Tomato yellow leaf curl virus in Tomato Crop of Khyber Pakhtunkhwa Province: Virus and Vector Prevalence and Transmission Properties
Tomato yellow leaf curl virus in Tomato Crop of Khyber Pakhtunkhwa Province: Virus and Vector Prevalence and Transmission Properties
Ghufran ul Haq1*, Muhammad Arif2, Asad Ali2 and Mian Inayatullah3
1Principal Research Officer, Agriculture Research Institute Tarnab, Peshawar, Pakistan; 2Department of Plant Pathology, Faculty of Crop Protection Sciences, The University of Agriculture, Peshawar, Pakistan; 3Department of Entomology, Faculty of Crop Protection Sciences, The University of Agriculture, Peshawar, Khyber Pakhtunkhwa, Pakistan.
Abstract | The present study was conducted to determine the prevalence of Tomato yellow leaf curl virus (TYLCV) and the population of its vector, whiteflies (Bemisia tabaci), and determine the transmission properties of the prevalent isolate of the virus in the major tomato growing areas of Khyber Pakhtunkhwa (KP). For this purpose, surveys were conducted in the hilly areas of Mohmand Agency and Malakand Agency and plains of Shabqadar, Charsadda and Peshawar during 2011, 2012 and 2013, to determine the incidence of TYLCV and the population of its vector. Triple antibody sandwich-enzyme linked immunosorbent assay (TAS-ELISA) was successful in detecting TYLCV in naturally and whiteflies inoculated tomato plants. Transmission properties of the virus were determined by inoculation of tomato plants of susceptible cultivar, Roma-VF, by whiteflies, determining the minimum time required for acquisition and inoculation of the virus, latency period and the persistence capacity. The highest incidence of 9.47 percent of TYLCV was recorded in Mohmand Agency, followed by Malakand Agency with 8.2 percent, Shabqadar with 7.7 percent, Charsadda with 4.9 and Peshawar with 5.8 percent. The average whiteflies population was the highest, 22.13, in Mohmand Agency, followed by Malakand Agency with 18.5, Shabqadar with 15.6, Charsadda with 14.9 and Peshawar with 14.2. A positive correlation was found between the whiteflies population and the viral incidence. Studies on the transmission properties of TYLCV revealed that, given an acquisition access peiod (AAP) of 48 hours, a single viruliferous whitefly had the capacity to transmit the virus, but for 100 percent transmission a minimum of five whiteflies per seedling were required. The minimum requirement of AAP was 30 minutes, which was longer than inoculation access period (IAP) of 20 minutes. For 100 percent acquisition and transmission, a minimum of 10 hours was required. The latent period was five to seven hours. The virus persisted in its vector, Bemisia tabaci, upto 10 days after which the whiteflies were dead, indicating a persistent type of transmission. The viral incidence and whiteflies population were higher in the cooler hilly areas than the plains and the values for the transmission parameters were different from those reported elsewhere in the world.
Received | October 13, 2017; Accepted | May 14, 2018; Published | July 02, 2018
*Correspondence | Ghufran ul haq, Agriculture Research Institute Tarnab, Peshawar, Khyber Pakhtunkhwa, Pakistan; Email: [email protected]
Citation | Haq, G., M. Arif, A. Ali and M. Inaaullah. 2018. Tomato yellow leaf curl virus in tomato crop of Khyber Pakhtunkhwa province: virus and vector prevalence and transmission properties. Sarhad Journal of Agriculture, 34(3): 500-508.
DOI | http://dx.doi.org/10.17582/journal.sja/2018/34.3.500.508
Keywords | Tomato, TYLCV, Incidence, Virus, Vector, Pakistan
Introduction
Tomato (Lycopersicon esculentum L. Mill), a member of the family Solanaceae, is an important vegetable crop for its high nutritive value and consumption throughout the world (Rice et al., 1987). The viral diseases of tomato are not only prevalent in Pakistan but are present worldwide causing severe economic losses (Golnaraghi et al., 2004). About 146 viruses are reported to infect tomato worldwide (Czosneck et al., 2001; Petrov, 2014). TYLCV is one of the most devastating problems of tomato worldwide (Moriones and Navas-Castillo, 2000).
