The Impact of Dietary Minerals on Indian Spiny Loach Growth and Spawning in Captivity
Pankaj Gargotra1*, C. Judith Betsy1 and J. Stephen Sampath Kumar2
1TNJFU, Department of Aquaculture, Fisheries College and Research Institute, Tuticorin, India
2TNJFU, Directorate of Sustainable Aquaculture, Nagapattinam, India.
ABSTRACT
Aquaculture is vital to feed the world’s rapidly growing population. We cannot secure long-term industrial growth unless we diversify our species and production systems. Loaches are an important category of freshwater fish with a global distribution, and studies have shown that loach meat has great nutritional value. There is a scarcity of data on their population structure, breeding behavior, and growth biology. Lepidocephalichthys thermalis, a member of the superfamily Cobitidae and the order Cypriniformes, is a significant alternative species for Indian aquaculture. Aquaculture breeding strategies for loaches must be carefully investigated in order to meet their rising demand. Owe to the aquaculture potential of L. thermalis, the current study was conducted to establish the importance of various minerals (Mn, Se, and Zn) in improving its growth, maturity, and spawning. According to the findings of this study, Se is a crucial mineral impacting the capacity of L. thermalis to develop in captivity. Mn and Zn, on the other hand, may boost the loach’s capacity to develop and reproduce. Dietary additions of Mn, Se, and Zn to the diet of loaches at rates of 10 mg/kg, 0.35 mg/kg, and 10 mg/kg resulted in the best growth performance, whereas dietary additions of Mn, Se, and Zn at rates of 15 mg/kg, 0.35 mg/kg, and 5 mg/kg resulted in the best maturity and breeding performance. To determine statistical significance, all recorded parameters were submitted to the ‘F’ test. The growth and maturation parameters were analyzed in detail using Regression analysis and compared using one-way ANOVA in SPSS- 22.0.
Article Information
Received 08 January 2023
Revised 22 January 2023
Accepted 22 February 2023
Available online 01 June 2023
(early access)
Published 18 October 2024
Authors’ Contribution
PG acquisition of data, data analysis, and interpretation, preparation of the manuscript. CJB design of the study, data analysis, and interpretation, manuscript correction. JSSK conception and design of the study, data analysis, and manuscript correction.
Key words
Loach, Mineral, Hormone, Histology, Gonads
DOI: https://dx.doi.org/10.17582/journal.pjz/20230108130148
* Corresponding author: [email protected]
0030-9923/2024/0006-2743 $ 9.00/0
Copyright 2024 by the authors. Licensee Zoological Society of Pakistan.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
India is currently the world’s second-largest fish producer, with the ability to meet global animal protein demands. Though carp production remains the backbone of Indian aquaculture production, there are numerous key constraints, such as the introduction of exotic carps, which leads to the extinction of indigenous fish species, habitat alteration, and the destruction of important indigenous freshwater fish fauna breeding grounds (Swain et al., 2017).
Therefore, diversifying the aquaculture species with indigenous species is the need of the hour, and species diversification, especially in Southeast Asian countries has been well-established. L. thermalis is an important indigenous freshwater candidate species that belong to the superfamily Cobitidae and the order Cypriniformes (Nelson, 2006; Helfman, 2013). It is indigenous to India and Sri Lanka and is known as the common spiny loach, spotted loach, or Indian spiny loach (Shaji, 2000). Indian spiny loach is omnivorous and shows a preference towards insect larvae, hence known as a micro predator (Keskar et al., 2014).
Loaches are an important group of freshwater species that have global distribution. Mostly, these small loach fishes are used for aquarium purposes, but studies have also highlighted the nutritional importance of loach flesh (You et al., 2011; Keskar et al., 2014; Wang et al., 2018). However, the culture techniques have not been standardized for loaches but wild-collected catch can fetch attractive market prices, especially in the Indian subcontinent.
Reports on the maturation and reproduction of spiny loaches are scanty. Broodstock nutrition influences the gamete quality and contributes to quality seed production (Bromage et al., 1992; Izquierdo et al., 2001). Hence, broodstock nutrition is an important aspect while studying the reproductive biology of any new species. Despite the fact that research on fish broodstock nutrition has gained momentum in recent years, knowledge of broodstock nutrition and the dietary micronutrient needs of fish broodstock is limited (Watanabe et al., 1997; Izquierdo et al., 2001).
Micronutrients are least studied when compared to macronutrients and only a few studies have demonstrated their requirements in fish (Watanabe, 1988; Hilton, 1989; Lall, 1989). The importance of Mn in fish nutrition has been recognized in the normal functioning of the brain, lipid and carbohydrate metabolism, activation of various enzymes, such as glycosyltransferase, kinases, transferases, hydrolases, and decarboxylases (Watanabe et al., 1997), and in reproduction (Satoh et al., 1987a; Lall, 1989). Se is an integral part of all selenoenzymes, such as glutathione peroxidase (GSH-Px), phospholipid hydroperoxide glutathione peroxidase (PHG-Px), and types I tetra iodothyronine 5 deiodinase has been shown by Zuberbuehler et al. (2006). Zn is considered essential for approximately 1000 regulatory and catalytic proteins promoting animal growth and development (Maage and Julshamn, 1993; Eide, 2006; Maret and Krężel, 2007). Zn has been found to regulate oxidative stress, immunity, and reproductive processes (Domínguez et al., 2019). Zn can be supplemented in many forms in aquafeeds, but European Food Safety Authority recommended zinc oxide as the safest source in all animals (FEEDAP, 2012). According to Watanabe et al. (1997), the mineral requirement for fish is 2-20 mg Mn/kg of diet, 0.15-0.5 mg Se/kg of diet, and 15-40 mg Zn/kg of diet.
Recognizing the aquaculture potential of L. thermalis, the present study has been planned to find out the importance of selected minerals (Manganese, Selenium, and Zinc) in enhancing its growth, maturation, and spawning.
Materials and Methods
Experimental fish
Wild collected specimens of L. thermalis from Parakkai, Kanyakumari district of Tamil Nadu, India (8.1519oN, 77.4544oE) was used in the experiment. They were brought to the research center at Kanyakumari Parakkai Centre for Sustainable Aquaculture, Parakkai and acclimatized for 2 weeks, and their length and weight were recorded immediately after they were stabilized.
