The Quality and Proportion of Spermatozoa X and Y in Sexed Frozen Semen Separated with Percoll Density Gradient Centrifugation Method on Friesian Holstein Bull
Research Article
The Quality and Proportion of Spermatozoa X and Y in Sexed Frozen Semen Separated with Percoll Density Gradient Centrifugation Method on Friesian Holstein Bull
Aulia Puspita Anugra Yekti1, Sri Rahayu3, Gatot Ciptadi2, Trinil Susilawati2*
1Doctoral Student, Faculty of Animal Science, Universitas Brawijaya, Malang 65145, East Java, Indonesia; 2Faculty of Animal Science, Universitas Brawijaya, Malang 65145, East Java, Indonesia; 3Faculty of Mathematics and
Natural Sciences, Universitas Brawijaya, Malang 65145, East Java, Indonesia.
Abstract | This study aimed to evaluate the semen quality and the proportion of spermatozoa X and Y from sexed semen using the Percoll Density Gradient Centrifugation method. The quality observed included individual motility, viability, abnormalities, concentration, total motile spermatozoa, chromatin damage, intact acrosome hood of spermatozoa, and sperm capacitation. The treatments consisted of T1 = unsexed semen, T2 = sexed semen X-bearing sperm, and T3 = sexed semen Y-bearing sperm. A general linear model with three treatments and ten replications was performed in this research. The data were analyzed using a general linear model using SAS Demand on Academics, and the least significant differences were applied if there is significance. The result showed that semen quality, including individual motility, viability, abnormality, concentration, and total motile spermatozoa in sexed semen, is lower than in unsexed semen. In addition, the sexed semen had a lower percentage of intact acrosome hood and higher chromatin damage, acrosome reactions, and sperm capacitation than unsexed semen. In conclusion, the quality of unsexed frozen semen was higher than sexed frozen semen both in sexed X sperm and sexed Y sperm. While the proportion of sperm in sexed X sperm had a proportion of sperm X higher than sperm Y, indicating that Percoll Density Gradient Centrifugation can separate with an accuracy of more than 80% based on the head measurement.
Keywords | Sexed semen, Percoll gradient, Motility, Acrosome hood, Capacitation
Received | November 15, 2022; Accepted | January 08, 2023; Published | February 13, 2023
*Correspondence | Trinil Susilawati, Faculty of Animal Science, Universitas Brawijaya, Malang, East Java, Indonesia; Email: tsusilawati@ub.ac.id
Citation | Yekti APA, Rahayu S, Ciptadi G, Susilawati T (2023). The quality and proportion of spermatozoa X and Y in sexed frozen semen separated with percoll gradient density centrifugation method on Friesian Holstein bull. Adv. Anim. Vet. Sci. 11(3):371-378.
DOI | https://dx.doi.org/10.17582/journal.aavs/2023/11.3.371.378
ISSN (Online) | 2307-8316
Copyright: 2023 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
INTRODUCTION
The Percoll density gradient centrifugation sexing method (PDGC) is one of the methods to separate sperm effectively. The principle of this method is the difference in density between sperm X-bearing and sperm Y-bearing Y. the density of sperm X-bearing is higher than sperm Y-bearing which will make the sperm X-bearing will reach the bottom fraction. At the same time, the Y-sperm will remain on the upper fraction (Kusumawati et al., 2019). The sexing process can lead the changes in motility and viability, accelerate the acrosome reaction, increase spermatozoa capacitation, membrane damage (Susilawati et al., 2017; Yekti et al., 2022), and fragmentation (Bermejo-Alvarez et al., 2008). Furthermore, the sexing process also reduces the concentration of spermatozoa, including the sexing process using the percoll method. It is because there is a complex process starting from separation through gradient levels, centrifugation, dilution, and freezing. The results of previous studies showed that the concentration of percoll sexing results in frozen Limousine Y cattle semen was 12.125 million/straw (Mahfud et al., 2019) and did not meet the Indonesian national standard of 25 million/straw.
Decreasing the concentration of spermatozoa can reduce the success of artificial insemination. In vertebrates with large reproductive organ sizes, competition for spermatozoa is more influenced by the number of spermatozoa than the size of the spermatozoa. The higher the number of spermatozoa, the higher the competition for spermatozoa in fertilizing the ovum (Parker, 2020). In addition, the success of AI semen sexing cannot be separated from the success of the sexing process itself, which the proportion of X and Y spermatozoa produced can prove. To observed the proportion of spermatozoa, it can be measured by various methods, one of which is by using the size of the head of the spermatozoa (Kusumawati et al., 2019), it is because there is a difference in size between the X and Y spermatozoa (Seidel, 2007). This study aims to evaluated the semen quality and also the proportion of spermatozoa X and Y from sexed semen using Percoll Density Gradient Centrifugation method.