Tomato yellow leaf curl virus (TYLCV) is a type species of the genus, Begomovirus, and family, Geminiviridae, is one of the most devastating begomoviruses of tomato which has long been known in the Middle East (Czosnek et al., 1990), and spread to Southern Europe where severe outbreaks of TYLCV occurred (Moriones and Navas-Castillo, 2000). It has also been identified in the Carribean region (Nakhla et al., 1994), Mexico (Ascencio-Ibanez, 1999) and the United States and in Georgia (Mamol et al., 1999; Lapidot et al., 2001). Among the viral diseases infecting tomato in Pakistan, TYLCV has emerged as the most important geminivirus (Haider et al., 2007). The yield reduction caused by TYLCV was 5-70 percent in crop sown in February to May. A yield loss of 100 percent was reported in sub tropical region (Lapidot et al., 1997; Sawalha, 2013). Symptoms produced by TYLCV include vein clearing accompanied by twisting and upward and downward rolling of the leaf lamina (Cercauskas, 2004). Fruits are smaller in size and their number is markedly reduced (Anfoka et al., 2005). The most prominent symptoms of the TYLCV in tomato crop are leaf yellowing and curling (Melzer et al., 2009).
The host range of TYLCV is quite broad infecting plants belonging to Solanaceae, Malvaceae and Leguminoseae (Zakay et al., 1991; Sawalha, 2013). TYLCV has been shown to infect tomato and at least 30 other plant species in over 12 plant families (Salati et al., 2002; Polston and Lapidot, 2007). Weeds can also play a role in TYLCV survival and spread in most parts of the world (Sawalha, 2009). Some weeds, such as Datura stramonium and Cynanchum acutum, produce distinct symptoms, whereas others, such as Malva parviflora serve as symptomless carriers (Salati et al., 2002).
The transmission properties of TYLCV have been extensively studied and these parameters can vary depending on the virus, its strain and the aleyrodid biotype. The virus primarily is transmitted by the sweetpotato whitefly (Bemisia tabaci). These whiteflies can acquire the virus by feeding on infected plants for as little as 5 minutes, and remain infective for life. The virus, however, is not passed on to their progeny. TYLCV is not spread by other whitefly species, such as the greenhouse whitefly (Trialeurodes vaporariorum) (Melzer et al., 2009). A single insect has the efficiency to acquire and transmit the virus. The minimum acquisition access and inoculation access periods are 30 minutes and 15 minutes. The rate of transmission increases with increase in acquisition and inoculation access periods. A minimum latent period of 8 hours from the start of acquisition is required for B. tabaci to transmit it to tomato test plants. (Martin, 1987). Other studies on these parameters showed that the AAP and IAP were approximately 10 to 20 minutes (Ghanem et al., 2001; Lapidot et al., 2001; Salati et al., 2002; Goldman and Czosneck, 2002). The minimal latent period reported was 21 hours but was 24 hours for the closely related TYLCV strain from Egypt (Mehta et al., 1994) and 17 hours for the more distant virus from Sardinia (Caciagli et al., 1995). Due to the presence of vectors in the tomato growing area of KP, TYLCV has spread widely in the crop causing heavy yield losses, but no work was done on the population of vectors and incidence of TYLCV and its transmission properties in the specific agro-climate of the area. Determining these parameters together with the knowledge of the virus hosts allows to elucidate the epidemiology of different geminivirus diseases as well as to design disease management strategies. This paper reports the incidence of TYLCV and population of its vector, B. tabaci, and its transmission properties of the severe isolate of the virus prevalent in our surveyed areas of KP.
Materials and Methods
Survey and plant sampling
Three comprehensive surveys were conducted during 2011, 2012, and 2013, in the major tomato growing areas of KPK which comprised hilly areas in Malakand and Mohmand Agencies and plains of Shabqadar, Charsadda and Peshawar (Figure 1). In each zone, three to nine villages were surveyed. In each village three fields were surveyed and in each field, 4m2 areas in three sites were randomly selected and the number of plants for characteristic symptoms of Tomato yellow leaf curl virus and the total number of plants were counted. The characteristic symptoms of TYLCV used during sampling were yellowing, curling and upward cupping of leaves (Figure 2). TYLCV in all plant samples was detected by Triple antibodies sandwich-Enzyme linked immunosorbent assay (TAS-ELISA). The percent incidence and distribution of TYLCV were calculated using the following formula:
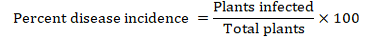
Whitefly population
Data on the population of whiteflies were recorded. Eight plants were randomly selected and whiteflies were counted on the upper, middle and lower leaf of selected plants and their average number was calculated.