Acclimatization and experiment feed
Fishes were acclimatized to prepared diets gradually before the commencement of the experiment as done by Garling and Wilson (1976). No specific feed has been either recommended or experimented with L. thermalis so far. As a result, Keskar et al. (2014) studies on the diet and feeding behavior of L. thermalis were used as the foundation for formulating the feed. The feed was prepared with locally available low-cost feed ingredients, and the feed formulation details are presented in Table I. Ten Iso-nitrogenous and iso-caloric feeds were prepared following methods prescribed by Luo et al. (2011), Khan et al. (2016), and Domínguez et al. (2019). Food grade minerals ‘Mn’ as ‘Manganese dioxide’ (Research-Lab Fine Chem Industries, Mumbai), Se as Sodium selenite (Medizens Lab Pvt Ltd. Bengaluru), and Zn as Zinc sulfate (Nice Chemicals Pvt Ltd., Kochi) were procured. Doses of minerals were finalized based on the studies on other species with these minerals (Table I).
Table I. Feed formulation of the experimental diets (%).
|
Ingredients |
Diet (% of inclusion) |
|||||||||
|
C |
T1 |
T2 |
T3 |
T4 |
T5 |
T6 |
T7 |
T8 |
T9 |
|
|
GNOC |
20 |
20 |
20 |
20 |
20 |
20 |
20 |
20 |
20 |
20 |
|
Rice bran |
40 |
40 |
40 |
40 |
40 |
40 |
40 |
40 |
40 |
40 |
|
Corn flour |
10 |
10 |
10 |
10 |
10 |
10 |
10 |
10 |
10 |
10 |
|
Soy flour |
20 |
20 |
20 |
20 |
20 |
20 |
20 |
20 |
20 |
20 |
|
Wheat bran |
10 |
10 |
10 |
10 |
10 |
10 |
10 |
10 |
10 |
10 |
|
Manganese (mg/kg) |
0 |
5 |
10 |
15 |
0 |
0 |
0 |
0 |
0 |
0 |
|
Selenium (mg/kg) |
0 |
0 |
0 |
0 |
0.2 |
0.35 |
0.5 |
0 |
0 |
0 |
|
Zinc (mg/kg) |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
5 |
10 |
15 |
|
Proximate composition |
||||||||||
|
Crude protein (%) |
22.72 |
22.70 |
22.73 |
22.69 |
22.79 |
22.80 |
22.78 |
22.78 |
22.77 |
22.77 |
|
Crude fibre (%) |
16.20 |
16.22 |
16.29 |
16.34 |
16.25 |
16.22 |
16.24 |
16.28 |
16.24 |
16.27 |
|
Moisture (%) |
6.37 |
6.4 |
6.39 |
6.41 |
6.42 |
6.42 |
6.41 |
6.41 |
6.43 |
6.41 |
|
Ash (%) |
8.66 |
8.64 |
8.65 |
8.62 |
8.61 |
8.62 |
8.61 |
8.64 |
8.62 |
8.61 |
|
Gross energy (Kcal/Kg) |
4106 |
4120 |
4100 |
4129 |
4124 |
4127 |
4126.5 |
4120.9 |
4126 |
4125.4 |
Small plastic tubs of 30 L capacity were used for the experiment and a water level of 12 cm was maintained in the tubs throughout the experiment. The treatments were carried out in replicates along with control and each tank was stocked with 30 female fish. Female brooders were fed with the experimental feed twice a day ad libitum for a period of 60 days.
Assessment of growth performance
Growth parameters such as mean weight gain (MWG), daily growth rate (DGR), survival rate (SR), and specific growth rate (SGR) were calculated and documented carefully.
Assessment of maturation
Ten fishes per treatment were dissected carefully, and bilobed gonads were abscised at the end of the feeding trial to assess the levels of maturation as explained below. The mean of all the recorded values was taken for the analysis.
Gonad histology
Histological sections were prepared, mounted, and stained in the histology lab and histological examinations were observed under the light microscope and documented carefully. Ovarian development was studied according to Kumari and Nair (1978a, b). The maturity stages of the ovary subjected to the experiment were observed and estimated based on the proportions of each oocyte stage. Individual oocyte stage was counted and recorded to find out the maturity status of each particular fish in different treatments.
Gonadosomatic index value
GSI was calculated during initial and final sampling. The GSI was estimated by using the following equation as suggested by Kumari and Nair (1978a, b).
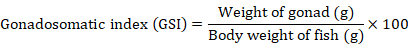
Absolute fecundity
Absolute fecundity was calculated by dividing the mean total fecundity and mean body weight of the fish (Muchlisin et al., 2011).
Hormone assays
Hormone assays were performed as described by Zhang et al. (2021). Gonad samples were homogenized in cold PBS (Phosphate buffer saline) and were centrifuged at 3000 rpm for 20 min in the refrigerated centrifuge (4oC). The supernatants were collected and analyzed for cortisol and progesterone levels using Accubind ELISA kits.
Assessment of breeding performance
After the completion of 60 days of the feeding experiment, males were introduced in the tanks at a ratio of 1:1 and were assessed for breeding performances for another 30 days. The number of spawns produced under different treatments was calculated carefully and was collected and stocked in separate tanks. Initial length and weight data of spawn produced were recorded carefully and were fed with powdered GNOC.
Statistical analysis
The data and parameters recorded were subjected to ‘F’ test to find statistical significance. The growth and maturation parameters were analyzed in detail using Regression analysis and compared using one-way ANOVA in SPSS- 22.0.
Results
Significant differences (p<0.05) in the mean estimated growth and maturity L. thermalis ovaries were recorded in the present study when fed with selected minerals at three different concentrations (Tables II-VI).