Materials and Methods
Ethical approval
The authors confirmed that in this study, there are no ethical issues involved with ethical clearance number 1071.55/UN10.C10/TU/2022.
Percoll density gradient centrifugation sexing method
The method of sperm separation was Percoll Density Gradient Centrifugation which the artificial insemination center of Singosari Malang produced. The separation method of percoll gradient was followed (Kusumawati et al., 2017, 2019) of X and Y sperms using medium density percoll with ten gradient levels arranged from the highest to the lowest density (65, 60, 55, 50, 45, 40, 35, 30, 25, 20%) and centrifugation 2250 rpm for 5 minutes. The extender used was tris aminomethane egg yolk.
Experimental design
The materials used in this study were 30 straws of frozen semen of Friesian Holstein (FH) bull produced by the Artificial Insemination Center of Singosari consisting of 10 straws of unsexed semen, ten straws of sexed sperm X-bearing sperm X, and ten straws of sperm Y. The treatments consisted of T1 = unsexed semen, T2 = sexed semen X-bearing sperm, and T3 = sexed semen Y-bearing sperm. Frozen sexing semen produced by Singosari Artificial Insemination Center used the Percoll Density Gradient Centrifugation method. The frozen semen was thawed using warm water 37o C for 29 seconds, followed by the Indonesian national standard. The observed parameters consist of motility, viability, abnormality, concentration, total motile spermatozoa (TMS) and intact acrosome hood.
Spermatozoa motility
Individual motility was observed by dripping one drop of semen on the object glass and then covered with a cover glass. Then the samples were observed under a microscope with 400x magnification in 5 fields of view using an Olympus CX 33 binocular microscope. Individual motility assessment was based on the percentage of progressively moving spermatozoa (Adiputra et al., 2022; Aldini et al., 2022).
Spermatozoa viability
The viability of spermatozoa was observed by making preparations on an object glass. One drop of semen is placed on the edge of the object glass, and then one drop of eosin-nigrosin is added to the semen. Furthermore, it is slowly homogenized and made thin review preparations on object glass, then dried and observed using a microscope with a magnification of 400x. Transparent spermatozoa characterize live spermatozoa head color, while dead spermatozoa are characterized by purplish red in spermatozoa heads (Aldini et al., 2022; Handayani et al., 2021). The number of live and dead spermatozoa was counted by hand tally counter. The percentage of viability is calculated using the formula:
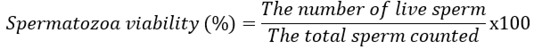
Abnormal spermatozoa
Spermatozoa abnormalities were calculated by placing one drop of semen and eosin nigrosine each on a slide, then homogenized and made a thin smear. Then observed under a microscope with a magnification of 400x and counted the number of abnormal and normal spermatozoa using a hand tally counter with a total of 200 spermatozoa in five fields of view (Aldini et al., 2022). The percentage of abnormality is calculated using the formula:
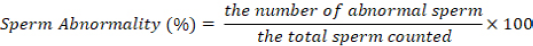
Spermatozoa concentration
Spermatozoa concentration was calculated using the Neubauer chamber. The concentration test procedure follows the method (Mahendra et al., 2018; Aldini et al., 2022).
The concentration of spermatozoa per straw were calculated using following formula:
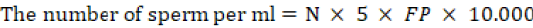
Where; N= the mean number of spermatozoa in the chamber; 5= correction factor because it only counts five boxes out of 25 boxes; FP= diluent factor (1:100); 10.000= neubauer chamber depth of 0.0001 ml/ Neubauer chamber (correction factor).
Chromatin damage
A chromatin test was carried out by making a smear of fresh semen on an object glass, air-dried, and fixed in a 96% ethanol and acetone solution in a ratio of 1:1 for 30 minutes at 4oC. After fixation, the preparations were air-dried and then hydrolyzed in 0.1 N HCl solution for five minutes at 4oC. Then the preparations were rinsed three times using aquabidest, stained with 0.05% Toluidine blue stain, and left for 10 minutes. Next, the preparations were washed with aquabidest, dehydrated using t-butanol twice, and then cleaned with xylol. After that, the preparations were observed using a microscope with 400x magnification. Good chromatin integrity is indicated by bright blue spermatozoa heads, while those with reduced chromatin integrity will be dark blue (Priyanto et al., 2015).