Sources of Virus isolate
Plant samples of naturally infected tomato plants showing symptoms of TYLCV were collected from plain areas of Peshawar, Charsadda, Shabqadar and hills of Mohmand agency and Malakand agency. These plants were used as virus source after with TAS-ELISA.
Diagnosis of TYLCV
TYLCV was diagnosed in all plant samples by TAS-ELISA (Pico et al., 1999; Kashina et al., 2003).
Source of whiteflies
Non viruliferous whitiflies were collected from Sarson (Brassica spp.) and were identified to be Bemisia tabacii in Entomology Research Section, FATA, Agricultural Research Institute Tarnab, Peshawar, KP, Pakistan. A group of 15 whiteflies were provided an IAP of 24 hours on tomato test plants. Symptoms were routinely observed and TYLCV was confirmed by TAS-ELISA (Figure 4).
Acquisition and transmission of TYLCV by Bemisia tabaci
Adult viruliferous whiteflies were collected from TYLCV infected tomato plants in groups of one, two, three, four, five, seven and ten individuals. Each group of whiteflies was caged on 30 tomato test plants inside a nylone net for an IAP of 48 hours. Virus transmission was evaluated by development of symptoms and confirmed by TAS-ELISA (Figure 4).
For determination of AAP of TYLCV, 30 test plants
Table 1: Incidence of Tomato yellow leaf curl virus in tomato growing areas of KP province during 2011, 2012 and 2013.
| Year 2011 | Year 2012 | Year 2013 | |||||
| Areas | Infected/ tested | Percent incidence | Infected/ tested | Percent incidence | Infected/ tested | Percent incidence | Mean Percent incidence |
| Mohmand Agency | 14/300 | 4.6 | 21/160 | 13.12 | 24/200 | 12.0 | 9.47 |
| Malakand Agency | 13/500 | 2.6 | 16/500 | 10.0 | 48/400 | 12.0 | 8.2 |
| Shabqadar | 12/500 | 2.4 | 15/700 | 2.1 | 25/700 | 3.5 | 7.7 |
| Charsadda | 11/300 | 3.6 | 15/200 | 7.5 | 19/500 | 3.8 | 4.9 |
| Peshawar | 10/300 | 3.3 | 28/280 | 3.2 | 38/350 | 10.9 |
5.8 |
of tomato cultivar, Roma-VF, found TYLCV-susceptible in our three years survey, were selected. Virus free whiteflies were caged on TYLCV infected tomato plants for different times, such as 10, 20, 30, 40 minutes, and 01, 05, 12, 18, 25, 48 and 72 hours to acquire the virus. To determine the IAP, the whiteflies were then caged on tomato test plants of susceptible variety, Roma-VF, using 30 plants per each treatment for various time periods, such as 10, 20, 30, 40 minutes, 01, 05, 10, 12, 18, 25, 48 and 72 hours. Each group of 30 plants was separately covered in nylone net, inside a screen house equipped with an insect proof screen, so that insects may not pass through it (Firmino et al., 2009).
For determining the latency period, five whiteflies were given an AAP of one hour and then transferred to each group of 20 test plants, covered in nylone net, for various IAPs, such as one, two, four, six, eight, ten, 12, 16, 20, and 24 hours. After each period, the insects were eliminated by spraying with the insecticide, Imidachloprid. Periodical symptoms evaluation were conducted and plants infection were confirmed by symptoms expression and TAS-ELISA (Munniyapa et al., 2000; Firmino et al., 2009).
For determining the persistence of TYLCV in its vectors, the whiteflies were given an AAP of 24 hours on infected tomato seedlings. The whiteflies were then caged on tomato test plants of variety, Roma VF, for successive IAPs of 24 hours. Each group of five whiteflies was allowed access to 20 test plants after one, two, three, four, five, six, seven, eight, nine, ten, 11, 12, 13 and 14 days. After each trial, the virus transmission was evaluated by symptoms expression and TAS-ELISA. The longest period after which the whiteflies transmitted the virus showed the persistence capacity of TYLCV inside the whiteflies (Firmino et al., 2009).
Results and Discussion
Incidence and distribution of TYLCV and its whitefly vectors in Khyber Pakhtunkhwa province
The naturally infected tomato plants, exhibiting marginal chlorosis, upward or downward leaf curling, flower abortion and stem upright as well as stunted plant growth, were counted (Figure 2). The results of the three years survey revealed that the highest mean incidence of 9.47 percent of TYLCV was recorded in Mohmand Agency, followed by Malakand Agency with 8.2 percent, Shabqadar with 7.7 percent, Charsadda with 4.9 and Peshawar with 5.8 percent. The viral incidence in 2013 was relatively higher than 2011 and 2012. Howevere, it was detected in all the areas surveyed (Table 1).
