Toxicity, Histological, Hepato-Somatic Index and Growth Responses of Cadmium Stressed Labeo rohita
Toxicity, Histological, Hepato-Somatic Index and Growth Responses of Cadmium Stressed Labeo rohita
Sumaira Fiaz1*, Huma Naz1*, Tanveer Ahmed2*, Iqra Zulfiqar1, Khalid Mehmood1, Muhammad Jabbar1, Syed Qaswar Ali Shah1, Muhammad Usman4
1Department of Zoology, Cholistan University of Veterinary and Animal Sciences, Bahawalpur, Pakistan
2Department of life Sciences, Khawaja Fareed University of Engineering and Technology, Rahim Yar Khan, Pakistan
3Department of Anatomy and Histology, Cholistan University of Veterinary and Animal Sciences, Bahawalpur, Pakistan.
Abstract | The concentration of heavy metals accelerated every day in aquatic surrounding causing serious health problem in fish and ultimately in human. So, current study was planned to evaluate effects of cadmium on the growth performance, liver histology and hepato-somatic index of Labeo rohita. The test was conducted in two phases. In the primary phase, the 96-hr LC50 and lethal concentration of water borne cadmium for fish L. rohita were determined as 12.38mgL-1 and 23.6mgL-1, respectively. In secondary phase, fish was subjected to sub-lethal (1/3rd of LC50) value of cadmium for 60-days. Result showed significant decrease in daily weight gain, daily length gain, condition factor, specific growth rate and feed conversion ratio of Cd-treated fish as compared to control fish. In liver, the diameter of hepatocytes was significantly lesser in Cd-treated fish in comparison with control fish. The width of sinusoids in Cd-treated fish was significantly greater in comparison with control fish. Some other histological disorders were also seen in liver of Cd-treated fish viz. infiltration of sinusoid, dilation and increased hemorrhage in sinusoids, inflammation and vacuolation in hepatocytes and ruptured hepatocytes after 60-days of experimental period. Hepatosomatic index was significantly less in Cd-treated fish than control fish throughout the experimental period. Addressing this problem requires a coordinated effort among governments, industries, and individuals to reduce heavy metal pollution, protect aquatic ecosystems, and safeguard human health.
Novelty Statement | The study is novel for fish farming as it finds out that cadmium has clear negative effects on the growth performance, liver histology and hepato-somatic index of fish.
Article History
Received: September 07, 2023
Revised: March 20, 2024
Accepted: April 12, 2024
Published: May 24, 2024
Authors’ Contributions
SF executed the research work. HN planned the research work. TA, IZ, KM and MJ equally contribute in writing article. SQAS and MU worked as member of supervisory committe.
Keywords
Acute, Chronic, Toxicity, Metals, FCR, Liver
Copyright 2024 by the authors. Licensee ResearchersLinks Ltd, England, UK. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Corresponding authors: Sumaira Fiaz, Huma Naz and Tanveer Ahmed
dr.humanaz98@gmail.com, tanvirahmeduaf@gmail.com
To cite this article: Fiaz, S., Naz, H., Ahmed, T., Zulfiqar, I., Mehmood, K., Jabbar, M., Shah, S.Q.A. and Usman, M., 2024. Toxicity, histological, hepato-somatic index and growth responses of cadmium stressed Labeo rohita. Punjab Univ. J. Zool., 39(1): 79-85. https://dx.doi.org/10.17582/journal.pujz/2024/39.1.79.85
Introduction
These days, heavy metals pollution is a worldwide issue due to continuous accumulation and persistence of metallic pollution in aquatic environment (Hyun et al., 2006). In the aquatic biota, significant consideration of heavy metal is due to their amassing tendency and severe toxicity. Both the ecological equilibrium of the receiver environment and the variety of water creatures may be severely impacted by heavy metals (Javed, 2004).