Intact acrosome hood
The intact acrosome hood was observed by introducing one drop of semen into the formol-saline solution (2.54 g potassium dihydrogen phosphate, 5.41 g sodium chloride, 6.19 g di-sodium hydrogen phosphate dehydrate, 125 mL formaldehyde solution (37 %), and 875 mL of distilled water). Fresh semen was added to the formol-saline solution in a ratio of 1:100. It was left for 1 hour, and one drop was taken and then placed on an object glass and covered with a cover glass. Observations were made on 200 spermatozoa under a phase contrast microscope with a magnification of 400x. Spermatozoa characterized by 1/2 to 2/3 of the anterior part of the head darker than the posterior have an intact acrosomal hood (Prabowo et al., 2021).
Chlortetracycline (CTC) staining preparation
45μl of semen and 45μl of CTC staining were put into a 1.5 ml Eppendorf tube which had been wrapped in aluminum foil, then vortexed 20 times for homogenization. Added 10μl of CTC fixative and vortexed 20 times, allowed to stand for 5 minutes. The standard is 30 minutes or a maximum of 3 hours. Then take 10μl of Dabco solution on a glass object, add 10μl CTC staining on the top of Dabco, and slowly homogenize using the tip. Then close with a cover glass, and lock the edges of the cover glass using transparent nail polish. Then rolled up using tissue and pressed slowly. Preparations can be directly observed using a fluorescence microscope with as many as 200 spermatozoa using 400x magnification. Preparations can also be put in a preparation box wrapped in aluminum foil and then stored in the freezer for later observation (Yekti et al., 2022).
Proportion of spermatozoa X and Y
The sample used was an FH semen straw produced by the Artificial Insemination Center of Singosari Malang, which consisted of non-sexing frozen semen straw, X sexing and Y sexing. Head measurements were made by making preparations for eosin nigrosin staining, and then the sample was observed using an Olympus BX-53 microscope. Morphometric measurements, including the length and width of the spermatozoa head, were carried out using the Olympus celSens Dimension software. Spermatozoa observations were carried out on 200 spermatozoa in each sample. The X and Y chromosome spermatozoa are determined based on the length times the width of the spermatozoa head. If the head of the spermatozoa is larger than or equal to the average (l x w x̄), then it is categorized as an X-chromosome spermatozoa. If the head size of the spermatozoa is smaller than the average (l x w < x̄) then it is categorized as spermatozoa with a Y chromosome (Kusumawati et al., 2019):
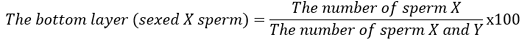
Data analysis
A general linear model (GLM) was performed using SAS OnDemand for Academics (ODA, Cary, NC, USA). The results were presented as standard error mean (SEM). Moreover, probability values were calculated using the least significant different testing. The following model was used:
Yij = μ + Ti + eij (Ardiansyah et al., 2022)
Where; Yij was the parameters observed, μ was the overall mean, Ti the unsexed and sexed semen, and eij was the amount of error number.
RESULTS AND DISCUSSION
The quality of frozen semen in unsexed and sexed semen
Spermatozoa quality is an essential factor in determining artificial insemination (AI’s) success. The progressive motility of sperm is needed in the reproductive tract of female cattle so that spermatozoa can fertilize the ovum and affect the pregnancy success rate. Bulls for artificial insemination must qualify as a superior bull that has good genetic quality and semen production (Susilawati et al., 2020). The results of frozen semen quality in unsexed and sexed semen can be seen in Table 1. Based on Table 1 shows that the motility of unsexed semen and sexed semen on X and Y sperm were 54.20%, 44.60%, and 42.10%, respectively. The highest percentage of individual motility is in unsexed semen at 54.20%. This study showed that the individual motility in sexed semen bot in T2 and T3 was decreased compared to control unsexed semen (T1) (Figure 2). The influence of the centrifugation process can cause a decrease in individual motility in sexed semen during sexing process. The centrifugation process decreased quality and damaged the membrane of spermatozoa (Kusumawati et al., 2019). The study by (Susilawati et al., 2017) reported that centrifugation in the process of separating X and Y spermatozoa could damage the cell membranes of spermatozoa which can affected the function of sperm to fertilize the egg. First, the Function membrane is a cell protector, which acts as protector against organelle such as mitochondria and lysosomes. Then mitochondria began to respirator as a consequence of energy cells. Furthermore, this cell influenced spermatozoa to decreasing the progressive of sperm motility.