Whiteflies were observed in naturally infected tomato crop in the plain areas as well as in the hilly areas (Figure 3). The mean whiteflies population, over the three years, was the highest, 22.13, in Mohmand Agency, followed by Malakand Agency with 18.5, Shabqadar with 15.6, Charsadda with 14.9 and Peshawar with 14.2. The population of whiteflies was higher in the cooler higher altitudes of the hills of Mohmand and Malakand Agencies than the plains of Peshawar, Charsadda and Shabqadar. Whiteflies population showed a positive correlation with the incidence of TYLCV (Table 2).
Table 2: Population of whiteflies in tomato growing areas of KP province during 2011, 2012 and 2013.
| Zones | Year 2011 | Year 2012 | Year 2013 | Mean |
| Mohmand Agency | 22.4 | 22.0 | 22.0 | 22.13 |
| Malakand Agency | 18.0 | 14.5 | 23.0 | 18.5 |
| Shabqadar | 17.0 | 10.0 | 20.0 | 15.6 |
| Charsadda | 11.0 | 14.5 | 19.2 | 14.9 |
| Peshawar | 14.0 | 11.6 | 17.0 |
14.2 |
Acquisition and transmission of TYLCV
Our results showed that, given a 24 hours AAP, a single viruliferous whitefly had the capacity to transmit TYLCV, but the rate of transmission, which was 25 percent, was very low. By increasing the number of whiteflies, the rate of transmission increased. Using different number of whiteflies, the rate of transmission increased by 55, 70, 80 and 100 percent. In increasing the number of whiteflies beyond 5, such as 7 and 10, the transmission rate remained constant, that is, 100 percent. It was concluded from these studies that even a single whitefly had the capacity to transmit the virus, but for 100 percent transmission, a minimum of 5 whiteflies per seedling were required (Table 3).
Table 3: Effect of the number of whiteflies on the transmission of TYLCV.
| Number of whiteflies | Infected/Inoculated | Percent Transmission |
| 01 | 3/12 | 25 |
| 02 | 11/20 | 55 |
| 03 | 14/20 | 70 |
| 04 | 16/20 | 80 |
| 05 | 20/20 | 100 |
| 07 | 20/20 | 100 |
| 10 | 20/20 |
100 |
Effect of number of whiteflies was determined by giving them 24 hours of each AAP and IAP; A 25 days old tomato seedlings of the susceptible variety, Roma-VF, were used.
Table 4: Effect of different acquisition access periods (AAPs) and inoculation access periods (IAPs) on transmission efficiency of TYLCV to TYLCV- susceptible tomato, cv. Roma VF, by B. tabaci.
| Acquisition access periods | Inoculation access periods | ||||
|
Time
|
Infected/ Inoculated | Percent transmision | Time | Infected/ Inoculated | Percent transmision |
| 10min | 0/30 | 0.0 | 10min * | 0/30 | 0.0 |
| 20 min | 0/30 | 0.0 | 20 min | 1/30 | 3.4 |
| 30 min | 2/30 | 6.7 | 30 min | 3/30 | 10 |
| 40 min | 4/30 | 13.4 | 40 min | 5/30 | 16.7 |
| 1h | 8/30 | 26.7 | 1h ** | 7/30 | 23.4 |
| 5 h | 25/30 | 93.4 | 5 h | 21/30 | 70 |
| 10 h | 30/30 | 100 | 10 h | 30/30 | 100 |
| 12 h | 30/30 | 100 | 12 h | 30/30 | 100 |
| 18 h | 30/30 | 100 | 18 h | 30/30 | 100 |
| 25 h | 30/30 | 100 | 25 h | 30/30 | 100 |
| 48 h | 30/30 | 100 | 48 h | 30/30 | 100 |
| 72 h | 30/30 | 100 | 72 h | 30/30 |
100 |
* min: Minutes; ** h: Hours.
The AAP required for the whiteflies to transmit the virus to tomato test plants was determined. Following each AAP on infected tomato plants, the whiteflies were allowed to feed on 20 tomato test plants, using five insects per plant (Figure 3). The results showed that the minimum AAP was 30 minutes when two out of 20 test plants were infected, showing a transmission rate of 6.7 percent. The transmission efficiency increased with the length of AAP. The minimum IAP required was also determined similarly. The minimum IAP was 20 minutes when one out of 30 test plants were infected showing a transmission rate of 3.4 percent. The transmission efficiency increased with the length of IAP. An IAP of 10 hours was required for 100 percent transmission (Table 4).
