Trace Elements Optimization for Production of Fibrinolytic Protein from Wild and Mutant Streptococcal Strains
Trace Elements Optimization for Production of Fibrinolytic Protein from Wild and Mutant Streptococcal Strains
Ghulam Akbar1*, Muhammad Anjum Zia1, Amer Jamil1 and Faiz Ahmad Joyia2
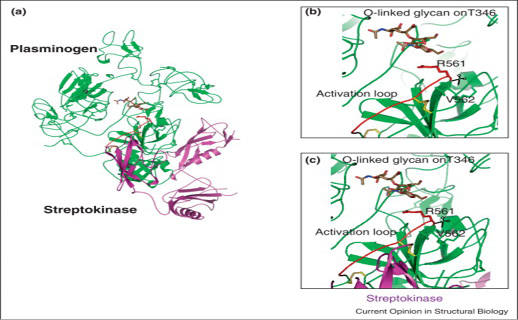
Proteolytic activation by breaking R561-V562 bond (a) Green color full-length plasminogen (plg) and SK superposition where SK covered the plasminogen catalytic domain. (b) Clear picture of plg activation domain of O-linked glycan on T346. (c) streptokinase-plasminogen binding view without steric hindrance and in yellow colored sticks disulphide bonds are expressed (Law et al., 2012).
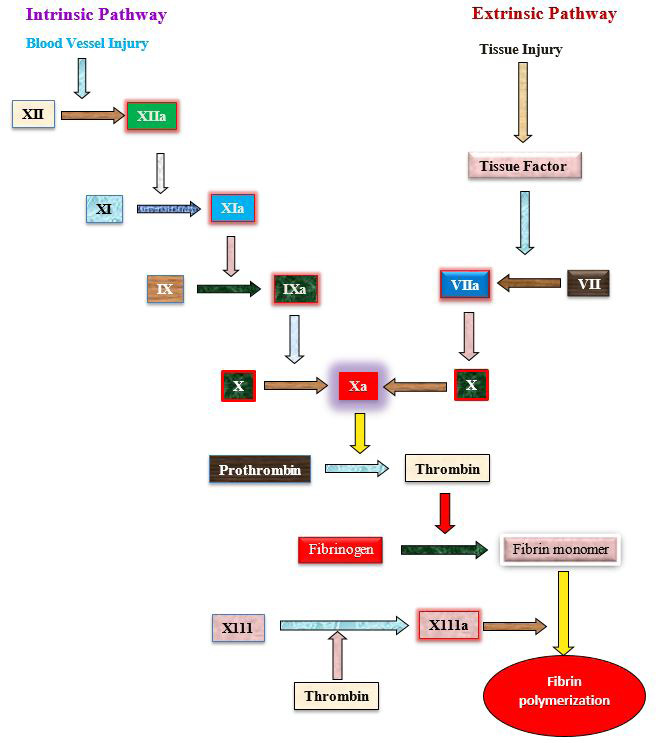
Extrinsic and intrinsic pathway involving in blood clotting (Schmaier et al., 2011).
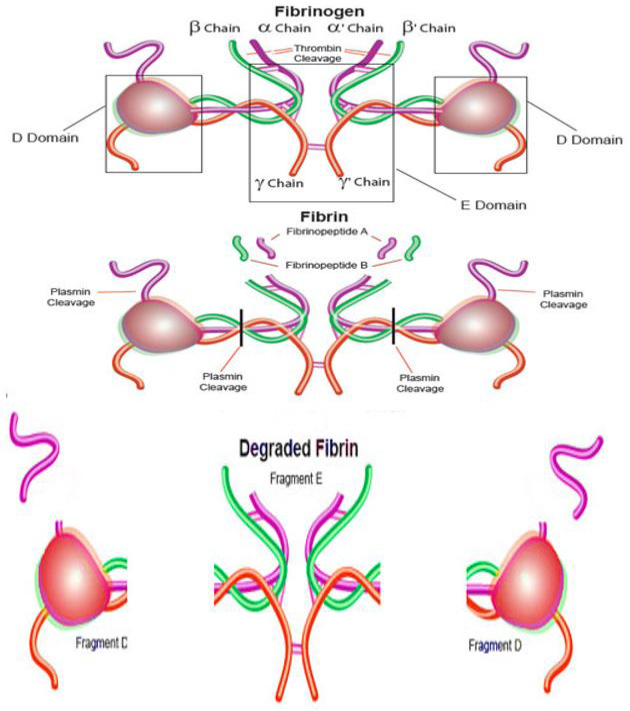
Fibrinogen transformation to fibrin by thrombin and fibrin cleavage by plasmin (https://www.sigmaaldrich.com/life-science/metabolomics/enzyme-explorer/analytical-enzymes/fibrinogen-and-fibrin.html).