Table 1: Evaluation of frozen semen in unsexed and sexed semen.
|
Parameters |
T0 |
T1 |
T2 |
R2 |
SEM |
|
Individual motility (%) |
54.20 |
44.60 |
42.10 |
0.61 |
4.31 |
|
Viability (%) |
75.35 |
72.24 |
71.25 |
0.12 |
4.98 |
|
Abnormality (%) |
3.38 |
5.94 |
7.05 |
0.32 |
2.30 |
|
Concentration (million/straw) |
36.31 |
28.31 |
27.19 |
0.47 |
4.54 |
|
Total motile spermatozoa (106/ml) |
19.55 |
12.64 |
11.38 |
0.76 |
2.07 |
Percoll Density Gradient Centrifugation (PDGC) is a method of separating spermatozoa based on differences in sperm density due to differences in mass and size of X and Y spermatozoa. X spermatozoa are larger and contain more Deoxyribonucleic acid (DNA) than Y spermatozoa, which is about 4% in cattle (Seidel, 2007). These differences cause X spermatozoa to be in the lower layers when centrifuged, and the Y spermatozoa will remain on the top layer (Kusumawati et al., 2019). Individual motility on T1 is higher than T2, and this is because the membrane damage in the lower layer is lower than in the upper layer, following the results of a study conducted by (Susilawati et al., 2017a, b). However, although sexed semen motility has a lower value than unsexed semen, it is still feasible to be used for artificial insemination based on the minimum motility requirement from the Indonesian national standard, which should be minimum motility of 40% (National Standardization Agency, 2017).
The viability of frozen semen in unsexed semen and sexed semen was 75.35%, X sperm was 72.24% and Y sperm was 71.25%. The data showed that the viability is in a standard category based on (Garner and Hafez, 2016) that live spermatozoa after thawing ranged from 60% to 75%. This study showed that the viability of sexed semen T1 and T2 were lower than unsexed semen T0. The percentage of spermatozoa viability after the sexing process has decreased because in the sexing process the spermatozoa are separated from the seminal plasma. Seminal plasma consists of various biochemical components such as glucose, protein, cholesterol, and antioxidants which are important for spermatozoa metabolism (Asadpour, 2012). Spermatozoa that are separated from the seminal plasma can cause the spermatozoa to lose energy so that the vitality of the spermatozoa will be weakened and even die. Added by (Pereira et al., 2016), the decrease in spermatozoa viability was also caused by cold temperatures during storage, reduced energy availability in the diluent, and damage to the plasma membrane and acrosome. In addition, the viability of spermatozoa is highly dependent on the integrity of the spermatozoa membrane. The membrane damage will lead to disruption of the intracellular metabolic processes of spermatozoa so that the ability of spermatozoa to survive will decrease and death (Aldini et al., 2022).
Spermatozoa abnormalities in Friesian Holstein Bulls showed the lowest abnormality in unsexed semen at 3.38%, followed by X sperm at 5.94% and Y sperm was 7.05%. The abnormalities percentage in those treatments are categories as good were suitable for insemination based on the statement of (Garner and Hafez, 2016) that post-thawing spermatozoa abnormalities should not be more than 20%. The percentage of spermatozoa abnormalities in sexed semen was higher than in unsexed semen because the centrifugation process during the separation of sperm can damage the membrane of sperm. The rotation that occurs during the centrifugation process causes the spermatozoa inside to collide with the tube wall, and friction with the medium can damage the spermatozoa membrane. If the spermatozoa membrane is damaged, it will cause an increase in spermatozoa abnormalities (Sujoko et al., 2009). The highest spermatozoa concentration was shown in unsexed semen at 36.31 million/straw, followed by X sperm at 28.31 million/straw and Y sperm at 27.19 million/straw (Figure 1). The average concentration of spermatozoa in unsexed semen was higher than in sexed semen. According to (Mahfud et al., 2019), the dilution process is the main factor that affects the concentration of spermatozoa in straw. Suppose the dilution process is carried out with precise calculations of the number of spermatozoa concentrations. As consequence of the number of spermatozoa in the straw will follow standard national Indonesia (SNI), which is 25 million/straw. In addition, the lower concentration in sexed semen is affected by the use of a pipette and the time of centrifugation when separating spermatozoa (Kusumawati et al., 2017, 2019). The total motile spermatozoa of Friesian Holstein Bull in unsexed semen was 19.55 million/straw, X sperm was 12.64 million/straw, and Y sperm was 11.38 million/straw. The results of analysis using the Chi-square test of the total motile spermatozoa in unsexed semen showed a very significant difference (P<0.01) higher than the SNI standard. In contrast, the total motile spermatozoa of X sperm and Y sperm after thawing showed no significant difference (P>0 0.05) with Indonesian national standards. Overall, the total motile spermatozoa of Friesian Holstein Bull in those treatments had an average above the standard frozen semen suitable for insemination according to the National Standardization Agency (SNI 4869-1-:2017), which is at least 10 million/straw or 40 million/ml. Based on the study’s results, the higher the percentage of spermatozoa motility and the concentration of spermatozoa, the higher the total motile spermatozoa obtained. This is in accordance with the opinion of (Heriyanta et al., 2013), which states that the number of spermatozoa and individual motility dramatically affect the total motile spermatozoa.
