Ardeola grayii |
Pelecaniformes |
Ardeidae |
R |
Carnivorous |
0.37 |
1.20 |
LC |
|
Cattle Egret |
Bubulcus ibis |
Pelecaniformes |
Ardeidae |
R |
Carnivorous |
- |
3.55 |
LC |
|
Little Egret |
Egretta garzetta |
Pelecaniformes |
Ardeidae |
R |
Carnivorous |
- |
0.06 |
LC |
|
Red-napped Ibis |
Pseudibis papillosa |
Pelecaniformes |
Thres-kiornithidae |
R |
Omnivorous |
- |
0.06 |
LC |
|
Coppersmith Barbet |
Megalaima haemacephala |
Piciformes |
Megalaimidae |
R |
Frugivorous |
0.12 |
- |
LC |
|
Brown-headed Barbet |
Megalaima zeylanica |
Piciformes |
Megalaimidae |
R |
Frugivorous |
- |
0.12 |
LC |
|
Plum-headed Parakeet |
Psittacula cyanocephala |
Psittaciformes |
Psittaculidae |
R |
Herbivorous |
- |
0.66 |
LC |
|
Alexandrine Parakeet |
Psittacula eupatria |
Psittaciformes |
Psittaculidae |
R |
Herviborous |
1.04 |
- |
NT |
|
Slaty-headed Parakeet |
Psittacula himalayana |
Psittaciformes |
Psittaculidae |
R |
Frugivorous |
- |
0.90 |
LC |
|
Rose-ringed Parakeet |
Psittacula krameri |
Psittaciformes |
Psittaculidae |
R |
herbivorous |
- |
0.78 |
LC |
|
Spotted Owlet |
Athene brama |
Strigiformes |
Strigidae |
R |
Carnivorous |
- |
0.06 |
LC |
|
Little Cormorant |
Microcarbo niger |
Suliformes |
Phalacroco-racidae |
R |
Carnivorous |
- |
0.12 |
LC |
dominant feeding guild in both seasons followed by omnivores whereas, herbivores and nectarivores were least dominant (Figure 3). Similarly, insectivores were also the dominant guild in both urban and suburban areas in each season.
Species richness was lower in urban area (n=39) than the suburban area (n=63) (Table 2). Significant difference was observed in species richness between urban and suburban areas for winter (t=3.40, p<0.05) as well as the post-monsoon (t=5.12, p<0.05). The overall abundance of birds in two areas did not differ significantly, however, some urban exploiter species like House sparrow (Passer domesticus), House crow (Corvus splendens) and Common pigeon (Columba livia) had much higher abundance in the urban habitat (Table 1). The evenness indices were lower in urban areas for both the seasons. Diversity indices were higher in suburban habitats than in urban areas irrespective of the seasons (Table 3). Species richness, abundance, as well as both the Simpson’s and Shannon-Weiner diversity indices were higher in winter than that of post-monsoon.
Table 2: Diversity in urban and sub-urban areas for both the study seasons.
|
Measures |
Winter |
Post-monsoon |
Overall |
|||
|
Urban |
Sub urban |
Urban |
Sub urban |
Urban |
Sub urban |
|
|
Richness |
36 |
51 |
24 |
49 |
39 |
63 |
|
Simpson’s 1-D |
0.6 |
0.71 |
0.57 |
0.68 |
0.66 |
0.77 |
|
Shannon’s H |
1.16 |
1.51 |
1.04 |
1.42 |
1.39 |
1.88 |
|
Abundance |
869 |
935 |
764 |
729 |
1633 |
1664 |
|
Evenness |
0.74 |
0.77 |
0.81 |
0.79 |
0.677 |
0.767 |
The regression analysis between NDVI and overall species richness for respective seasons showed statistically significant positive association (p<0.05) (Figures 4a, b). There was negative association between species richness and human foot print index for both seasons (p<0.05) (Figures 4c, d). Among the environmental variables examined, the GLM showed their differential relations with bird richness for the two seasons. For the winter, the richness varied significantly in urban areas with the NDVI and precipitation; whereas in suburban areas it varied with the temperature. For the post-monsoon, only the temperature in suburban area appeared to shape the bird richness. Bird abundance in suburban area varied with temperature in winter and with both temperature and precipitation in post-monsoon season.