The latent period of TYLCV was determined. Following an AAP of 01 hour, the whiteflies were allowed to feed on tomato test plants for different IAPs. The whiteflies started to efficiently transmit the virus after six hours of IAP, which indicated that the insects got the ability to inoculate the plants between five and seven hours after the start of AAP (Table 5).
Table 5: Latent period of TYLCV in whiteflies.
| Time (hours) | Infected/ Inoculated | Percent transmision |
| 01 | 0/20 | 0 |
| 02 | 0/20 | 0 |
| 04 | 0/20 | 0 |
| 06 | 3/20 | 15 |
| 08 | 3/20 | 15 |
| 10 | 6/20 | 30 |
| 12 | 8/20 | 40 |
| 16 | 12/20 | 60 |
| 20 | 14/20 | 70 |
| 24 | 14/30 |
70 |
A 5 viruliferous insects were caged on each seedling following an AAP of 01 hour; Latent period: IAP+AAP for 01 hour.
The study revealed a persistant type of transmission of the virus. The whiteflies were provided a 24 hours AAP on infected tomato plants, using five insects per plant. The whiteflies were allowed to feed on tomato test plants for consecutive IAP of 24 hours and then on healthy tomato plants. The whiteflies transmitted the virus upto 10 days, after which they were dead, which indicated the presence of the virus in the whiteflies. As the whiteflies were dead after ten days, therefore, no further transmission was observed (Table 6).
Our data, for the three years, on the incidence of TYLCV and whiteflies showed a higher population of inoculative whiteflies and the higher incidence of TYLCV in the hilly areas than in the plains. As other tomato viruses were also infecting the crop causing mixed infection, however, plants with typical symptoms of TYLCV were counted for determining the incidence. These higher altitudes provide a cooler favourable environment for the whiteflies which caused a higher incidence of TYLCV. During 2011, the highest incidence of TYLCV and the highest whiteflies population was recorded in hills of Mohmand and Malakand agencies than the plains of Shabqadar, Charsadda and Peshawar. The data on these parameters in the following years, 2012 and 2013, showed the same trend. The study on the epidemiology of TYLCV in Palestine also showed that a favorable condition for multiplication of whiteflies and their higher population had a potential role in the outbreak of TYLCV in the region (Sawalha, 2013).
Table 6: Persistence of TYLCV in whiteflies after 24 hours of Acquisition access period on infected tomato plants of TYLCV susceptible cultivar, Roma VF.
| No. of days | Infected/ noculated | % infection | Transmission |
| 1 | 3/20 | 15 | T |
| 2 | 8/20 | 40 | T |
| 3 | 18/20 | 90 |
T |
| 4 | 20/20 | 100 | T |
| 5 | 20/20 | 100 | T |
| 6 | 20/20 | 100 | T |
| 7 | 20/20 | 100 |
T |
| 8 | 20/20 | 100 | T |
| 9 | 20/20 | 100 | T |
| 10 | 20/20 | 100 | T |
| 0/20 | 0.0 |
D |
T: Transmission; D: Death of whiteflies.
The acquisition, retention and transmission were, for the first time, studied for Tomato yellow leaf curl virus-Israel (TYLCV-Is) and were based on biological tests (Cohen and Nitzany, 1966). Our study on these parameters revealed several phenomena. Firstly, a single whitefly was efficient enough to transmit TYLCV, following a 24 hours AAP, though not all plants were infected this way. These results were supported by other studies which reported the same AAP enabling a single whitefly to transmit the virus (Czosnech et al., 2002). Secondly, increasing the insect number the efficiency of transmission increased and when 5 insects per seedling were used, the transmission efficiency reached 100 percent. Similar results were obtained in other studies in which 5 to 15 insects were used (Czosnech et al., 2002). Thirdly, it was observed in our study that a longer AAP of 30 minutes was required for virus than an IAP of 20 minutes. Other studies on TYLCV, mentioned that the minimum requirement of AAP was usually longer than IAP (Salati et al., 2002). Fourthly, increasing both the AAP and IAP increased the efficiency of transmission. The transmission efficiency was 100 percent when an AAP and IAP of 10 hours were applied. The transmission of Tomato yellow leaf curl virus-Saudi (TYLCV-SA) was 100 percent for an AAP and IAP of 48 hours.