Fish are the only animals that are unable to escape the negative consequences of metal contamination in water (Farombi et al., 2007; Vosyliene and Jankaite, 2006). Fish are able to quickly absorb dissolved metals, which may be used to determine how polluted an area is. By absorbing and metabolic activities as the storage metals may accumulate in the tissues of fish (Farkas et al., 2002; Shukla et al., 2007). Cadmium is considered among toxic heavy metals. To all living beings, it is a non-essential component. Cadmium contamination of aquatic habitats has expanded dramatically in recent decades, leading to an increase in Cd bioaccumulation in aquatic organisms across the food chain (Okocha and Adedeji, 2011).
Growth is a simple metric that can account for all of the effects within the fish. If it is primarily intended to know that in what way a toxicant will affect fish growth then it is important to know how loss of appetite occurs due to metallic toxicity (Javed and Saeed, 2010). Cadmium may also alter the growth of fish (Heydarnejad et al., 2013). Chronic exposure to Cd may cause sublethal effects in fish such as decreased growth that is more prominent when Cd concentration exceed from 3µgL-1 in fresh water (Levit, 2010). Altered behavior and slow growth rate were observed in fish at high dose of dietary cadmium after long time exposure (Nogami et al., 2000).
Studies on fish’s histopathology can help determine how toxicants affect the fish (Ahmed et al., 2015; Pimpao et al., 2007), and has been used widely in both field studies and in laboratory as a biomarker in the assessment of fish health (Thophon et al., 2003). For critical function of body such as accumulation and biotransformation of chemicals in fish, liver is considered a vital organ of body (Gernhofer et al., 2001). In heavy metal pollution, liver has been known as the storage site of cadmium (Gbem et al., 2001). Cadmium can cause observed effects such as physiological dysfunction and morphological disruption in exposed organ (Satarug et al., 2010; Huo et al., 2017).
The hepatosomatic index (HSI) is utilized in fisheries research as a measure of the liver’s ability to store and release energy reserves. Hepatosomatic index is the percentage of weight of fish liver to total weight of fish that offers information on the fish’s health and water quality (Pandit and Gupta, 2019; Dane and Sisman, 2020). In field and laboratory trials, the HSI has proven to be effective. The HSI had only been utilized by a few fish toxicologists as a fish health biomarker in connection to pollution (Javed and Usmani, 2017). Therefore, this study was designed to estimate the growth performance, liver histology and hepatosomatic index of L. rohita exposed to Cd.
Materials and Methods
Fish collection and acclimatization
The 150 days old Labeo rohita was collected from Fisheries Research and Training Complex, Bahawalpur. Fish was brought to Fisheries Research Laboratory at Cholistan University of Veterinary and Animal Sciences, Bahawalpur, Pakistan in plastic bags and handled with proper care. Fish was adjusted to laboratory conditions for 14 days before starting a trial. Water medium was refreshed every 24 h to eliminate feeding debris and fecal matter.
Acute toxicity test
The 96-h LC50 and lethal concentration of cadmium for L. rohita were checked by carrying out the acute toxicity bioassay tests. Stock solutions (1000 mg/L) of Cd were prepared by mixing Cdcl2 in 1L distilled water. Cadmium concentration in each aquarium was gradually increased by 0, 2.5, 7.5, 12.5, 17.5 and 20mgL-1. The LC50 was the Cd concentration at which 50% of fishes were died and the concentration at which 100% fishes were dead was lethal concentration.