Intact acrosome hood, chromatin damage, acrosome reaction and capacitation status in unsexed and sexed semen
The intact acrosome hood test was performed to determine whether the spermatozoa have intact components (Adiputra et al., 2022). Acrosome intact has an important role in the ability of the spermatozoa to fertilize the egg. Spermatozoa that have a damaged acrosome head will not be able to fertilize an egg because the ability of spermatozoa to penetrate the egg is determined by the acrosome head of the spermatozoa (Susilawati et al., 2017a; Priyanto et al., 2015) The Intact Acrosome Hood and chromatin damage of sperm can be seen in Table 2.
Table 2: Intact acrosome hood, chromatin damage, acrosome reaction and capacitation status in unsexed and sexed semen.
|
Parameters |
Mean |
||||
|
T0 |
T1 |
T2 |
R2 |
SEM |
|
|
Intact acrosome hood (%) |
78.40 |
74.08 |
70.70 |
0.35 |
4.56 |
|
Chromatin damage (%) |
5.34 |
6.59 |
7.09 |
0.17 |
1.63 |
|
Acrosome reaction (%) |
7.93 |
11.07 |
9.90 |
0.15 |
3.21 |
|
capacitation (%) |
15.13 |
16.52 |
18.39 |
0.08 |
4.62 |
|
Non capacitation (%) |
76.94 |
72.41 |
71.71 |
0.11 |
0.53 |
Table 2 showed that the intact acrosomal hood of Friesian Holstein bull in unsexed semen was 78.40%, X sperm was 74.08%, and Y sperm was 70.70%. Overall, the hood acrosome intact in all treatments (T0, T1, T2) were categorized as good criteria based on (Vijayalakshmy et al., 2018) stated that the minimum intact acrosome hood in frozen semen is at least 65%. In addition, the acrosome contains several hydrolytic enzymes, including proacrosin, hyaluronidase, esterase, and acid hydrolase. Spermatozoa need these enzymes to penetrate the oocyte (Sitepu and Marisa, 2019). In this study, the percentage of intact acrosome hood in sexed semen has a lower value than in unsexed semen. This condition could be caused by the cold shock during the processing of frozen semen (Baust et al., 2009) and membrane damage while the separation process (Susilawati et al., 2017). In addition, the freezing process also damages the integrity of the spermatozoa due to changes in the structure and function of the membrane and cell metabolism (Baust et al., 2009). Priyanto et al. (2015) stated that the damage on the plasma membrane and acrosome cap may lead to damage or a decrease in spermatozoa DNA. This study also evaluated the chromatin damage that can be seen in Table 3. The percentage of chromatin damage in Friesian Holstein bull in unsexed semen was 5.34%, X sperm was 6.59%, and Y sperm was 7.09%. The percentage of chromatin damage in sexing frozen semen has a higher value than in unsexed frozen semen. Sexing frozen semen has passed various processes such as dilution, separation of spermatozoa, cooling, and freezing, all of which can damage the structure of the spermatozoa membrane and cause a decrease in quality. In this study, the acrosomal reaction on sexed semen in T1 and T2 was higher than in unsexed semen T0, and this result is in line with the research of (Yekti et al., 2022) that the process of sexing and cooling sperm also increased capacitation, acrosomal reactions, and hyperactivation. Based on Table 3, it was found that the spermatozoa that had not been capacitated with the highest value were in frozen non-sexing semen of 76.94% while the average of spermatozoa that had not been capacitated with the lowest value was in Y sperm of 71.71%. The average spermatozoa that have not undergone capacitation obtained in this study are still relatively high, so the semen used is still suitable for AI. This is because the process of capacitation of spermatozoa should occur in the female reproductive tract in order be able to penetrate the oocyte (Yekti et al., 2022).