We assessed the bird community structure for winter and post-monsoon seasons in Butwal Sub-Metropolitan City at central lowland Nepal and tested the effects of urbanization on bird assemblage along the rural-urban gradient. The findings followed the rural-urban gradient trend as illustrated by Chace and Walsh (2006) and Filloy et al., (2019). Higher diversity and richness was recorded in the suburban area than in the urban area, which supports the intermediate disturbance theory as depicted in multiple other studies (Tratalos et al., 2007; Katuwal et al., 2018; Gillings, 2019).
Table 3: Summary of the GLM between environmental variables and species richness and abundance for winter and post monsoon seasons.
|
Season |
Area |
Variable |
Avian richness |
Avian abundance |
||||
|
Slope (a) |
Intercept (b) |
P value |
Slope (a) |
Intercept (b) |
P value |
|||
|
Winter |
Urban |
NDVI |
19.439 |
2.748 |
0.024 |
72.87 |
12.076 |
0.344 |
|
Precipitation |
-0.725 |
9.329 |
0.018 |
-2.464 |
35.15 |
0.374 |
||
|
Temperature |
2.181 |
-34.616 |
0.078 |
10.666 |
-172.99 |
0.325 |
||
|
Sub-urban |
NDVI |
15.833 |
3.317 |
0.083 |
42.707 |
12.603 |
0.287 |
|
|
Precipitation |
0.538 |
3.769 |
0.678 |
1.7487 |
12.308 |
0.754 |
||
|
Temperature |
-21.884 |
405.96 |
0.003 |
-92.69 |
1713.1 |
0.004 |
||
|
Post-monsoon |
Urban |
NDVI |
8.5919 |
2.858 |
0.060 |
-11.58 |
18.597 |
0.774 |
|
Precipitation |
-0.205 |
21.226 |
0.372 |
0.159 |
3.803 |
0.935 |
||
|
Temperature |
1.060 |
-23.705 |
0.129 |
1.330 |
-17.12 |
0.826 |
||
|
Sub-urban |
NDVI |
0.267 |
5.704 |
0.972 |
11.694 |
14.581 |
0.736 |
|
|
Precipitation |
-0.602 |
55.065 |
0.157 |
-6.684 |
563.52 |
< 0.001 |
||
|
Temperature |
9.00 |
-241.51 |
0.049 |
54.054 |
-1403.1 |
0.008 |
||
The most dominant feeding guild was insectivores, which was also observed in other studies (MacGregor-Fors and Schondube, 2011; Katuwal et al., 2018; Dangaura et al., 2020). On the contrast, this finding is opposite to some other studies because generalist such as omnivorous species can exploit different resources and are most abundant (Clergeau et al., 1998; Chace and Walsh, 2006; Silva et al., 2016); however, omnivores were second most abundant guild in this study. Similarly, insectivores and other feeding guilds assemblage were most in suburban habitats owing to the habitat heterogeneity that altogether fosters insect richness and bird assemblage (Aronson et al., 2017).
In the context of Nepal, seasonality plays crucial role in determining bird species distribution as one-third of birds are summer and winter migratory where winter migrants exceed summer migrants (Grimmett et al., 2000; Inskipp et al., 2016). This might be the reason the study recorded higher species richness in winter than in post-monsoon. Higher species richness in winter was also illustrated by Carbó-Ramírez and Zuria, (2011) and Katuwal et al., (2018) in their respective research. But Keten et al. (2020) recorded low richness in winter while Verma and Murmu (2015) recorded high richness in spring that are in contrast with this study. These seasonal fluctuations in species diversity is associated with resources availability, climatic conditions and seasonal movement (Katuwal et al., 2016; Pandey et al., 2020).