Table II. ANOVA for bio growth parameters of L. thermalis fed with Mn, Se and Zn at three different levels.
|
Parameter |
C |
Treatments |
||||||||
|
T1 |
T2 |
T3 |
T4 |
T5 |
T6 |
T7 |
T8 |
T9 |
||
|
Weight gain (g) |
0.13± 0.00i |
0.32± 0.16c |
0.14± 0.11g |
0.19± 0.14e |
0.39± 0.08a |
0.14± 0.04h |
0.14± 0.06h |
0.18± 0.05f |
0.23± 0.13d |
|
|
Weight gain % |
0.17± 0.00i |
0.43± 0.22c |
0.19± 0.16g |
0.25± 0.19e |
0.53± 0.12a |
0.19± 0.05h |
0.18± 0.09h |
0.24± 00.06f |
0.30± 0.17d |
|
|
DGR |
0.59± 0.00i |
1.41± 0.71c |
0.62± 0.51g |
0.82± 0.62e |
1.75± 0.38a |
0.61± 0.16h |
0.60± 0.28h |
0.8± 0.21f |
1.00± 0.55d |
|
|
SGR |
0.23± 0.00i |
0.49± 0.22c |
0.24± 0.19g |
0.31± 0.22e |
0.60± 0.10a |
0.24± 0.06h |
0.24± 0.10h |
0.30± 0.08f |
0.37± 0.19d |
|
Data expressed as Mean ± S.E. (n=30, r=3, t=10); Mean values in the same row with different superscripts differ significantly (p<0.05). One-way ANOVA was used following Duncan’s multiple range test in SPSS- 22.0.
Effect of Mn incorporation on the growth and maturation of L. thermalis
When L. thermalis were fed with diets incorporated with different levels of manganese, maximum values of mean weight gain (0.3350 ± 0.145g), mean weight gain percentage (0.4550 ± 0.1950), mean DGR (1.510 ± 0.650), and mean SGR (0.5250 ± 0.195) were recorded with diet containing 10 mg Mn/kg diet (T2), followed by 5 mg Mn/kg of diet (T1) (Table II). The maximum mean biomass production rate (34.90 ± 21.36 g/m2) was recorded in the group fed with 10 mg Mn/kg of diet, followed by 5 mg Mn/kg of diet (32.55 ± 23.20) and 15 mg Mn/kg of diet (34.90 ± 21.36). A direct relationship between the dietary incorporation levels of Mn and mean production rates were noticed up to 10 mg Mn/kg of diet, after which there was a decline in the production rates (Table III).
Table III. Biomass estimation in the trials done with L. thermalis fed with Mn, Se and Zn at three different levels.
|
Treatments |
Biomass (g) |
Net biomass production (g) |
Production rate/m2 |
|
|
Initial |
Final |
|||
|
C |
3.7 ± 0.00 |
4.35±0.00 |
0.65 ± 0.00 |
13.54 ± 0.00i |
|
T1 |
3.7 ± 0.00 |
5.26 ± 1.11 |
1.56 ± 1.11 |
32.55 ± 23.20c |
|
T2 |
3.7 ± 0.00 |
5.38 ± 1.03 |
1.68 ± 1.03 |
|
|
T3 |
3.7 ± 0.00 |
4.39 ± 0.80 |
0.69 ± 0.80 |
14.32 ± 16.57g |
|
T4 |
3.7 ± 0.00 |
4.61 ± 0.97 |
0.91 ± 0.97 |
19.01 ± 20.26e |
|
T5 |
3.7 ± 0.00 |
5.64 ± 0.58 |
1.94 ± 0.58 |
40.36 ± 12.15a |
|
T6 |
3.7 ± 0.00 |
4.38± 0.25 |
0.68 ± 0.18 |
14.06 ± 5.16h |
|
T7 |
3.7 ± 0.00 |
4.36 ± 0.44 |
0.66 ± 0.44 |
13.80 ± 9.21h |
|
T8 |
3.7 ± 0.00 |
4.59 ± 0.34 |
0.89 ± 0.34 |
18.49 ± 7.00f |
|
T9 |
3.7 ± 0.00 |
4.81 ± 0.87 |
1.11 ± 0.87 |
23.18 ± 18.05d |
*Data expressed as Mean ± S.E. (n= 30, r=3, T = 10); Mean values in the same row with different superscripts differ significantly (p<0.05). One-way ANOVA was used following Duncan’s multiple range test in SPSS- 22.0.
While comparing the effect of different concentrations of Mn on the maturation of L. thermalis, it was found that diet supplemented with 15 mg Mn/kg diet showed maximum numbers of secondary oocytes followed by 10 mg Mn/kg diet and 5 mg Mn/kg diet (Table IV and Fig. 1). Similarly, maximum mean GSI (19.53±0.92) was recorded 15 mg Mn/kg diet, followed by 10 mg Mn/kg diet and 5 mg Mn/kg diet. The mean absolute fecundity of L. thermalis, when fed with Mn-incorporated diets, was in the range of 3689.6 - 4683.8/gm body weight of the female, with maximum fecundity corresponding to a higher Mn dose (15mg Mn/Kg diet). A direct relation between the incorporation levels of Mn with estimated GSI and absolute fecundity was recorded in the present study (Table V).
Effect of selenium incorporation on the growth and maturation
While evaluating the dietary impact of Se-incorporated feed on the growth performance of L. thermalis, we recorded, maximum mean weight gain (0.3900 ± 0.080 g), mean weight gain percentage (0.5250 ± 0.115), mean DGR (1.7450 ± 0.375) and mean SGR (0.6050 ± 0.105) when fed with 0.35 mg/kg diet, followed by 0.2 mg Se/kg of diet and 0.5 mg Se/kg of diet. Also, fishes fed with 0.35 mg Se/kg of diet recorded the maximum mean production of 40.36 ± 12.15 g/m2, followed by 0.2 mg Se/kg of diet (19.01 ± 20.26) and T6 0.5 mg Se/kg of diet (14.06 ± 5.16) (Tables II and III).