Growth determination
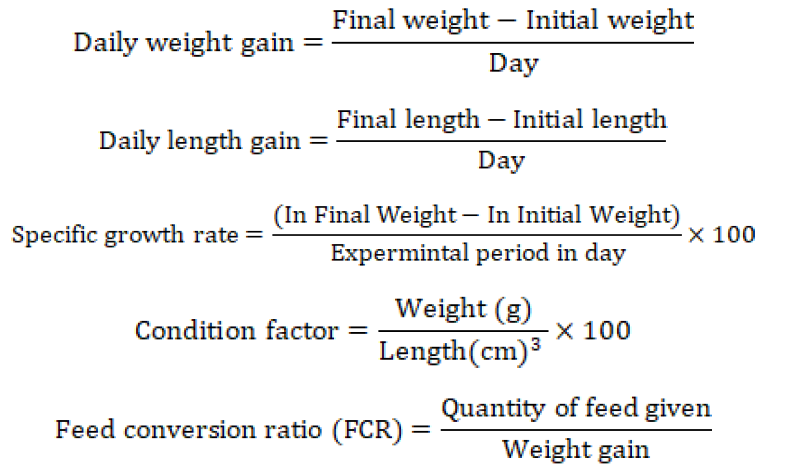
After determination of 96-h LC50, fish was exposed to cadmium for 60 days at 1/3rd of LC50 value. Just before exposure, the length and weight of L. rohita were measured. To measure the growth, daily weight gain (DWG), daily length gain (DLG), condition factor, specific growth rate (SGR) and feed conversion ratio (FCR) was measured on a weekly basis throughout the experimental period.
Liver histology
Liver tissues were isolated from both experimental and control fish. Samples were preserved in a 10 percent neutral-buffering formalin solution then dehydrated and followed by paraffin wax embedding. For histological analysis, the 5µm thick slices were cut, stained with hematoxylin-eosin, and observed under a light microscope. as described by Bancroft et al. (2013). The diameter of the hepatocytes (50 hepatocytes per slide) and width of sinusoids (20 sinusoids per slide) were measured at random (Kasperk et al., 1994).
Hepato-somatic index (HSI)
To examine the health state of fish, HSI was calculated. By using the method of (Rajaguru, 1992) HSI was calculated by following formula:
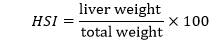
Statistical analyses
Probit analysis was implemented to find out the 96-h LC50 and lethal concentration of cadmium for L. rohita. Data of growth performance and hepatosomatic index was analyzed statistically by One-way analysis of variance (ANOVA) through software program Statistix 8.1 version. While for histological examination of liver Tukey test was used through software program SPSS to check the effect of treatment. Histological observations were made on Optika microscope and image J software was used for histomorphometry.
Results and Discussion
Acute toxicity
The 96-h LC50 and lethal concentration of L. rohita were determined through acute toxicity bioassay as 12.38mgL-1 and 23.6mgL-1, respectively. The lower and upper limits for LC50 were 9.95 and 14.48, respectively. The lower and upper limits for lethal value were 20.26 and 31.01, respectively. Figure 1 shows the fix mortality against cadmium metal concentration. Figure 2 shows the probability plot for 96-h LC50 and lethal value of Cd for L. rohita.
Growth performance
The growth performance of both the treated and control fish group fluctuate significantly during the trial period of 60 days. All the growth parameters daily weight gain, daily length gain (standard length, fork length and total length), condition factor, feed conservation ratio and specific growth rate were significantly lesser in Cd-treated fish as compared to control fish.
Table 1: Growth performance of control and Cd treated L. rohita.
|
Parameters |
Control |
Cd treated |
|
Daily weight gain |
1.23±0.68a |
0.03±0.04b |
|
Standard length gain |
0.04±0.00a |
0.006±0.00b |
|
Fork length gain |
0.03±0.00a |
0.006±0.005b |
|
Total length gain |
0.04±0.009a |
0.01±0.012b |
|
Specific growth rate |
1.95±1.61a |
0.02±0.07b |
|
Condition factor |
2.53±0.43a |
1.43±0.03b |
|
Feed conservation ratio |
1.23±0.09a |
-0.02±0.22b |
Means with similar letters in a single row are statistically similar at p<0.05.