Urbanization affects the species diversity and richness as a result of landscape manipulation that alters habitat and resources (Blair and Johnson, 2008; Grimm et al., 2008). Species diversity and richness both decrease but abundance of urban exploiters increases with urban expansion (Reis et al., 2012; Rodrigues et al., 2018). This reduction in richness and diversity indices may be due to environmental pollution, and lack of green spaces and fruiting trees (Crooks et al., 2004). Urban expansion supports urban dwellers or generalist species compensating the loss of other species (Fontana et al., 2011; MacGregor-Fors and Schondube, 2011; Oliveira Hagen et al., 2017) as the urban adapters are well adapted to the residential and open areas (Keten et al., 2020) while specialist species are the least urban tolerant (Callaghan et al., 2019). The most abundant bird species observed during this study were House sparrow, House crow and Common pigeon which are known to be commensals with human (Jokimäki and Suhonen, 1998). Abundance of such urban exploiters was much higher in urban areas than in the suburban periphery. The ability to feed on diverse food, nesting, roosting on urban built structures (building, poles and wires) and withstand anthropogenic pressure are the major causes of abundant presence of urban dwellers (MacGregor-Fors and Schondube, 2011; Rodrigues et al., 2018).
Human built infrastructures or sealed areas (houses and buildings) exert the major effects on bird diversity and richness (MacGregor-Fors and Schondube, 2011; Menon and Mohanraj, 2016; Filloy et al., 2019). Anthropogenic pressure is second to none when it comes to decreasing richness and diversity (Zhou and Chu, 2012), which negative influence was also found in this study but the supplementary feeding habits somehow compensate the bird communities (Lepczyk et al., 2008; Galbraith et al., 2015). Similarly, noise of any kind, either vehicular or anthropogenic, are also detrimental to bird richness (Rodrigues et al., 2018; Filloy et al., 2019). Vegetative cover, mainly trees, is the most prominent factor to increase diversity and richness (Fontana et al., 2011; Haedo et al., 2017) be it either in urban or suburban areas. The positive association of NDVI with richness in this study also supports this view. In urban sites, green spaces or areas hosted more diverse species including urban adapters. These vegetative covers provide the species with nesting, roosting, shelter and foraging, ultimately uplifting the species richness and diversity (Marzluff and Ewing, 2008; Menon et al., 2015). Suburban areas characterized by the presence of more open or green spaces, farmlands and less impervious surfaces provided heterogeneous habitat, increasing the bird diversity and richness of the area as reported in multiple other studies (Verma and Murmu, 2015; Ferenc et al., 2016).
Conclusions and Recommendations
Land cover and seasonality exert influence upon bird community assemblage and distribution. Suburban sites host more diverse species and higher feeding guild distribution. Bird species richness and diversity has positive association with the vegetation cover whereas the same has negative association with human pressure. Cities experiencing decline in bird richness should focus on increasing green spaces and parks. In urban settings, urban green spaces are pivotal to foster the bird richness.
Acknowledgements
We are thankful to Binod Bhattarai and Milan Aryal for their support in field works.
Conflict of interests
The authors have declared no conflict of interest.