Table 3: The proportion of spermatozoa X and Y in unsexed and sexed semen.
|
Spermatozoa |
Fresh semen (%) |
T0 (%) |
T1 (%) |
T2 (%) |
|
X |
49,50 |
49,7 |
77,1 |
19,4 |
|
Y |
50,50 |
50,3 |
22,9 |
80,6 |
|
Total |
100 |
100 |
100 |
100 |
The proportion of spermatozoa X and Y in unsexed and sexed semen
The proportion of spermatozoa can confirm the success of the sexing method in separating spermatozoa. The higher the separation, the higher the success of the AI in getting the sex of the child expected. In this study, The proportion of X and Y spermatozoa was confirmed by head size by calculating the length and width of each spermatozoa using a micrometer (Kusumawati et al., 2019; Yekti et al., 2019). The proportion of spermatozoa X and Y in unsexed and sexed semen can be seen in Table 3.
The percentage of the proportion of unsexed semen resulted in the proportion of X spermatozoa at 49.7% and the proportion of Y spermatozoa of 50.3%. The results of the Chi-Square test obtained showed that non-sexing frozen semen gave an insignificant difference (P>0.05) on X and Y spermatozoa. This result is the same as the research conducted by (Kusumawati et al., 2019) revealed that in unsexed semen, the proportion of X spermatozoa and Y spermatozoa are 50,4% and 49,6%, respectively. The percentage of unsexed semen for X sperm (bottom layer) resulted in a higher X spermatozoa proportion of 77.1 %, while the proportion of Y spermatozoa was lower by 22.9%. These results indicate that the proportion of spermatozoa follows the expected value of frozen semen sexing X, 80% X, and 20% Y. The average percentage of frozen semen proportions Y produces a higher proportion of Y spermatozoa by 80.6%, while the proportion of X spermatozoa is lower by 19.4%. These results indicate that the proportion of spermatozoa is in accordance with the expected value of frozen semen of Y sperm, 80% Y and 20% X. The accuracy of semen sexing can be seen based on the proportion of X and Y spermatozoa after the sexing process. Several studies have stated that Percoll density gradient
“The observation of capacitation sperm, non capacitation sperm and acrosomal reaction in unsexed and sexed semen can bee seen on Figure 3”.
centrifugation can separate the X and Y spermatozoa ratio with high separation. The top layer produces Y spermatozoa as much as 72%. In comparison, the lower layer produces X spermatozoa 77.5% compared to non-sexing, which has a proportion of X and Y spermatozoa, respectively, 50.4% and 49.6% (Kusumawati et al., 2019). Yekti et al. (2019) reported that the proportion of X and Y spermatozoa after sexing in the upper fraction was around 80.79% compared to non-sexing, which only had 52.77% Y spermatozoa. Silva et al. (2017) stated that the success rate of the SGDP method varies from 86% to 94%. Resende et al. (2009) mentioned that in cattle, the accuracy of the SGDP method is around 70% and has high viability for in vitro fertilization.
CONCLUSIONS
In conclusion, this study indicates that the quality of spermatozoa which includes individual motility, viability, abnormality, concentration, and total motile spermatozoa in sexed semen, is lower than in unsexed semen. However, the quality ranges in the standard category according to Indonesian national standards and is suitable for artificial insemination. Furthermore, sexed semen had a lower percentage of intact acrosome hood and higher chromatin damage, acrosome reactions, and sperm capacitation than unsexed semen.
ACKNOWLEDGEMENTS
We would like to thank the Directorate General of Higher Education, The Ministry of Education, Culture, Research and Technology, which provides funding through the Doctor Dissertation Research scheme 2022.