Histology of liver
The histomorphometric parameters, diameter of hepatocytes in Cd-treated fish was significantly less in comparison with control fish (Table 2, Figures 3-4). The width of sinusoids in Cd-treated fish was significantly greater in comparison with control fish (Table 2, Figures 5-6). Some other histological disorders viz. infiltration in sinusoids, congestion in sinusoids, increased dilation in sinusoids (Figure 7) vacuolation in hepatocytes, inflammation in hepatocytes and abnormal shape of hepatocytes (Figure 8).
Table 2: Morphological parameters of control and Cd-treated L. rohita.
|
Parameters |
Control |
Cd-treated |
|
Diameter of hepatocytes |
10.95±0.18a |
8.64±0.23b |
|
Width of sinusoids |
5.91±0.41b |
8.44±0.04a |
MEAN± SEM: In the row, superscripts show values are significantly different (p< 0.05).
Hepato-somatic index (HSI)
Throughout the 60-days trial period, the HSI of both the treated and control fish fluctuated significantly. The HSI of Cd-treated fish firstly decreased then slightly increase. The HSI of control fish increased throughout experimental period. Figure 4 shows HSI of Cd-treated and control fish.
Acute toxicity
Acute toxicity tests are used to assess the hazards of chemical pollutants to organisms. The LC50 value is the concentration of a toxic substance at which 50% of the exposed population died and lethal value (LC100) is the concentration of a toxic substance at which 100% of the exposed population died. Through acute toxicity test, the 96 h LC50 and lethal value of Cr for fish L. rohita were calculated as 12.38 and 23.6mgL-1, respectively. According to the findings of Bekmezeci (2010), the acute toxicity of metals fluctuates greatly amongst different fish species. At the same dosage, a fish that is more susceptible to one metal may exhibit greater resistance to another metal. This can happen even if the two metals are in the same environment. Previous studies showed 96-h LC50 of cadmium for aquatic species liable on the type of species and metal. Yalsuyi et al. (2017) calculated 96-h LC50 of cadmium for Carassius auratus and Cyprinus carpio as 9.202 and 8.845 mgL-1, respectively. Younis et al. (2013) stated that the 96-hr LC50 value of CdCl2 for Oreochromis niloticusis as 14.8 mgL-1. Rani et al. (2015) determined the 96-hr LC50 value of Cd to Puntius ticto as 26 mgL-1.
Growth performance
Growth is a direct expression of the influence that metal has on the fish since growth incorporates all of the stimuli within the body (Azmat and Javed, 2011). In this study, fish growth parameters viz. daily weight gain, daily length gain, specific growth rate, condition factor, and feed conversion ratio of Cd-treated fish were significantly lesser in comparison with control fish. The findings of the present study were justified by Hansen et al. (2002) who concluded that growth reduction in fish occurred as a consequence of behavioral and physiological pressures during long-term exposure to heavy metals. Rahman et al. (2018) concluded that Cd stress decreased O. niloticus growth. Naz et al. (2013) concluded that chronic heavy metals exposure to L. rohita, C. catla, H. molitrix, C. mrigala, and C. idella gave significantly lower average weights and fork and total lengths in comparison to unstressed fish. They also concluded that condition factor was significantly better in unstressed fish species. According to (Javed, 2012) fish health and growth were adversely affected by metal exposure at sub-lethal concentrations. Verma and Prakash (2019) concluded that there is a significant decrease in fish appetite due to heavy metal toxicity that resulted into decrease in feed conversion efficiency and condition factor of fish. Abdel-Hakim et al. (2016) showed a substantial decrease in WG, SGR, FCR, and survival rate of O. niloticus after chronic exposure to Hg, Cu, Cd, and Pb. Rahman et al. (2018) investigated dropped growth rate of O. niloticus’s when exposed to cadmium stress. Fish health and growth are negatively impacted by metal exposure at sub-lethal levels, according to (Javed, 2012).