References
Altaf, M., Javid, A., Khan, A.M., Khan, M., Umair, M. and Ali, Z., 2018. Anthropogenic impact on the distribution of the birds in the tropical thorn forest, Punjab, Pakistan. J. Asia Pac. Biodiv., 11: 229–236. https://doi.org/10.1016/j.japb.2018.03.001
Anderies, J.M., Katti, M. and Shochat, E., 2007. Living in the city: Resource availability, predation, and bird population dynamics in urban areas. J. Theor. Biol., 247: 36–49. https://doi.org/10.1016/j.jtbi.2007.01.030
Aronson, M.F., Lepczyk, C.A., Evans, K.L., Goddard, M.A., Lerman, S.B., MacIvor, J.S., Nilon, C.H. and Vargo, T., 2017. Biodiversity in the city: key challenges for urban green space management. Front. Ecol. Environ., 15: 189–196. https://doi.org/10.1002/fee.1480
Blair, R.B. and Johnson, E.M., 2008. Suburban habitats and their role for birds in the urban rural habitat network: Points of local invasion and extinction? Landsc. Ecol., 23: 1157–1169. https://doi.org/10.1007/s10980-008-9267-y
Callaghan, C.T., Major, R.E., Lyons, M.B., Martin, J.M. and Kingsford, R.T., 2018. The effects of local and landscape habitat attributes on bird diversity in urban greenspaces. Ecosphere, 9: e02347. https://doi.org/10.1002/ecs2.2347
Callaghan, C.T., Major, R.E., Wilshire, J.H., Martin, J.M., Kingsford, R.T. and Cornwell, W.K., 2019. Generalists are the most urban-tolerant of birds: A phylogenetically controlled analysis of ecological and life history traits using a novel continuous measure of bird responses to urbanization. Oikos, 128: 845–858. https://doi.org/10.1111/oik.06158
Carbó-Ramírez, P. and Zuria, I., 2011. The value of small urban greenspaces for birds in a Mexican city. Landsc. Urban Plan., 100: 213–222. https://doi.org/10.1016/j.landurbplan.2010.12.008
Chace, J.F. and Walsh, J.J., 2006. Urban effects on native avifauna: A review. Landsc. Urban Plan., 74: 46–69. https://doi.org/10.1016/j.landurbplan.2004.08.007
Clergeau, P., Mennechez, G., Sauvage, A. and Lemoine, A. 2001. Human perception and appreciation of birds: A motivation for wildlife conservation in urban environments of France. In: (Eds) Avian ecology and conservation in an urbanizing world. Springer: 69–88. https://doi.org/10.1007/978-1-4615-1531-9_4
Clergeau, P., Savard, J.P.L., Mennechez, G. and Falardeau, G., 1998. Bird abundance and diversity along an urban-rural gradient: A comparative study between two cities on different continents. Condor, 100: 413–425. https://doi.org/10.2307/1369707
Crooks, K.R., Suarez, A.V. and Bolger, D.T., 2004. Avian assemblages along a gradient of urbanization in a highly fragmented landscape. Biol. Conserv., 115: 451–462. https://doi.org/10.1016/S0006-3207(03)00162-9
Czech, B., Krausman, P.R. and Devers, P.K., 2000. Economic associations among causes of species endangerment in the United States: Associations among causes of species endangerment in the United States reflect the integration of economic sectors, supporting the theory and evidence that economic growth proceeds at the competitive exclusion of nonhuman species in the aggregate. BioScience, 50: 593–601. https://doi.org/10.1641/0006-3568(2000)050[0593:EAACOS]2.0.CO;2
Dale, S., 2018. Urban bird community composition influenced by size of urban green spaces, presence of native forest, and urbanization. Urban Ecosyst., 21: 1–14. https://doi.org/10.1007/s11252-017-0706-x
Dangaura, H.L., Pandey, N., Chand, D.B. and Bhusal, K.P., 2020. Avian richness of the Basanta Protected Forest, far-western lowland Nepal: Implication for conservation. Nepal. J. Zool., 4: 68–84. https://doi.org/10.3126/njz.v4i2.33886