Similarly, on comparing the dietary impact of Se on the maturation of L. thermalis, we found that maximum numbers of secondary oocytes were observed in a diet supplemented with 0.2 mg Se/kg diet, followed by 0.35 mg Se/kg diet and 0.5 mg Se/kg diet (Table IV and Fig. 2). Likewise, diet incorporated with 0.35 mg Se/kg of diet recorded maximum mean GSI (24.17±3.40) followed by 0.2 mg Se/kg of diet and 0.5 mg Se/kg of diet. The mean GSI recorded from Se treatments was in the range of
Table IV. Histology observations of L. thermalis gonads fed Mn, Se and Zn at three different concentrations.
|
Treatments |
Stages observed (nos) |
|||||
|
OG |
CNS |
PNS |
YVS |
PVS |
SVS |
|
|
C |
5 ± 0b |
7 ± 0a |
10 ± 0a |
3 ± 0c |
2 ± 0d |
3 ± 0g |
|
T 1 |
4.0 ± 1.0c |
2.0 ± 1.0d |
9.0 ± 2.0b |
5 ± 1.0a |
3.0 ± 0b |
3.5 ± 1.0f |
|
T 2 |
8.0 ± 1.0a |
2.5 ± 1.5c |
5.0 ± 1.0d |
2.5 ± 0.5d |
2.0 ± 0d |
3.5 ± 0.5f |
|
T 3 |
2.5 ± 0.5e |
1.5 ± 0.5e |
4.0 ± 1.5f |
2 ± 1.0e |
2.5 ± 0.5c |
9.5 ± 0.5a |
|
T 4 |
2.0 ± 0.0f |
1.0 ± 0.0f |
4.5 ± 0.5e |
2.5 ± 0.5d |
4.0 ± 0a |
6.5 ± 1.0c |
|
T 5 |
3.0 ± 0.0d |
1.0 ± 1.0f |
5.5 ± 0.5c |
3 ± 1.0c |
3.0 ± 1.0b |
6.0 ± 0.5d |
|
T 6 |
3.0 ± 0.0d |
3.0 ± 1.0b |
4.5 ± 1.5e |
2 ± 0.0e |
4.0 ± 0a |
5.5 ± 2.0e |
|
T 7 |
2.5 ± 0.5e |
1.5 ± 0.5e |
3.5 ± 1.5g |
2 ± 0.0e |
2.5 ± 0.5c |
7.0 ± 1.5b |
|
T 8 |
2.5 ± 0.5e |
2.5 ± 0.5c |
3.0 ± 1.0h |
2.5 ± 0.5d |
4.0 ± 1.0a |
5.5 ± 1.0e |
|
T 9 |
2.5 ± 0.5e |
1.0 ± 0.0f |
4.0 ± 0.0f |
4.50 ± 0.5b |
2.5 ± 0.5c |
5.5 ± 0.5e |
*Data expressed as Mean ± S.E. (n= 10, r=3, T = 10); Mean values in the same row with different superscripts differ significantly (p<0.05). One-way ANOVA was used following Duncan’s multiple range test in SPSS- 22.0. ** Oogonia cells (OG), Chromatin Nucleolus Stage (CNS), Perinucleolus stage (PNS), Yolk vesicle stage (YVS), Primary vitellogenin stage (PVS) and Secondary vitellogenin stage (SVS).
Table V. Estimated maturation parameters of L. thermalis fed with Mn, Se and Zn at three different concentrations.
|
Treatments |
GSI |
Absolute fecundity (per BW) |
Hormone level (ng/ml) |
||
|
Initial |
Final |
Cortisol |
Progesterone |
||
|
C |
0.14 ±0.00 |
12.64 ± 3.5 |
4172.4 |
24.966 ±0.081 |
2.5661 ±0.004 |
|
T 1 |
0.14 ±0.00 |
15.44 ±1.80 |
3689.6 |
24.973 ±1.80 |
2.5702 ±0.004 |
|
T 2 |
0.14 ±0.00 |
16.24±6.43 |
4283.2 |
24.914 ±0.07 |
2.5646 ±0.000 |
|
T 3 |
0.14 ±0.00 |
19.53 ±0.92 |
4683.8 |
24.713 ±0.39 |
2.5649 ±0.003 |
|
T 4 |
0.14 ±0.00 |
22.22 ±4.82 |
4543.2 |
24.873 ±0.02 |
2.5568 ±0.001 |
|
T 5 |
0.14 ±0.00 |
24.17 ±3.40 |
4827.7 |
24.902 ±0.07 |
2.5637 ±0.005 |
|
T 6 |
0.14 ±0.00 |
17.14 ±4.69 |
3780.6 |
24.884 ±0.00 |
2.5626 ±0.002 |
|
T 7 |
0.14 ±0.00 |
18.62 ±2.08 |
4741.3 |
24.978 ±0.01 |
2.5649 ±0.000 |
|
T 8 |
0.14 ±0.00 |
17.44 ±1.88 |
4457.4 |
24.929 ±0.02 |
2.5649 ±0.009 |
|
T 9 |
0.14 ±0.00 |
14.81 ±0.07 |
4194.4 |
24.862 ±0.05 |
2.5715 ±0.003 |
(17.14±4.69-24.17±3.40). Also, the mean absolute fecundity recorded was in the range of 3780.6-4827.7/gm body weight of the female brooder, with the maximum value from 0.35 mg Se/kg diet followed by 0.2 mg Se/kg diet and 0.5 mg Se/kg diet (Table V).
Effect of zinc incorporation on the growth and maturation
While evaluating the dietary impact of Zn on the growth performance of L. thermalis, we found that a diet incorporated with 15 mg Zn/kg of diet showed maximum mean weight gain (0.2250 ± 0.125 g), mean weight gain percentage (0.3050 ± 0.165), mean SGR (1.00 ± 0.550) and mean DGR (0.3650 ± 0.1850), followed by 10 mg Zn/kg of diet and 5 mg Zn/kg of diet (Table II). The maximum mean production rate of 23.18 ± 18.05 g/m2 was recorded in the group of L. thermalis fed with a diet incorporated with 15 mg Zn/kg of diet, followed by 10 Zn/kg of diet (18.49 ± 7.00) and 5 Zn/kg of diet (13.80 ± 9.21) (Table III).
Likewise, the incorporation of 5 mg Zn/kg of diet resulted in the maximum number of secondary oocytes followed by 10 mg Zn/kg of diet and 15 mg Zn/kg of diet (Table IV and Fig. 3). Also, diet incorporated with Zn at 5 mg Zn/kg diet exhibited a higher mean GSI of 18.62 ± 2.08 followed by a 10 mg Zn/kg diet and a 15 mg Zn/kg diet. The mean absolute fecundity recorded in the present study was in the range of 4194.4-4741.3, with the maximum recorded from a 5 mg Mn/kg diet followed by 10 mg Mn/kg diet and 15 mg Mn/kg diet (Table V). In the present study, a direct relationship between dietary incorporation levels of Zn and growth parameters was recorded. On the contrary dietary incorporations levels of Se have shown an indirect relationship with the maturation parameters (GSI and absolute fecundity).