Histology of liver
Fish liver is more vulnerable to aquatic pollutants than other organs because most of pollutants have tendency to accumulate in liver in higher concentration in comparison with other organs (Al-Balawi et al., 2013). The current investigation shows that there are no pathological abnormalities in liver of control fish and it has a normal structure. During chronic trial, diameter of hepatocytes in Cd-treated fish was significantly lesser than control fish. Width of sinusoids in Cd-treated fish was significantly greater than control fish. Some other histological disorders in liver were also seen in Cd-treated fish viz. infiltration of sinusoid, dilated sinusoid and increased hemorrhage in sinusoids, inflammation and vacuolation in hepatocytes and ruptured hepatocytes. Liver is the most affected organ by water pollutants due to its role in detoxification and biotransformation processes, as well as its position and contact with bloodstream. These histological changes are typically linked to the hepatocytes response to toxins (Dyk et al., 2007). Olojo et al. (2005) also reported different variations in the liver of C. gariepinus including hepatocyte shrinkage and increase of sinusoidal spaces due to heavy metal exposure. Dyk et al. (2007) concluded that exposure of cadmium and zinc to O. mossambicus showed histological changes in liver included ruptured hepatocytes, inflammation and hepatocyte vacuolation and the degree of these histological alterations was determined by the duration of the exposure period. Prabhahar et al. (2012) evaluated the cadmium impact on the liver histology of C. mrigala. The findings revealed that the treated fish liver contained ruptured hepatocytes with lost polygonal structure and enlarged vacuoles in the hepatocytes with displaced nuclei. Fatima and Usmani (2013) reported that exposure of fishes (Channa striatus and Heteropneustes fossilis) to heavy metals including Cr showed various histological alterations in liver including vacuolization and hemorrhages. Alshkarchy et al. (2021) investigated the histopathological effects of Zn, Fe and Cu on the liver of the Euphrates River’s C. carpio. Major histopathological changes in liver were disintegration of the hepatocytes with spaces between the cells and several areas filled with degraded cells from the visceral tissues.
Hepatosomatic index
Hepato-somatic index reflects the metabolic status of the organism as well as the stress situation that has arisen as a result of metal exposure, and the excessive use of energy reserves in response to increased demand may result in a reduction in HSI (Verma and Prakash, 2019). The result of this study shows that hepato-somatic index of Cd exposed fish was significantly less than control fish throughout the experimental period of 60 days. The results of the present study were supported by (Verma and Prakash, 2019) who stated that HSI is the key indicator of metabolic activity and stress condition developed due to heavy metals and the surplus usage of energy assets in response to increase in requirement might cause the reduction in HSI. Bekmezci (2010) also reported that HSI of fish decreased due to heavy metals probably because of depletion of energy reserves in liver. Similarly, Singh and Srivastava (2015) reported that sub lethal exposure of heavy metals to H. fossilis caused a reduction of HSI however the control fish showed an increase in HSI. Messaoudi et al. (2009) also concluded that when Salaria basilisca was treated with cadmium for 14 and 28 days, the liver somatic index drastically reduced.
Conclusions and Recommendations
In this research work, Labeo rohita was used to check the effects of cadmium on fish. Cadmium had clear negative effects on the growth performance, liver histology and hepato-somatic index of fish. To mitigate the effects of heavy metals pollution on fish, it is crucial to implement strict regulations and practices to minimize the release of these contaminants into water bodies. Efforts should focus on reducing industrial emissions, improving waste management systems, and implementing sustainable agricultural practices.
Acknowledgement
Authors are grateful to the Department of Zoology, CUVAS, Bahawalpur for providing the lab facility.
Conflict of interest
The authors have declared no conflict of interest.