NOVELTY STATEMENT
Information of semen quality, including motility, viability, abnormalities, concentration, total motile spermatozoa, intact acrosome hood, chromatin damage, acrosome reaction, and capacitation in unsexed and sexed semen using percoll density gradient centrifugation method produced by artificial insemination Singosari Malang is still very limited. Furthermore, the study of the identification of X and Y proportions in sexed and unsexed semen is rarely used and still has limited information. Therefore, this study was conducted to provide information on semen quality and the proportion of X and Y sperm in sexed semen with the percoll density gradient centrifugation method.
AUTHOR’S CONTRIBUTION
APA conceptualization, drafting the original manuscript, collecting data, SR conceptualization, supervision, GC conceptualization, supervision, TS conceptualization, supervision.
Conflict of interest
The authors have declared no conflict of interest.
REFERENCES
Adiputra K, Maulana DD, Kaiin TM, HasbiE, Sonjaya H (2022). Bali bull, fresh semen, frozen semen, semen quality, simmental bull. Adv. Anim. Vet. Sci., 10(12). https://doi.org/10.17582/journal.aavs/2022/10.12.2562.2570
Aldini SA, Isnaini N, Yekti APA, Susilawati T (2022). Study of the quality and integrity of spermatozoa acrosome caps in frozen sexing semen Friesian Holstein cattle. J. Ilmu Ilmu Peternakan, 32(2): 233-240. https://doi.org/10.21776/ub.jiip.2022.032.02.09
Ardiansyah W, Sjofjan O, Widodo E, Suyadi S, Adli DN (2022). Effects of combinations of α-Lactobacillus sp. and Curcuma longa flour on production, egg quality, and intestinal profile of Mojosari ducks. Adv. Anim. Vet. Sci., 10(8): 1668-1677. https://doi.org/10.17582/journal.aavs/2022/10.8.1668.1677
Asadpour R (2012). Relationship between mineral composition of seminal plasma and semen quality in various ram breeds. Acta Sci. Vet., 40(2): 1027.
Baust JG, Gao D, Baust JM (2009). Cryopreservation: An emerging paradigm change. Organ, 5(3): 90-96. https://doi.org/10.4161/org.5.3.10021
Bermejo-Alvarez P, Rizos D, Rath D, Lonergan P, Gutierrez-Adan A (2008). Can bovine in vitro-matured oocytes selectively process X- or Y-sorted sperm differentially? Biol. Reprod., 79(4): 594-597. https://doi.org/10.1095/biolreprod.108.070169
Garner DL, Hafez ESE (2016). Spermatozoa and seminal plasma. In: Reproduction in farm animals. Lippincott Williams and Wilkins. https://doi.org/10.1002/9781119265306.ch7
Handayani E, Supriatna I, Tumbelaka LITA, Kaiin EM (2021). Analisis komparatif kualitas semen Beku yang Telah dan Belum Bersertifikasi Standar Nasional Indonesia. J. Vet., 22(2): 207-215. https://doi.org/10.19087/jveteriner.2021.22.2.207
Heriyanta E, Ihsan MN, Isnaini N (2013). Pengaruh umur kambing peranakan etawah (PE) terhadap kualitas semen segar. J. Trop. Anim. Prod., 14(2): 1-5.
Kusumawati ED, Isnaini N, Yekti APA, Luthfi M, Affandhy L, Pamungkas D, Kuswati, Ridhowi A, Sudarwati H, Susilawati T, Rahayu S (2017). The quality of sexed semen on filial ongole bull using percoll density gradient centrifugation method. Asian J. Microbiol. Biotech. Environ. Sci., 19(1): 189-199.
Kusumawati ED, Isnaini N, Yekti APA, Luthfi M, Affandhy L, Pamungkas D, Kuswati, Ridhowi A, Sudarwati H, Rahadi S, Rahayu S, Susilawati T (2019). The motility and ratio of X and Y sperm filial ongole cattle using different sexed semen methods. Am. J. Anim. Vet. Sci., 14(2): 111-114. https://doi.org/10.3844/ajavsp.2019.111.114
Mahendra HC, Samsudewa D, Ondho YS (2018). Evaluation of semen quality buffalo frozen semen produced by artificial insemination center. J. Ind. Trop. Anim. Agr., 43(1): 26-34. https://doi.org/10.14710/jitaa.43.1.26-34
Mahfud A, Isnaini N, Yekti APA, Kuswati K, Susilawati T (2019). Kualitas spermatozoa post thawing semen beku sperma Y hasil sexing pada sapi limousin. J. Trop. Anim. Prod., 20(1): 1-7. https://doi.org/10.21776/ub.jtapro.2019.020.01.1
National Standardization Agency (2017). SNI Frozen Semen- Part 1: Bulls. BSN. BSN.