Hormone assays
No significant difference (p>0.05) in the mean cortisol and progesterone concentrations was recorded in any of the mineral-incorporated diets (Table IV). Mean cortisol concentration recorded from individual treatments was in the range of 24.713– 24.973 ng/ml for Mn, 24.873–24.966 ng/ml for Se, and 24.862–24.978 ng/ml for Zn incorporated diets (Table IV).
Similarly, a negligible difference was noticed in the mean progesterone concentrations of L. thermalis ovary from all the treatments. It was recorded in the range of 2.5646–2.5702 ng/ml for Mn, 2.5568–2.5661 ng/ml for Se, and 2.5661–0.5715 ng/ml in Zn treatments (Table IV).
Breeding performance
Breeding could not be recorded from all the treatments, and only a few fishes spawned. A total of 166 spawns were collected in the present study, and the details are given in Table VI. Eggs were not physically observed in any of the treatments. Maximum numbers of spawns (97) were recorded from Mn-incorporated diets followed by 38 Zn-incorporated diets and 31 Se-incorporated diets. Mn incorporation at the level of 5 Mn mg/kg diets yields the maximum number of spawns (65) among all treatments. Spawns were collected during morning hours (10:00 am – 11:30 am). The average initial length of spawns recorded in the present study was 6 ± 0.01 mm.
Table VI. Breeding performance observed in L. thermalis fed with Mn, Se and Zn at three different concentrations.
|
Treatments |
Breeding occurrence |
No. of young ones observed |
Larval survival rate (%) |
|
|
Day 1 |
Day 15 |
|||
|
C |
No |
- |
- |
- |
|
T 1 |
Yes |
65 |
100 |
93.84 |
|
T 2 |
No |
22 |
- |
- |
|
T 3 |
Yes |
10 |
90 |
70 |
|
T 4 |
Yes |
9 |
88.9 |
77.8 |
|
T 5 |
Yes |
16 |
100 |
81.25 |
|
T 6 |
Yes |
6 |
100 |
100 |
|
T 7 |
Yes |
18 |
100 |
100 |
|
T 8 |
No |
12 |
- |
- |
|
T 9 |
Yes |
8 |
100 |
100 |
Discussion
Dietary impact of minerals on the growth
There was a distinct impact noted in the present study (Tables II and III) when different levels of minerals such as Mn, Se, and Zn were incorporated into the diet of L. thermalis, endorsing the observations and reports of Domínguez et al. (2019), Izquierdo et al. (2017) and Watanabe et al. (1980), who highlighted the importance of minerals in various biological and physiological functions and stated that these are the key components for synthesis and functioning of hormones and enzymes in fishes. In the present study when fishes were fed with different incorporation levels of Mn, maximum mean growth performance was recorded when fed with 10 mg, Mn/kg of diet. It has also been noted that further increment in the incorporation level of manganese in the diet of L. thermalis showed a reduction in the growth performance. The dietary manganese requirements reported in other fishes are 13–15 mg/kg for common carp and rainbow trout (Ogino and Yang, 1980; Satoh et al., 1991), 15 mg/kg for fingerling grass carp (Wang and Zhao, 1994), 2.4 mg/kg for channel catfish (Gatlin and Wilson, 1984b), 7.5–10.5 mg/kg for Atlantic salmon (Maage et al., 2000) and 17.8 mg/kg for red sea bream.
In the present study, manganese at the rate of 15 mg Mn/kg of diet has resulted in poor growth performance of L. thermalis and similar incidence has also been described in mammals (Underwood, 1963). Supplementation of high manganese (15mg Mn/kg) in the fish diet resulted in the reduction of growth performance in juvenile mulloway (Argyrosomus japonicus) due to poor feed utilization efficiency as well as reduced food intake in A. japonicus, which could also be attributed as a possible reason for poor growth in L. thermalis.
In the present study, when fishes were fed with feed incorporated with elevated levels of selenium from 0 to 0.35 mg Se/kg of diet, a direct relationship with the mean estimated growth parameters were observed. The incorporation of 0.35 mg Se/kg of diet has recorded maximum mean growth parameters which are well within the limits reported for other freshwater fishes. Dietary Se requirements reported for other fish species were 0.25 mg Se/kg for channel catfish (Gatlin and Wilson, 1984a); 0.48 to 0.50 mg Se/kg for loach (Hao et al., 2014); 0.15 to 0.38 mg Se/kg for rainbow trout (Hilton et al., 1980). Also, the best performance was seen with Se lesser than 0.5 mg Se/kg of diet which is in line with the recommendation of Gatlin and Wilson (1984a) and Lemly (1997). The positive role of Se in L. thermalis can be compared with the results of Hilton et al. (1980), and Khan et al. (2016), who witnessed the positive role of selenium on the growth performance of fish.
It was recorded that, the incorporation of zinc when escalated from 0 to 15 mg Zn/kg of diet, a direct relationship with mean growth parameters could be noted. The best growth performance was obtained in 15 mg Zn/kg of diet (Tables II and III). Optimum dietary zinc requirements described for other fishes were 15-30 mg/kg diet for rainbow trout (Ogino and Yang, 1978), 8-18 mg/kg for salmon (Tacon and De Silva, 1983), 20 mg/kg diet for channel catfish (Gatlin and Wilson, 1983), 37–57 mg/kg diet for Atlantic salmon (Maage and Julshamn, 1993), 20 mg/kg for channel catfish (Gatlin and Wilson, 1983), 30 mg Zn/kg purified diet for Nile tilapia (Eid and Ghonim, 1994) and 17.12-20.86 mg/kg for yellow catfish (Luo et al., 2011). The Brazilian animal nutrition industry recommended the supplementation of 30–150 mg Zn/kg in commercial diets for omnivorous fishes. The results of the present study are in accordance with previous works. Low growth response recorded with respect to the high dose of zinc (15mg/kg) is due to the toxicity of zinc on L. thermalis impairing normal physiological function as reported by Mohanty et al. (2009).