On the other hand great variations have been reported in the values of these parameters for TYLCV from different parts of the world. Our values for the minimum times required for AAP and IAP were 30 and 20 minutes compared to 15 to 60 minutes and 15 to 30 minutes for TYLCV-Is, as then reported (Cohen and Nitzany, 1966; Mansour and Al-Musa, 1992; Mehta et al., 1994). The minimum AAP and IAP of Tomato leaf curl virus-Banglore (ToLCV-Ban4), a begomovirus, were 10 and 20 minutes respectively (Muniyappa et al., 2000). Similar values were reported for TYLCV-SA from Italy (Casiagli et al., 1995) and from India (Butter and Rataul, 1977; Reddy and Yaraguntarah, 1981). The AAP and IAP required for 100 percent transmission of TYLCV-SA was 48 hours.
The latent period in our study was 7 hours compared to latent period of 8 hours for TYLCV-Is (Ghanem et al., 2001). The latent period longer than 24 hours was required for TYLCV from Egypt (Mehta et al., 1994), while 6 hours reported for ToLCV-Ban4 (Munniyappa et al., 2000). The parameters of acquisition and transmission reported for TYLCV isolates from the Middle East also showed greater heterogenith (Cohen and Nitzany, 1966). The latent period varied between 6 and 24 hours and the virus was retained by its vector for the entire life span.
The persistence capacity in our results was 10 days after which the whiteflies were dead, indicating a persistent type of transmission. The parameters of acquisition and transmission of TYLCV isolates from the Middle East were also heterogeneous (Cohen and Nitzany, 1966). The latent period varied between 6 and 24 hours and the virus was retained by its vector for the entire life span which indicates persistence of the virus.
Variables such as the TYLCV species, geoghraphic origin of the virus isolate and the whiteflies may also affect these parameters as reported in studies on other begomoviruses (Firmino et al., 2009). These variations may also be due to the frequency of whiteflies, the way the insects are handled and the nature of the test plants. (Rosell et al., 1999).
From our results and studies conducted elsewhere it can be inferred that the persistence of TYLCV in its vector and the efficiency of a single whitefly to inoculate the virus, intensive cultivation of tomato throughout the year and weed hosts harboring the virus are the causes for the high incidence of TYLCV in the nature.
Acknowledgements
The authors acknowledge the administrative staff of the Department of Plant Pathology, the University of Agriculture Peshawar-Pakistan, for providing laboratory facilities; Director General, Agricultural Research Institute Tarnab, Peshawar, for providng field and screen house; Mr. Akhtar Munir, Field Officer, Sher Ali Seed Company Farm, Akorha Khattak, for providing inoculative whiteflies and seeds of tomato, cv. Roma VF.
Conclusions
The incidence of TYLCV and whitefly population was higher in the hilly areas than in the plains was because of the cooler favourable environment for the multiplication of the whiteflies. A positive correlation was found between the whitefly population and the viral incidence. A single viruliferous whitefly had the capacity to transmit the virus, though not all plants were infected this way, however, five whiteflies per plant caused 100 percent transmission. The minimum requirement of acquisition access period (AAP) was 30 minutes, which was longer than inoculation access period (IAP) of 20 minutes. The values for the transmission parameters in our study were different from those reported elsewhere in the world which was due to difference in the strain.
Author’s Contribution
Muhammad Arif and Asad Ali conceived and designed the experiments; Ghufranul Haq performed the experiments; Muhammad Arif analyzed the data; Mian Inayatullah contributed reagents and materials; Ghufranul Haq wrote the first draft of the paper: All authors read and approved the final manuscript.
References
Anfoka, G.H., M. Abhary and M.K. Nakhla. 2005. Molecular identification of the species of Tomato yellow leaf curl virus complex in Jordan. J. Pl. Pathol. 87: 65-70.
Ascencio-Ibanez., J.T, R. Diaz-Plaza, J. Mendez-Lozano, Z.I. Monslave- Fonnerga, G.R. Arguello-Astroga and R.F. Rivera-Bustamante. 1999. First report of Tomato yellow leaf curl geminivirus in Yucatan Mexico. Pl. Dis. 83:1178. https://doi.org/10.1094/PDIS.1999.83.12.1178A
Butter, N.S. and H.S. Rataul. 1977. The virus vector relationship of Tomato leaf curl virus (ILCV) and its vector Bemisia tabaci (Homoptera: Aleyrodidae). Phytoparasit. 5:170–186. https://doi.org/10.1007/BF02980351
Caciagli, P., D. Bosco and L. Albitar. 1995. Relationships of the Sardinian isolate of tomato yellow leaf curl geminivirus with its whitefly vector Bemisia tabaci Gen. Euro. J. Pl. Pathol. 101: 163-170. https://doi.org/10.1007/BF01874762
Cercauskas, R. 2004. Tomato yellow leaf curl virus. Fact Sheet. AVRDC. The World Vegetable Centre. Thaiwan.