References
Abdel-Hakim, N.F., Helal, A.F., Salem, M.F., Zaghloul, A.M. and Hanbal, M.M., 2016. Effect of some heavy metals on physiological and chemical parameters in Nile tilapia (Oreochromis niloticus L.). J. Egypt. Acad. Soc. Environ. Dev. D. Environ. Stud., 17: 81-95. https://doi.org/10.21608/jades.2016.63382
Ahmed, S.I., Ahmmed, M.K., Ghosh, S.K., Islam, M.M. and Shahjahan, M., 2015. Histo-architectural changes of intestinal morphology in Zebra fish (Daniorerio) exposed to Sumithion. Res. Agric. Livest. Fish., 2: 499-506. https://doi.org/10.3329/ralf.v2i3.26174
Al-Balawi, H.F.A., Al-Akel, A.S., Al-Misned, F., Suliman, E.A.M., Al-Ghanim, K.A., Mahboob, S. and Ahmad, Z., 2013. Effects of sub-lethal exposure of lead acetate on histopathology of gills, liver, kidney and muscle and its accumulation in these organs of Clarias gariepinus. Braz. Arch. Biol. Technol., 56: 293-302. https://doi.org/10.1590/S1516-89132013000200015
Alshkarchy, S.S., Raesen, A.K. and Najim, S.M., 2021. Effect of heavy metals on physiological and histological status in liver of common carp Cyprinus carpio, reared in cages and wild in the Euphrates River, Babil/Iraq. Earth Environ. Sci., 779: 12-66. https://doi.org/10.1088/1755-1315/779/1/012066
Azmat, H., Javed, M ., 2011. Acute toxicity of chromium to Catla catla, Labeo rohita and Cirrhina mrigala under laboratory conditions. Int. J. Agric. Biol., 13: 961-965.
Bancroft, J.D., Floyd, A.D. and Suvarna, S.K., 2013. Bancroft’s theory and practice of histological techniques. 7th ed. New York (NY) USA: Elsevier Health Sci.,
Bekmezci, H.D., 2010. Asago Scyhan Ovasr Drenaj Sistemlerindeki Kirlilik Etmenlerinin Clarias gariepinus’da Toksik etkileri, Cukurova Universitesi, Fen Biyoliji ABD, Doktora Tezi, 145.
Dane, H. and Sisman, T., 2020. Effects of heavy metal pollution on hepatosomatic index and vital organ histology in Alburnus mossulensis from Karasu River. Turk. J. Vet. Anim. Sci., 44: 607-617. https://doi.org/10.3906/vet-1904-50
Dyk, V.J.C., Pieterse, G.M. and Vuren, J.H.J.V., 2007. Histological changes in the liver of Oreochromis mossambicus (Cichlidae) after exposure to cadmium and zinc. Ecotoxicol. Environ. Saf., 66: 432-440. https://doi.org/10.1016/j.ecoenv.2005.10.012
Farkas, A., Salanki, J. and Specziar, A., 2002. Relation between growth and the heavy metal concentration in organs of bream Abramisbrama L. populating Lake Balaton. Arch. Environ. Contam. Toxicol., 43: 236-243. https://doi.org/10.1007/s00244-002-1123-5
Farombi, E.O., Adelowo, O.A. and Ajimoko, Y.R., 2007. Biomarkers of oxidative stress and heavy metal levels as indicators of environmental pollution in African cat fish (Clarias gariepinus) from Nigeria Ogun River. Int. J. Environ. Res. Publ. Hlth., 4: 158-165. https://doi.org/10.3390/ijerph2007040011
Fatima, M. and Usmani, N., 2013. Histopathology and bioaccumulation of heavy metals (Cr, Ni and Pb) in fish (Channa striatus and Heteropneustes fossilis) tissue: A study for toxicity and ecological impacts. Pak. J. Biol. Sci., 16: 412-420. https://doi.org/10.3923/pjbs.2013.412.420