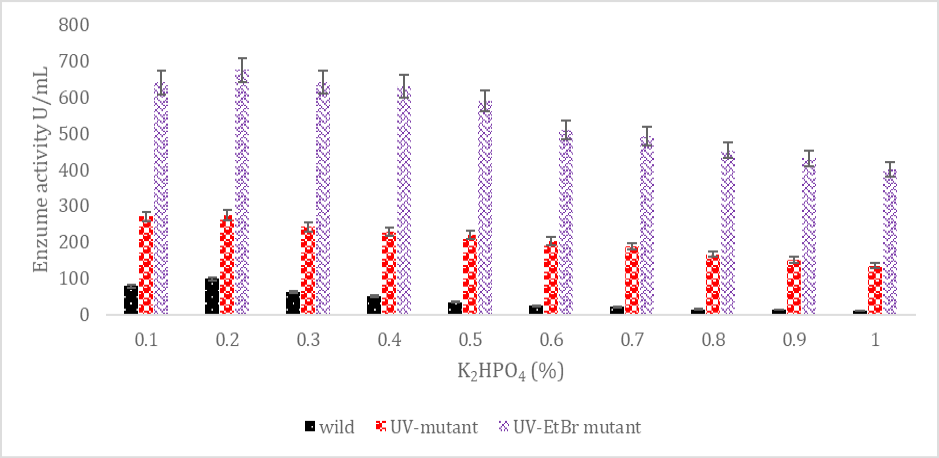
SK production from wild and mutated Streptococcus mutans strains from various K2HPO4 amounts.
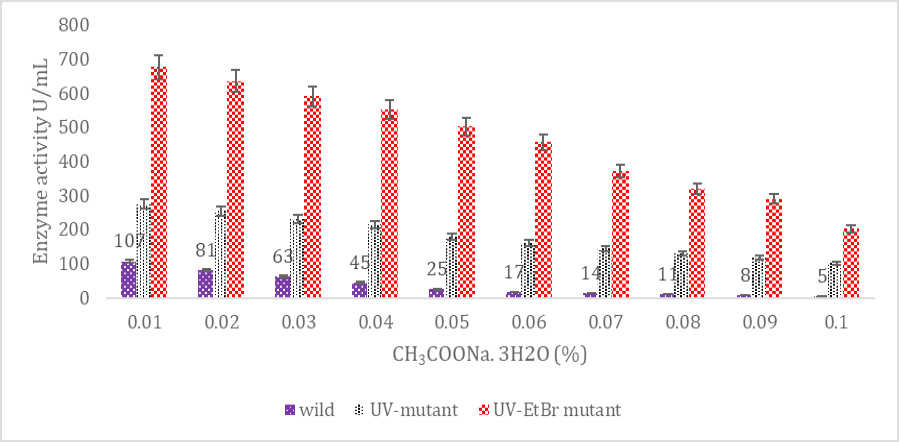
SK yield from wild and mutagenic Streptococcus mutans on diverse CH3COONa.3H2O amount.
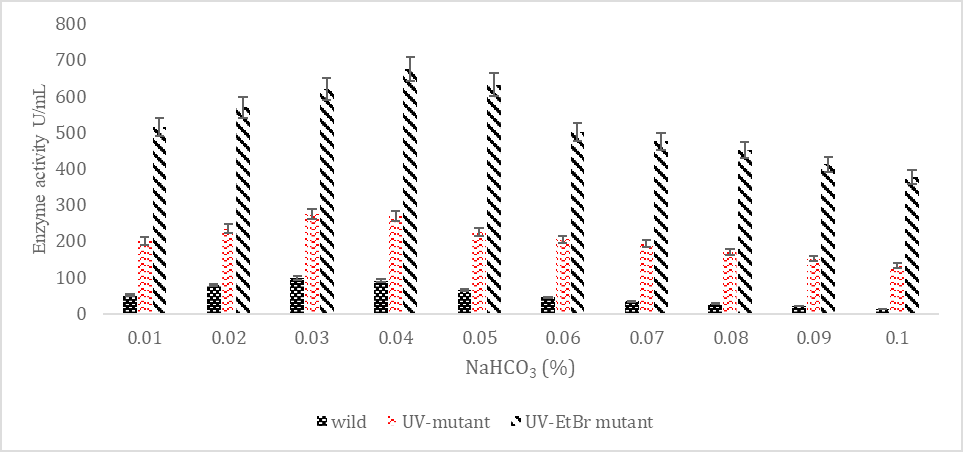
SK yield from wild and mutagenic Streptococcus mutans on diverse NaHCO3 amount.