Cohen, S. and E.E. Nitzany. 1966. Transmission and host range of Tomato yellow leaf curl virus. Phytopathol. 56: 1 127-1 131.
Czosnek, H., N. Navot and H. Laterrot. 1990. Geographical distribution of Tomato yellow Leaf curl virus. A first survey using a specific DNA probe. Phytopathol. Mediterr. 29:1-6
Czosneck, H., M. Ghanem and M. Ghanem. 2002. The circulative pathways of begomoviruses in whiteflies vector, Bemisia tabaci-insight from studies with Tomato yellow leaf curl virus. Ann. Appl. Biol. 140: 215-231. https://doi.org/10.1111/j.1744-7348.2002.tb00175.x
Firmino, A.C., A.Y. Valdir, G.M. Adriana and A.M.R. Jorge. 2009. Tomato yellow vein streak virus: relationship with Bemisia tabaci biotype B and host range. Pl. Pathol. 66: 1-10. https://doi.org/10.1590/S0103-90162009000600011
Ghanem, M., S. Morin, and H. Czosnek, 2001. Rate of Tomato yellow leaf curl virus translocation in the circulative transmission pathway at its vector, the whitefly Bemisia tabaci. Phytopathol. 91: 188- 196. https://doi.org/10.1094/PHYTO.2001.91.2.188
Goldman, V. and H. Czosnek. 2002. Whiteflies (Bemisia tabaci) issued from eggs bombarded with infection DNA clones of tomato yellow leaf curl virus from Israel are able to infect tomato plants. Archiv. Viro. 147: 787-801. https://doi.org/10.1007/s007050200026
Golnaraghi, A.R., N. Shahreen, S.H. Pourahim and A. Ghamesi. 2004. Occurrence and relative incidence of viruses infecting soybean in Iran. Pl. Dis. 88: 1069-1074. https://doi.org/10.1094/PDIS.2004.88.10.1069
Haider, M.S., I.D. Bedford, A.A.F. Evans and P.G. Marakham. 2003. Geminivirus transmission by different biotypes of the whitefly Bemisia tabaci (Gennadius). Pak. J. Zool. 35: 343-351.
Kashina, B.D., R.B. Mabagala, A.A. Mpunami. 2003 b. Biomolecular relationships among isolates of Tomato yellow leaf curl Tanzania virus. Phytoparasit. 31(2): 188–199 https://doi.org/10.1007/BF02980789.
Lapidot, M., M. Friedmann, O. Lachman, A. Yehezkel, S. Nahon, S. Cohen and M. Pilowsky 1997. Comparison of resistance to tomatoyellow leaf curl virus among commercial cultivars and breeding lines. Pl. Dis. 81:1425-1428. https://doi.org/10.1094/PDIS.1997.81.12.1425
Lapidot, M., M. Friedmann, M. Pilowsky, R.B. Joseph and S. Cohen. 2001. Effect of host plant resistance to Tomato yellow leaf curl virus (TYLCV) on virus acquisition and its transmission by its whitefly vector. Virol. 91: 1209-1213. https://doi.org/10.1094/PHYTO.2001.91.12.1209
Mansour, A. and A. Al-Musa. 1992. Tomato Yellow Leaf Curl Virus: host-range virus and vector relationships. Pl. Pathol. 41: 122-125. https://doi.org/10.1111/j.1365-3059.1992.tb02328.x
Martin, J.H. 1987. An identification guide to common whitefly pest species of the wordld(Homoptera : Aleyrodidae). Trop. Pest Manag. 33: 298-322. https://doi.org/10.1080/09670878709371174
Mehta, P., J.A. Wyman, M.K. Nakhla and D.P. Maxwell. 1994. Transmission of Tomato yellow leaf curl geminivirus by Bemisia tabaci (Homoptera: Aleyrodidae). J. Econ. Ento. 87: 1291–1297. https://doi.org/10.1093/jee/87.5.1291
Melzer, M.J., D.Y. Ogata, S.K. Fukuda, R. Shimabuku, W.B. Borth, D.M. Sether and John S. Hu. 2009. Tomato Yellow Leaf Curl virus. Pl. Dis. 2009. PD-70.