Dietary impact of minerals on the maturation and breeding
Dietary fatty acids are the strongest determinants of reproductive performance, and fish eggs contain a high level of polyunsaturated fatty acid lipids; their structures make them more sensitive to the attack of reactive oxygen species (ROS), as reported by Izquierdo et al. (2001). These ROS can thus damage cell membranes, and macromolecules (DNA and RNA) and inactivate within the developing embryo, thus affecting early larval development (Mourente et al., 1999). ROS alters fertilization in germinal vesicles and may cause the failure of zygote segmentation in embryo development (Marik, 2000). It is well known that superoxide dismutase (SOD) is the first line of defense against oxygen toxicity, as reported by Fattman et al. (2003). A direct influence of Mn supplementation in the diets of fish and hepatic Mn-SOD activity has been reported in the juvenile tilapia (Lin et al., 2008), Atlantic salmon (Maage et al., 2000), and rainbow trout (Knox et al., 1982). In the present study, we have recorded the maximum maturity of L. thermalis under Mn and Zn treatments which could be because Mn acts as a cofactor for essential metalloenzymes and helps prevent harmful effects of the free radicals by forming part of Mn superoxide dismutase and supplementation of oxide forms of Mn (Domínguez et al., 2019).
Similarly, Zn has also shown strong effects in regulating the oxidative stress, immunity, and reproductive processes of fish species (Domínguez et al., 2019). Watanabe et al. (1997) highlighted Zn as an essential trace element in fish nutrition by contributing to many metabolic pathways such as the specific cofactor and integral part of 20 metalloenzymes (alkaline phosphatase, alcohol dehydrogenase, and carbonic anhydrase), involved in prostaglandin metabolism, plays an essential structural role in nucleoproteins and controls normal growth (Chesters, 1991).
The incorporation of maximum concentrations of Mn and Se in the diets of L. thermalis has resulted in the maximum mean absolute fecundity and GSI (direct relationship) of L. thermalis in the present study. In contrast, dietary incorporation of Zn in the feed of L. thermalis has shown an indirect relationship with its mean absolute fecundity and mean GSI values. Further, we also found that fishes fed Mn and Se incorporated diets showed more fecundity and GSI than control, which proves the importance of Mn and Se in controlling a fish’s reproductive performance. The results of the present study could be compared with the investigations of Gatlin and Wilson (1984a, b), Lemly (1997) and Hao et al. (2014).
Also, Se incorporation in the diets of L. thermalis has shown maximum mean absolute fecundity, which could be due to its importance as an oxidizing agent and preventing the oocytes from oxidative damage by activation of seleno-enzymes and selenoprotein as suggested by Watanabe et al. (1997), Zuberbuehler et al. (2006), and Khan et al. (2016).
Chassaigne et al. (2002) and Połatajko et al. (2006) postulated that Se requirement and toxicity level is the smallest of any element, which is also shown in the present study in terms of reduction in mean absolute fecundity of L. thermalis fed with higher doses of Se. Recommended Se level in the fish diet has been 0.1–0.5 mg Se/kg feed as highlighted by Gatlin and Wilson (1984a), and Lemly (1997). The optimum dietary requirement of Se for loach is 0.48–0.50 mg Se/kg diet, as reported by Hao et al. (2014). Similarly, in the present study, we have found that dietary incorporation of 0.5 mg Se/kg diet has resulted in maximum mean absolute fecundity of L. thermalis.
Histological changes in the maturing oocytes of different species have been recorded by many authors (Kumari and Nair,1978b, 1979). Gonad’s histology helped the workers understand the origin and development of the reproductive element at the ultrastructural level. Histology sections of the female ovary helped to understand the prolonged spawning season of L. thermalis. The presence of oocytes from the different development stages by studying histology slides is a widely accepted tool to define the final maturity of fish species (Ismail et al., 2011; Ferreira et al., 2012; Hismayasari et al., 2015; Singh et al., 2019).
Ismail et al. (2011) reported the maturity of mahseer based on the proportion of final stages of oocytes during the breeding season. They have explained different development stages of female mahseer based on the histology observations of the female brooder. Ferreira et al. (2012) described the oogenesis in Lipophrys pholis by reporting the proportion of different development stages of oocytes. They defined the early oogenesis, mid oogenesis, and spawning based on the total numbers of primary and secondary oocytes.
Kumari and Nair (1978b, 1979) described the gonad developments of L. thermalis female brooders by studying their histology sections. They were the first to report the reproductive biology of female Indian spiny loach from the Western Ghats. According to them, female L. thermalis attained maturity at their TL of 45 mm, and 5 maturity stages could be identified. The results of the present study are in accordance with Kumari and Nair (1978b, 1979), Ismail et al. (2011), Ferreira et al. (2012), and Singh et al. (2019).
Studies have shown the effectiveness of Mn supplementation in influencing the breeding performance of finfish and shellfish (Ogino and Yang, 1980; Knox et al., 1982; Satoh et al., 1987a; Watanabe et al., 1997; Domínguez et al., 2019). The positive role of Mn in influencing the GSI could be attributed to its importance as a cofactor for essential metalloenzymes and helps prevent adverse effects of free radicals by forming part of Mn superoxide dismutase. Supplementation of oxide forms of Mn results in the highest growth rate and protein deposition of gilthead seabream compared to its chelated forms (Domínguez et al., 2019).
The maximum concentration of Mn from gonads during the gametogenic activity of mangrove oyster (Crassostrea corteziensis) has been documented by Frıas-Frıas-Espericueta et al. (1999), suggesting its importance in gonad maturation. Similarly, Satoh et al. (1987a) stated that Mn affects the quality of eggs and hatchability rate in trout broodstock. Thus, the results obtained in the present study by the dietary incorporation of Mn at three different doses could be correlated with the findings of Ogino and Yang (1980), Knox et al. (1982), Satoh et al. (1987a), Watanabe et al. (1997), Frıas-Espericueta et al. (1999), and Domínguez et al. (2019).
The importance of Zn as an essential trace element has been well established in approximately 1000 regulatory and catalytic proteins for growth and development in animals, as reported by Eide (2006) and Maret and Krężel (2007). Zn has also been reported an active component in 20 metalloenzymes involved in lipid, carbohydrate, protein metabolism, regulating oxidative stress, immunity and reproductive processes (Domínguez et al., 2019).