Gbem, T.T., Balogun, J., Lawal, F.A. and Annune, P.A., 2001. Trace metal accumulation in Clarias gariepinus (Teugels) exposed to sublethal levels of tannery effluent. Total Environ., 271: 1-9. https://doi.org/10.1016/S0048-9697(00)00773-7
Gernhöfer, M., Pawert, M., Schramm, M., Müller, E. and Triebskorn, R., 2001. Ultrastructural biomarkers as tools to characterize the health status of fish in contaminated streams. J. Aquat. Ecosyst. Stress Recovery, 8: 241-260. https://doi.org/10.1023/A:1012958804442
Hansen, J.A., Welsh, P.G., Lipton, J. and Suedkamp, M.J., 2002. The effects of long-term cadmium exposure on the growth and survival of juvenile bull trout (Salvelinus confluentus). Aquat. Toxicol., 58: 165-174. https://doi.org/10.1016/S0166-445X(01)00233-8
Heydarnejad, M.S., Khosravian-Hemamai, M. and Nematollahi, A., 2013. Effects of cadmium at sub-lethal concentration on growth and biochemical parameters in rainbow trout (Oncorhynchus mykiss). Ir. Vet. J., 66: 1-7. https://doi.org/10.1186/2046-0481-66-11
Huo, J., Dong, A., Yan, J., Wang, L., Ma, C. and Lee, S., 2017. Cadmium toxicokinetics in the freshwater turtle, Chinemysreevesii. Chemosphere, 182: 392-398. https://doi.org/10.1016/j.chemosphere.2017.05.029
Hyun, S., Lee, T., Lee, C.H. and Park, Y.H., 2006. The effects of metal distribution and anthropogenic effluents on the benthic environment of Gwangyang Bay, Korea. Mar. Pollut. Bull., 52: 113-120. https://doi.org/10.1016/j.marpolbul.2005.10.011
Javed, M. And Saeed, M.A., 2010. Growth and bioaccumulation of iron in the body organs of Catla catla, Labeo rohita and Cirrhina mrigala during chronic exposures. Int. J. Agric. Biol., 12: 881-886.
Javed, M. and Usmani, N., 2017. An overview of the adverse effects of heavy metal contamination on fish health. Proc. Natl. Acad. Sci. India B Biol. Sci., 89: 389-403. https://doi.org/10.1007/s40011-017-0875-7
Javed, M., 2004. Comparison of selected heavy metals toxicity in the planktonic biota of the river Ravi.Indus J. Biol. Sci., 1: 59-62.
Javed, M., 2012. Growth responses of fish under chronic exposures of water-borne and dietary metals. Int. J. Agric. Biol., 14: 281-285.
Kasperk, R., Füzeti, L., Müller, M. and Schumpelick, V., 1994. Morphometric study of centrilobular vessels in the rat liver after continuous hypothermic perfusion with Euro-Collins and University of Wisconsin solution. Res. Exp. Med., 194: 383-390. https://doi.org/10.1007/BF02576401
Levit, S.M., 2010. A literature review of effects of cadmium on fish. Nat. Conserv., 15.
Messaoudi, I., Barhoumi, S., Saïd, K. and Kerken, A., 2009. Study on the sensitivity to cadmium of marine fish Salaria basilisca (Pisces: Blennidae). J. Environ. Sci., 21: 1620-1624. https://doi.org/10.1016/S1001-0742(08)62464-X
Naz, S., Javed, M. and Tahir, A., 2013. Assessing growth responses of fish exposed to heavy metals mixture by using regression analyses. Pakistan J. Zool., 45: 921-928.
Nogami, E. M., Kimura, C. C., Rodrigues, C., Malagutti, A. R., Lenzi, E. and Nozaki, J., 2000. Effects of dietary cadmium and its bioconcentration in tilapia Oreochromisniloticus. Ecotoxicol. Environ. Saf., 45: 291-295. https://doi.org/10.1006/eesa.1999.1858
Okocha, R.C. and Adedeji, O.B., 2011. Overview of cadmium toxicity in fish. Res. J. appl. Sci., 7: 1195-1207.