Momol, M.T., G.W. Simone, W. Dankers, R.K. Sprenkel, S.M. Olson, E.A. Momol, J.E. Polston and E. Hiebert. 1999. First report of Tomato yellow leaf curl virus in tomato in Georgia. Pl. Dis. 83:487. https://doi.org/10.1094/PDIS.1999.83.5.487C
Moriones, E. and J. Navas-Castillo. 2000. Tomato yellow leaf curl virus, an emerging virus complex causing epidemics worldwide. Virus Res. 71:123-134.
Muniyapa, V., H.M. Venkatesh, H.K. Ramappa, R.S. Kulkarni, M. Zeidan, C.Y. Tarba, M. Ghanim and H. Czosnel. 2000. Tomato leaf curl virus from Bangalore (ToLCV-Ban4): sequence comparison with Indian ToLCV isolates, detection in plants and insects, and vector relationships. Archiv. Virol. 145: 1583-98. https://doi.org/10.1007/s007050070078
Momol, M.T., G.W. Simone, W. Dankers, R.K. Sprenkel, S.M. Olson, E.A. Momol, J.E. Polston and E. Hiebert. 1999. First report of Tomato yellow leaf curl virus in tomato in Georgia. Pl. Dis. 83:487. https://doi.org/10.1094/PDIS.1999.83.5.487C
Nakhla, M.K., D.P. Maxwell, R.T. Martinez, M.G. Carvalho and R.L. Gilbertson. 1994. Widespread occurrence of the eastern Mediterraneanstrains of Tomato yellow leaf curl geminivirus in tomatoes in theDominican Republic. Pl. Dis. 78:926. https://doi.org/10.1094/PD-78-0926D
Petrov, N. 2014. Damaging effects of Tomato mosaic virus and Potato virus Y on tomato plants. Pl. Stud. 4:56-59.
Pico, B., M.J. Diez and F. Nuez. 1999. Improved diagnostic techniques for Tomato yellow leaf curl virus in tomato breeding programmes. Pl. Dis. 83 : 1006–1012. https://doi.org/10.1094/PDIS.1999.83.11.1006
Polston, J.E. and P.K. Anderson. 1997. The emergence of whitefly transmitted geminiviruses in tomato in the western hemisphere. Pl. Dis. 81: 1358-1369. https://doi.org/10.1094/PDIS.1997.81.12.1358
Reddy, K.S and R.C. Yaraguntaiah. 1981. Virus vector relationships in leaf curl disease of tomato. India. Phytopath. 34: 310–313.
Rice, R.P., L.W. Rice and L.D.T. Tindall. 1987. Fruits and vegetable production in Africa. Mackmillan Publishers. U.K. 371 P.
Rosell, R.C., I. Torres-Jerez and J.K. Brown. 1999. Tracing the geminivirus-whitefly transmission pathway by polymerase chain reaction in whitefly extracts, saliva, hemolymph, and honeydew. Phytopath. 89: 239–246. https://doi.org/10.1094/PHYTO.1999.89.3.239
Salati, R., M.K. Nahkla, M.R. Rojas, P. Guzman, J. Jaquez, D.P. Maxwell and R.L. Gilbertson. 2002. Tomato yellow leaf curl virus in the Dominican Republic: Characterization of an infectious clone, virus monitoring in whiteflies, and identification of reservoir hosts. Phytopath. 92: 487-496. https://doi.org/10.1094/PHYTO.2002.92.5.487
Sawalha, H. 2009. Occurrence of Tomato Yellow Leaf Curl Virus on volunteer tomato, Jimsonweed, and tobacco in North West Bank: distribution of virus natural reservoirs in summer season. An-Najah Univ. J. Res. 23: 1-19.
Sawalha, H. 2013. Epidemiology of Tomato yellow leaf curl virus in the Northern region of the West Bank, Palestine. The Open Agric. J. 7:80-85. https://doi.org/10.2174/1874331501307010080
Zakay, Y., N. Navot, M. Zeidan, N. Kedar, H. Rabinowitch, H. Czosnek and D. Zamir. 1991. Screening Lycopersicon accessions for resistance to Tomato yellow leaf curl virus presence of viral DNA and symptom development. Pl. Dis. 75: 279-281. https://doi.org/10.1094/PD-75-0279
To share on other social networks, click on any share button. What are these?