Although there have been many reports correlating cortisol and progesterone levels with the maturity stages and spawning, in the present study, no such correlations could be established between cortisol levels and progesterone levels with the maturity stages recorded. This could be attributed to the multiple spawning behavior of L. thermalis or the living habitat where they might have been acclimatized to changes in the environmental parameters for heartening their breeding behaviour. Further studies are needed to establish this fact.
Among the selected minerals, Mn incorporation at the rate of 5 mg/kg of diet has shown the highest breeding performance in terms of total spawns collected. Production of free radicals during the maturation of fish is a well-known phenomenon that could be the possible cause of reproductive failures in many fish species. Mn is reported to play an essential role in anti-oxidant metabolism, as reported by Watanabe et al. (1997). Domínguez et al. (2019) highlighted that Mn acts as a cofactor for essential metalloenzymes. When fed with Mn-supplemented diets, Satoh et al. (1987a) observed a high hatchability rate in trout broodstock. Therefore, in the present study also Mn would have played a positive stimulation for the fish to breed. No eggs were physically observed in any of the treatments. It may be because these fishes preferred laying their eggs in the sand thus making evaluating hatching and fertilization rates challenging.
Conclusion
It is clear from the results of the current study that the performance of L. thermalis development and maturation was significantly affected by the addition of Mn, Se, and Zn. We also discovered that Se is a significant mineral influencing L. thermalis ability to develop in captivity. Mn and Zn, however, might improve the loach’s ability to mature and reproduce. The findings of the current study led us to conclude that adding Mn, Se, and Zn to the diet at rates of 10 mg/kg, 0.35 mg/kg, and 10 mg/kg could produce the highest growth performance in loaches, whereas adding Mn, Se, and Zn at rates of 15 mg/kg, 0.35 mg/kg, and 5 mg/kg leads to the highest maturation and breeding performance in loaches.
Acknowledgement
The SRF given by ICAR and the research facilities provided by TNJFU are warmly acknowledged by the authors.
Funding
Present Research Project was carried out under an ICAR-SRF fellowship.
Ethical statement
The experiment was conducted following the procedures of CPCSEA (Committee for the Purpose of Control and Supervision of Experiments on Animals), Ministry of Environment and Forests (Animal Welfare Division), Government of India on care and use of animals in scientific research.
Data availability statement
The data presented in this paper has not been shared with any other source.
Statement of conflict of interest
The authors have declared no conflict of interest.
References
Bromage, N., Jones, J., Randall, C., Thrush, M., Davies, B., Springate, J., Duston, J., and Barker, G., 1992. Broodstock management, fecundity, egg quality and the timing of egg production in the rainbow trout (Oncorhynchus mykiss). Aquaculture, 100: 141-166. https://doi.org/10.1016/0044-8486(92)90355-O
Chassaigne, H., Chéry, C.C., Bordin, G., and Rodriguez, A.R., 2002. Development of new analytical methods for selenium speciation in selenium-enriched yeast material. J. Chromatogr. A, 976: 409-422. https://doi.org/10.1016/S0021-9673(02)00945-7
Chesters, J.K., 1991. Trace element–gene interactions with particular reference to zinc. Proc. Nutr. Soc., 50: 123-129. https://doi.org/10.1079/PNS19910023
DAHDF, 2020. Handbook on fisheries statistics. Department of Fisheries Ministry of Fisheries, Animal Husbandry and Dairying Government of India, New Delhi.
Domínguez, D., Robaina, L., Zamorano, M.J., Karalazos, V., and Izquierdo, M., 2019. Effects of zinc and manganese sources on gilthead seabream (Sparus aurata) fingerlings. Aquaculture, 505: 386-392. https://doi.org/10.1016/j.aquaculture.2019.03.004
FEEDAP (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012. Scientific Opinion on safety and efficacy of zinc compounds (E6) as feed additive for all animal species: Zinc oxide, based on a dossier submitted by Grillo Zinkoxid GmbH/EMFEMA. EFSA J., 10: 2970.
Eid, A.E., and Ghonim, S.I., 1994. Dietary zinc requirement of fingerling Oreochromis niloticus. Aquaculture, 119: 59-264. https://doi.org/10.1016/0044-8486(94)90180-5
Eide, D.J., 2006. Zinc transporters and the cellular trafficking of zinc. Biochim. Biophys. Acta Mol. Cell Res., 1763: 711-722. https://doi.org/10.1016/j.bbamcr.2006.03.005
Fattman, C.L., Schaefer, L.M., and Oury, T.D., 2003. Extracellular superoxide dismutase in biology and medicine. Free Radic. Biol. Med., 35: 236-256. https://doi.org/10.1016/S0891-5849(03)00275-2
Fernández-Palacios, H., Izquierdo, M.S., Robaina, L., Valencia, A., Salhi, M., and Vergara, J., 1995. Effect of n− 3 HUFA level in broodstock diets on egg quality of gilthead sea bream (Sparus aurata L.). Aquaculture, 132: 325-337. https://doi.org/10.1016/0044-8486(94)00345-O
Ferreira, F., Santos, M.M., Reis-Henriques, M.A., Vieira, N.M., and Monteiro, N.M., 2012. The annual cycle of oogenesis in the shanny, Lipophrys pholis (Pisces: Blenniidae). Sci. Mar., 76: 273-280. https://doi.org/10.3989/scimar.03288.18C
Frıas-Espericueta, M.G., Osuna-López, J.I., and Páez-Osuna, F., 1999. Gonadal maturation and trace metals in the mangrove oyster Crassostrea corteziensis: seasonal variation. Sci. Total Environ., 231: 115-123. https://doi.org/10.1016/S0048-9697(99)00097-2
Garling, Jr, D.L., and Wilson, R.P., 1976. Optimum dietary protein to energy ratio for channel catfish fingerlings, Ictalurus punctatus. J. Nutr., 106: 1368-1375. https://doi.org/10.1093/jn/106.9.1368
Gatlin, D.M. and Wilson, R.P., 1983. Dietary zinc requirement of fingerling channel catfish. J. Nutri., 113: 630-635.