Evans, K.L., Chamberlain, D.E., Hatchwell, B.J., Gregory, R.D. and Gaston, K.J., 2011. What makes an urban bird? Glob. Chang. Biol., 17: 32–44. https://doi.org/10.1111/j.1365-2486.2010.02247.x
Ferenc, M., Sedláček, O., Mourková, J., Exnerová, A., Škopek, J., Formánek, J., and Fuchs, R., 2016. Disentangling the influences of habitat availability, heterogeneity and spatial position on the species richness and rarity of urban bird communities in a central European city. Urban Ecosyst., 19: 1265–1281. https://doi.org/10.1007/s11252-016-0558-9
Filloy, J., Zurita, G.A. and Bellocq, M.I., 2019. Bird diversity in urban ecosystems: the role of the biome and land use along urbanization gradients. Ecosystems, 22: 213–227. https://doi.org/10.1007/s10021-018-0264-y
Fontana, S., Sattler, T., Bontadina, F. and Moretti, M., 2011. How to manage the urban green to improve bird diversity and community structure. Landsc. Urban Plan., 101: 278–285. https://doi.org/10.1016/j.landurbplan.2011.02.033
Fuller, R.A., Irvine, K.N., Davies, Z.G., Armsworth, P.R. and Gaston, K.J., 2012. 16. Interactions between People and Birds in Urban Landscapes. In: (Eds) Urban bird ecology and conservation. University of California Press. pp. 249–266. https://doi.org/10.1525/california/9780520273092.003.0016
Galbraith, J.A., Beggs, J.R., Jones, D.N. and Stanley, M.C., 2015. Supplementary feeding restructures urban bird communities. Proc. Natl. Acad. Sci. U. S. A., 112: E2648–E2657. https://doi.org/10.1073/pnas.1501489112
Gibbons, D. and Gregory, R., 2006. Birds. In: W. Sutherland (Ed.), ecological census techniques: A handbook. Cambridge: Cambridge University Press. pp. 308-350. https://doi.org/10.1017/CBO9780511790508.010
Gillings, S., 2019. Bird responses to housing development in intensively managed agricultural landscapes. Urban Ecosyst., 22: 1007–1017. https://doi.org/10.1007/s11252-019-00895-1
Goddard, M.A., Dougill, A.J. and Benton, T.G., 2010. Scaling up from gardens: biodiversity conservation in urban environments. Trends Ecol. Evol., 25: 90–98. https://doi.org/10.1016/j.tree.2009.07.016
Grimm, N.B., Faeth, S.H., Golubiewski, N.E., Redman, C.L., Wu, J., Bai, X., and Briggs, J.M., 2008. Global change and the ecology of cities. Science, 319: 756–760. https://doi.org/10.1126/science.1150195
Grimmett, R., Inskipp, C., Inskipp, T. and Baral, H., 2000. Birds of Nepal. Helm field guide. Prakash Books, New Delhi.
Grimmett, R., Inskipp, C., Inskipp, T. and Baral, H.S., 2016. Birds of Nepal. Bloomsbury Publishing.
Haedo, J., Gioia, A., Aráoz, E., Paolini, L. and Malizia, A., 2017. Primary productivity in cities and their influence over subtropical bird assemblages. Urban For. Urban Green., 26: 57–64. https://doi.org/10.1016/j.ufug.2017.04.017
Inskipp, C., Baral, H., Phuyal, S., Bhatt, T., Khatiwada, M., Inskipp, T., Khatiwada, A., Gurung, S., Singh, P.B., Murray, L., Poudyal, L. and Amin, R., 2016. The status of Nepal’s birds: The national red list series. Zool. Soc. Lon., UK, 3.
Jokimäki, J. and Suhonen, J., 1998. Distribution and habitat selection of wintering birds in urban environments. Landsc. Urban Plan., 39: 253–263. https://doi.org/10.1016/S0169-2046(97)00089-3
Jokimaki, J., Suhonen, J., Jokimaki-Kaisanlahti, M.L., and Carbo-Ramirez, P., 2016. Effects of urbanization on breeding birds in European towns: Impacts of species traits. Urban Ecosyst., 19: 1565–1577. https://doi.org/10.1007/s11252-014-0423-7