Parker GA (2020). Conceptual developments in sperm competition: A very brief synopsis. Philos. Trans. R. Soc. Lon. B Biol. Sci., 375(1813): 20200061. https://doi.org/10.1098/rstb.2020.0061
Pereira GR, Becker EG, Siqueira LC, Ferreira R, Severo CK, Truzzi VS, Oliveira JFC, Gonçalves PBD (2016). Assessment of bovine spermatozoa viability using different cooling protocols prior to cryopreservation. Ital. J. Anim. Sci., 9(4). https://doi.org/10.4081/ijas.2010.e88
Prabowo TA, Indarjulianto S, Pertiwiningrum A, Sugiyanto C, Priyanto L (2021). Performen reproduksi dan produksi susu sapi perah di kecamatan tegalombo kabupaten pacitan Provinsi Jawa Timur. J. Peternakan Sriwijaya. 10(1): 29-36. https://doi.org/10.33230/JPS.10.1.2021.13762
Priyanto L, Arifiantini RI, Yusu TL (2015). Deteksi kerusakan DNA spermatozoa semen segar dan semen beku sapi menggunakan pewarnaan toluidine blue. J. Vet., 16(1): 48-55.
Resende MV, Bezerra MB, Perecin F, Almeida AO, Lucio AC, De Lima VFMH (2009). Separation of X-bearing bovine sperm by centrifugation in continuous percoll and optiprep density gradient: Effect in sperm viability and in vitro embryo production. Ciência Anim. Brasil., 10(2): 581-587.
Seidel GE Jr (2007). Overview of sexing sperm. Their, 68(3): 443-446. https://doi.org/10.1016/j.theriogenology.2007.04.005
Silva JCF, Moura MT, Basto SRL, Oliveira LRS, Caldas ELC, Filho MLS, Oliveira MAL (2017). Use of percoll density centrifugation for sperm sexing in small ruminants. Glob. J. Sci. Front. Res. D Agr. Vet., 6(1): 55-59.
Sitepu SA, Marisa J (2019). Percentage value of membrane integrity and acrosome integrity spermatozoa in simmental liquid semen with addition penicillin and sweet orange essential oil. IOP Conf. Ser. Earth Environ. Sci., 327: 012027. https://doi.org/10.1088/1755-1315/327/1/012027
Sujoko H, Setiadi MA, Anwar B (2009). Seleksi spermatozoa domba garut dengan metode sentrifugasi gradien densitas percoll. Veterinary, 10(3): 125-132.
Susilawati T, Kusumawati ED, Isnaini N, Yekti APA, Sudarwati H, Ridhowi A (2017a). Effect of sexing process using percoll density gradient centrifugation and frozen on motility and damage to spermatozoa membrane of filial ongole. Adv. Health Sci. Res. (AHSR), 1st Int. Conf. One Health, 5: 5.
Susilawati T, Kuswati, Rahayu S, Sudarwati H, Marjuki, Yekti APA, Udrayana S (2017b). Quality of ongole bull sperm after storage in CEP-2 extender containing different extracellular cryoprotectans. Asian J. Microbiol. Biotech. Environ. Sci., 19(7): 268-273.
Susilawati T, Sholikah NU, Wahjuningsih S, Herwiyanti E, Yekti APA (2020). Relationship of scrotal circumference with spermatozoa production in various breed of Indonesian local bulls. Am. J. Anim. Vet. Sci., 15(2): 102-107. https://doi.org/10.3844/ajavsp.2020.102.107
Vijayalakshmy K, Virmani M, Kumar D, Kumar P, Rahman H (2018). Different methods of assessing semen quality. Indian Farm., 5(12): 1383-1387.
Yekti APA, Bustari WO, Kuswati, Huda AN, Satria AT, Susilawati T (2019). Male calf proportion of artificial insemination results by using sexed sperm with double dose on Ongole crosbred cows. IOP Conf. Ser. Earth Environ. Sci., 387: 012029. https://doi.org/10.1088/1755-1315/387/1/012029
Yekti APA, Mustofa R, Lutfi M (2022). Relationship of motility and acrosome reaction on sexing sperm in Ongole crossbred bull. E3S Web of Conferences, 335. https://doi.org/10.1051/e3sconf/202233500042
To share on other social networks, click on any share button. What are these?