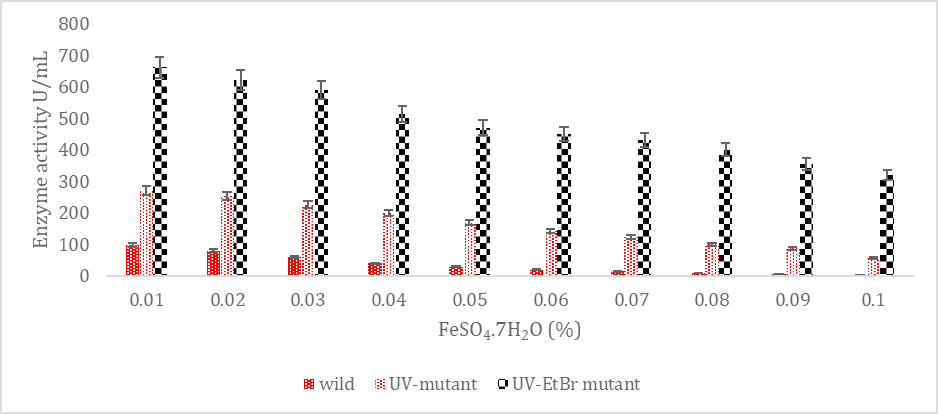
SK yield from wild and mutagenic Streptococcus mutans on diverse FeSO4.7H2O amount.
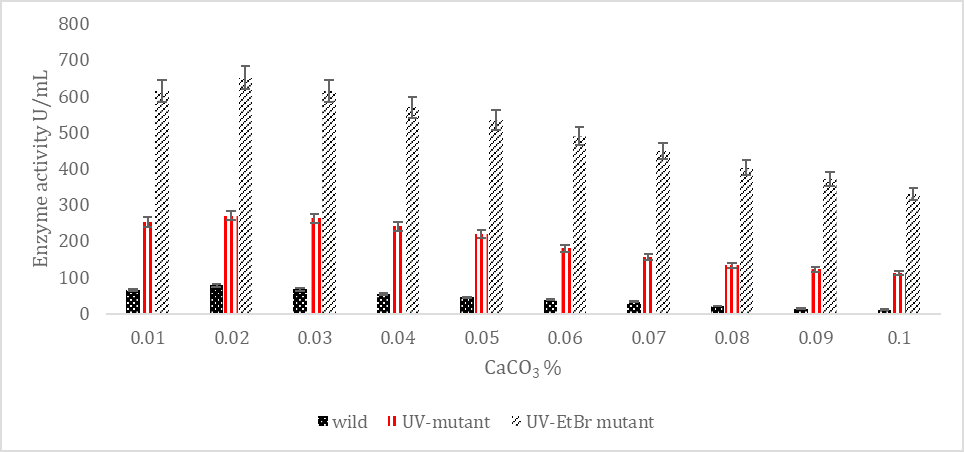
SK yield from wild and mutagenic Streptococcus mutans on diverse CaCO3 amount.
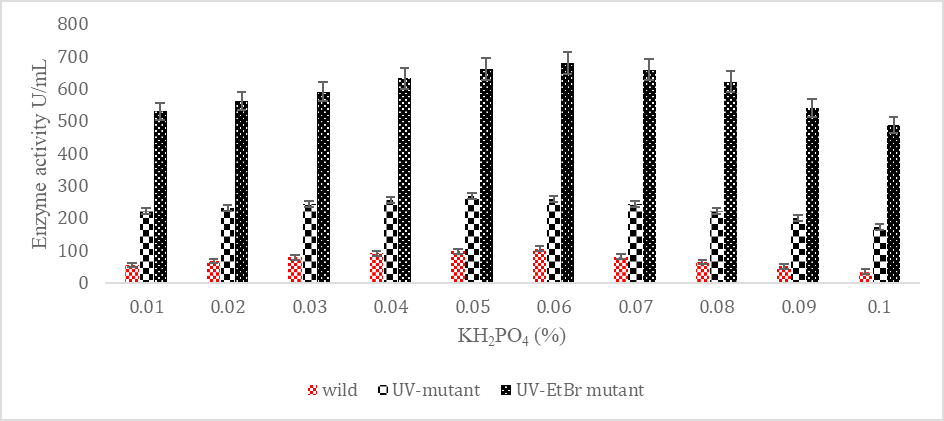
SK yield from wild and mutagenic Streptococcus mutans on diverse KH2PO4 amount.