Katuwal, H.B., Basnet, K., Khanal, B., Devkota, S., Rai, S.K., Gajurel, J.P., Scheidegger, C. and Nobis, M.P., 2016. Seasonal changes in bird species and feeding guilds along elevational gradients of the Central Himalayas, Nepal. PLoS One, 11: e0158362. https://doi.org/10.1371/journal.pone.0158362
Katuwal, H.B., Pradhan, N.M.B., Thakuri, J.J., Bhusal, K.P., Aryal, P.C. and Thapa, I., 2018. Effect of urbanization and seasonality in bird communities of Kathmandu Valley, Nepal. Proc. Zool. Soc., 71: 103–113. https://doi.org/10.1007/s12595-018-0265-z
Keten, A., Eroglu, E., Kaya, S. and Anderson, J.T., 2020. Bird diversity along a riparian corridor in a moderate urban landscape. Ecol. Indic., 118: 106751. https://doi.org/10.1016/j.ecolind.2020.106751
Lepczyk, C.A., Flather, C.H., Radeloff, V.C., Pidgeon, A.M., Hammer, R.B. and Liu, J., 2008. Human impacts on regional avian diversity and abundance. Conserv. Biol., 22: 405–416. https://doi.org/10.1111/j.1523-1739.2008.00881.x
Lin, Y.P., Yeh, M.S., Deng, D.P. and Wang, Y.C., 2008. Geostatistical approaches and optimal additional sampling schemes for spatial patterns and future sampling of bird diversity. Glob. Ecol. Biogeogr., 17: 175–188. https://doi.org/10.1111/j.1466-8238.2007.00352.x
MacGregor-Fors, I. and Schondube, J.E., 2011. Gray vs. green urbanization: relative importance of urban features for urban bird communities. Basic Appl. Ecol., 12: 372–381. https://doi.org/10.1016/j.baae.2011.04.003
Marzluff, J.M. and Ewing, K., 2008. Restoration of fragmented landscapes for the conservation of birds: a general framework and specific recommendations for urbanizing landscapes. In: (Eds) Urban Ecology. Springer: pp. 739–755. https://doi.org/10.1007/978-0-387-73412-5_48
Marzluff, J.M., 2001. Worldwide urbanization and its effects on birds. In: Marzluff, J.M., Bowman, R., Donnelly, R., (eds) avian ecology and conservation in an urbanizing world. Springer, Boston, MA. https://doi.org/10.1007/978-1-4615-1531-9
McKinney, M.L., 2006. Urbanization as a major cause of biotic homogenization. Biol. Conserv., 127: 247–260. https://doi.org/10.1016/j.biocon.2005.09.005
Menon, M. and Mohanraj, R., 2016. Temporal and spatial assemblages of invasive birds occupying the urban landscape and its gradient in a southern city of India. J. Asia Pac. Biodivers., 9: 74–84. https://doi.org/10.1016/j.japb.2015.12.005
Menon, M. and Rangaswamy, M., 2016. Avifaunal richness and abundance along an urban rural gradient with emphasis on vegetative and anthropogenic attributes in Tiruchirappalli, India. Landsc. Res., 41: 131–148. https://doi.org/10.1080/01426397.2014.910294
Menon, M., Devi, M.P., Nandagopalan, V. and Mohanraj, R., 2015. Species diversity and functional assemblages of bird fauna along the riverine habitats of Tiruchirappalli, India. In: (Eds) Environmental Management of River Basin Ecosystems. Springer: pp. 729–748. https://doi.org/10.1007/978-3-319-13425-3_32
Mortimer, J. A. and Clark, D., 2013. A comparison of bird counting techniques in an urban environment. Notornis, 60: 173–177
Neupane, J., Khanal, L., Gyawali, B. and Chalise, M.K., 2020. Elevational pattern and seasonality of avian diversity in Kaligandaki River Basin, central Himalaya. J. Threat. Taxa., 12: 16927–16943. https://doi.org/10.11609/jott.5815.12.14.16927-16943
Oksanen, J., Blanchet, F.G., Kindt, R., Legendre, P., Minchin, P.R., O’hara, R., Simpson, G.L., Solymos, P., Stevens, M.H. and Wagner, H., 2019. Vegan: community ecology package, version 2.4-4. 2.