Olojo, E., Olurin, K., Mbaka, G. and Oluwemimo, A., 2005. Histopathology of the gill and liver tissues of the African catfish Clarias gariepinus exposed to lead. Afr. J. Biotechnol., 4: 117-122.
Pandit, D.N. and Gupta, M.L., 2019. Heapto-somatic index, gonado-somatic index and condition factor of Anabas testudineus as bio-monitoring tools of nickel and chromium toxicity. Int. J. Innov. Eng. Technol., 12: 25-28.
Pimpão, C.T., Zampronio, A.R. and De Assis, H.S., 2007. Effects of deltamethrin on hematological parameters and enzymatic activity in Ancistrus multispinis (Pisces, Teleostei). Pestic Biochem. Physiol., 88: 122-127. https://doi.org/10.1016/j.pestbp.2006.10.002
Prabhahar, C., Saleshrani, K., Tharmaraj, K. and Vellaiyan, M., 2012. Effect of cadmium compound on the histological changes of various vital organs of the fresh water fish Cirrhinus mrigala. Int. J. Pharm. Biol. Arch., 3: 84-88.
Rahman, Z., Ahmad, I. and Rashid, I., 2018. Effects of cadmium exposure on growth and survival and accumulation in various organs of Nile Tilapia (Oreochromis niloticus, Linnaeus). J. Agric. Aquacult., 1: 1-10.
Rajaguru, A., 1992. Biology of two co-occurring tonguefishes, Cynoglossusarel and C. lida (Pleuronectiformes: Cynoglossidae), from Indian waters. Fish. Bull., 90: 328-367.
Rani, S., Gupta, R.K. and Rani, M., 2015. Heavy metal induced toxicity in fish with special reference to zinc and cadmium. Int. J. Fish. Aquat., 3: 118-123.
Satarug, S., Garrett, S.H., Sens, M.A. and Sens, D.A., 2010. Cadmium, environmental exposure, and health outcomes. Environ. Hlth. Perspect., 118: 182-190. https://doi.org/10.1289/ehp.0901234
Shukla, V., Dhankhar, M., Prakash, J. and Sastry, K.V., 2007. Bioaccumulation of Zn, Cu and cd in Channa punctatus. J. Environ. Biol., 28: 395.
Singh, S. and Srivastava, A.K., 2015. Variations in hepatosomatic index (HSI) and gonadosomatic index (GSI) in fish Heteropneustes fossilis exposed to higher sub-lethal concentration to arsenic and copper. J. Ecophysiol. Occup. Hlth, 15: 89-93.
Thophon, S., Kruatrachue, M., Upatham, E.S., Pokethitiyook, P., Sahaphong, S. and Jaritkhuan, S., 2003. Histopathological alterations of white seabass, Latescalcarifer, in acute and subchronic cadmium exposure. Environ. Pollut., 121: 307-320. https://doi.org/10.1016/S0269-7491(02)00270-1
Verma, A.K. and Prakash, S., 2019. Impact of arsenic on haematology, condition factor, hepatosomatic and gastrosomatic index of a fresh water cat fish, Mystus vittatus. Int. J. Biol. Sci., 10: 49-54.
Vosyliene, M.Z. and Mikalajūnė, A., 2006. Effect of heavy metal model mixture on rainbow trout biological parameters. Ekologija, 4: 12-17.
Yalsuyi, A.M., Hedayati, A., Vajargah, M.F. and Mousavi-Sabet, H., 2017. Examining the toxicity of cadmium chloride in common carp (Cyprinus carpio) and goldfish (Carassiusauratus). J. Environ. Treat. Tech., 5: 83-86.
Younis, E.M., Abdel-Warith, A.A., Al-Asgah, N.A., Ebaid, H. and Mubarak, M., 2013. Histological changes in the liver and intestine of Nile tilapia, Oreochromis niloticus, exposed to sublethal concentrations of cadmium. Pakistan J. Zool., 45: 833-841.
To share on other social networks, click on any share button. What are these?