Oliveira-Hagen, E., Hagen, O., Ibáñez-Álamo, J.D., Petchey, O.L. and Evans, K.L., 2017. Impacts of urban areas and their characteristics on avian functional diversity. Front. Ecol. Evol., 5: 84. https://doi.org/10.3389/fevo.2017.00084
Palacio, F.X., 2020. Urban exploiters have broader dietary niches than urban avoiders. Ibis, 162: 42–49. https://doi.org/10.1111/ibi.12732
Pandey, N., Khanal, L. and Chalise, M.K., 2020. Correlates of avifaunal diversity along the elevational gradient of Mardi Himal in Annapurna Conservation Area, Central Nepal. Avian Res., 11: 1–14. https://doi.org/10.1186/s40657-020-00217-6
Pandey, N., Khanal, L., Chapagain, N., Singh, K.D., Bhattarai, B.P. and Chalise, M.K., 2021. Bird community structure as a function of habitat heterogeneity: A case of Mardi Himal, Central Nepal. Biodiversitas, 22: 262–271. https://doi.org/10.13057/biodiv/d220132
Pickett, S.T., Cadenasso, M.L., Grove, J.M., Boone, C.G., Groffman, P.M., Irwin, E., Kaushal, S.S., Marshall, V., McGrath, B.P., Nilon, C.H., Pouyat, R.V., Szlavecz, K., Troy, A. and Warren, P., 2011. Urban ecological systems: Scientific foundations and a decade of progress. J. Environ. Manage., 92: 331–362. https://doi.org/10.1016/j.jenvman.2010.08.022
Prins, H.H. and Namgail, T., 2017. Bird migration across the Himalayas: Wetland functioning amidst mountains and glaciers. Cambridge University Press. https://doi.org/10.1017/9781316335420
R Core Team, 2013. A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing.
Reis, E., López-Iborra, G.M. and Pinheiro, R.T., 2012. Changes in bird species richness through different levels of urbanization: Implications for biodiversity conservation and garden design in Central Brazil. Landsc. Urban Plan., 107: 31–42. https://doi.org/10.1016/j.landurbplan.2012.04.009
Rodrigues, A.G., Borges-Martins, M. and Zilio, F., 2018. Bird diversity in an urban ecosystem: The role of local habitats in understanding the effects of urbanization. Iheringia Ser. Zool., 108. https://doi.org/10.1590/1678-4766e2018017
Sandström, U., Angelstam, P. and Mikusiński, G., 2006. Ecological diversity of birds in relation to the structure of urban green space. Landsc. Urban Plan., 77: 39–53. https://doi.org/10.1016/j.landurbplan.2005.01.004
Silva, C.P., Sepúlveda, R.D. and Barbosa, O., 2016. Nonrandom filtering effect on birds: Species and guilds response to urbanization. Ecol. Evol., 6: 3711–3720. https://doi.org/10.1002/ece3.2144
Thapa, M.S. and Poudel, G., 2018. Assessing the coverage of urban green space in Butwal Sub-Metropolitan City, Nepal: A GIS based approach. For. J. Inst. For. Nepal, 15: 77–86. https://doi.org/10.3126/forestry.v15i0.24923
Tratalos, J., Fuller, R.A., Evans, K.L., Davies, R.G., Newson, S.E., Greenwood, J.J., and Gaston K.J., 2007. Bird densities are associated with household densities. Glob. Chang. Biol., 13: 1685–1695. https://doi.org/10.1111/j.1365-2486.2007.01390.x
Venter, O., Sanderson, E., Magrach, A., Allan, J., Beher, J., Jones, K., Possingham, H., Laurance, W., Wood, P., Fekete, B., Levy, M. and Watson, J., 2018. Last of the Wild Project, Version 3 (LWP-3): 2009 Human Footprint, 2018 Release. Palisades, NY: NASA Socioeconomic Data and Applications Center (SEDAC).
Verma, S.K. and Murmu, T.D., 2015. Impact of environmental and disturbance variables on avian community structure along a gradient of urbanization in Jamshedpur, India. PLoS One, 10: e0133383. https://doi.org/10.1371/journal.pone.0133383
Zhou, D. and Chu, L., 2012. How would size, age, human disturbance, and vegetation structure affect bird communities of urban parks in different seasons? J. Ornithol., 153: 1101–1112. https://doi.org/10.1007/s10336-012-0839-x
To share on other social networks, click on any share button. What are these?