Gatlin, D.M., and Wilson, R.P., 1984a. Dietary selenium requirement of fingerling channel catfish. J. Nutr., 114: 627-633. https://doi.org/10.1093/jn/114.3.627
Gatlin, D.M., and Wilson, R.P., 1984b. Studies on the manganese requirement of fingerling channel catfish. Aquaculture, 41: 85-92. https://doi.org/10.1016/0044-8486(84)90085-1
Gatlin, D.M., and Wilson, R.P., 1984c. Zinc supplementation of practical channel catfish diets. Aquaculture, 41: 31-36. https://doi.org/10.1016/0044-8486(84)90387-9
Hao, X., Ling, Q., and Hong, F., 2014. Effects of dietary selenium on the pathological changes and oxidative stress in loach (Paramisgurnus dabryanus). Fish Physiol. Biochem., 40: 1313-1323. https://doi.org/10.1007/s10695-014-9926-7
Helfman, G.S., 2013. Fishes, biodiversity. Encyclopedia of biodiversity. 2nd Edition. pp. 456-476. https://doi.org/10.1016/B978-0-12-384719-5.00054-X
Hilton, J.W., 1989. The interaction of vitamins, minerals and diet composition in the diet of fish. Aquaculture, 79: 223-244. https://doi.org/10.1016/0044-8486(89)90463-8
Hilton, J.W., Hodson, P.V., and Slinger, S.J., 1980. The requirement and toxicity of selenium in rainbow trout (Salmo gairdneri). J. Nutr., 110: 2527-2535. https://doi.org/10.1093/jn/110.12.2527
Hismayasari, I.B., Marhendra, A.P.W., Rahayu, S., Saidin, S.D., and Supriyadi, D.S., 2015. Gonadosomatic index (GSI), hepatosomatic index (HSI) and proportion of oocytes stadia as an indicator of rainbowfish Melanotaenia boesemani spawning season. Int. J. Fish. aquat. Stud., 2: 359-362.
Ismail, M.F.S., Siraj, S.S., Daud, S.K., and Harmin, S.A., 2011. Association of annual hormonal profile with gonad maturity of mahseer (Tor tambroides) in captivity. Gen. Comp. Endocrinol., 170: 125-130. https://doi.org/10.1016/j.ygcen.2010.09.021
Izquierdo, M.S., Fernandez-Palacios, H., and Tacon, A.G.J., 2001. Effect of broodstock nutrition on reproductive performance of fish. Aquaculture, 197: 25-42. https://doi.org/10.1016/B978-0-444-50913-0.50006-0
Izquierdo, M.S., Ghrab, W., Roo, J., Hamre, K., Hernández-Cruz, C.M., Bernardini, G., Terova, G., and Saleh, R., 2017. Organic, inorganic and nanoparticles of Se, Zn and Mn in early weaning diets for gilthead seabream (Sparus aurata; Linnaeus, 1758). Aquacult. Res., 48: 2852-2867. https://doi.org/10.1111/are.13119
Keskar, A., Kumkar, P., Paingankar, M.S., Padhye, A., and Dahanukar, N., 2015. Length-weight and length-length relationships of seven loach species (Teleostei: Cypriniformes) from five localities in northern Western Ghats. India. J. Threat. Taxa., 7: 8025-8220. https://doi.org/10.11609/jott.2462.7.15.8025-8220
Keskar, A., Padhye, A., and Dahanukar, N., 2014. Fighting against all odds: The struggle for existence among hill stream loaches of northern Western Ghats. Min Newsl. FFSG, 2: 25-29.
Khan, K.U., Zuberi, A., Nazir, S., Fernandes, J.B.K., Jamil, Z., and Sarwar, H., 2016. Effects of dietary selenium nanoparticles on physiological and biochemical aspects of juvenile Tor putitora. Turk. J. Zool. Derg., 40: 704-712. https://doi.org/10.3906/zoo-1510-5
Knox, D., Cowey, C.B., and Adron, J.W., 1982. Effects of dietary copper and copper: Zinc ratio on rainbow trout Salmo gairdneri. Aquaculture, 27: 111-119. https://doi.org/10.1016/0044-8486(82)90130-2
Kumari, S.D.R., and Nair, N.B., 1978a. Length-weight relationship of the loaches Noemacheilus triangularis Day and Lepidocephalus thermalis (Cuv. and Val). Matsya, 4: 52–58.
Kumari, S.D.R., and Nair, N.B., 1978b. Maturation and spawning in a tropical loach, Lepidocephalus thermalis (Cuv. and Val). Proc. Indian natl. Sci. Acad., 44: 52–58.
Kumari, S.R., and Nair, N.B., 1979. Oogenesis in a tropical loach Lepidocephalus thermalis (Cuv. and Val.). J. Anim. Sci., 88: 45-54. https://doi.org/10.1007/BF03179623
Lall, S.P., 1989. The minerals, In: Fish nutrition (ed. J.E. Halver), 2nd edn. Academic Press, New York, pp. 219-257.
Lemly, A.D., 1997. A teratogenic deformity index for evaluating impacts of selenium on fish populations. Ecotoxicol. Environ. Saf., 37: 259-266. https://doi.org/10.1006/eesa.1997.1554
Lin, Y.H., Lin, S.M. and Shiau, S.Y., 2008. Dietary manganese requirements of juvenile tilapia, Oreochromis niloticus× O. aureus. Aquaculture, 284: 207-210.
Luo, Z., Tan, X.Y., Zheng, J.L., Chen, Q.L., and Liu, C.X., 2011. Quantitative dietary zinc requirement of juvenile yellow catfish Pelteobagrus fulvidraco, and effects on hepatic intermediary metabolism and antioxidant responses. Aquaculture, 319: 150-155. https://doi.org/10.1016/j.aquaculture.2011.06.047
Maage, A., and Julshamn, K., 1993. Assessment of zinc status in juvenile Atlantic salmon (Salmo salar) by measurement of whole body and tissue levels of zinc. Aquaculture, 117: 179-191. https://doi.org/10.1016/0044-8486(93)90134-K
Maage, A., Lygren, B. and El-Mowafi, A.F.A., 2000. Manganese requirement of Atlantic salmon (Salmo salar) fry. Fish. Sci., 66: 1-8.
Maret, W., and Krężel, A., 2007. Cellular zinc and redox buffering capacity of metallothionein/thionein in health and disease. In: Molecular medicine. BioMed. Centr., 13: 371-375. https://doi.org/10.2119/2007-00036.Maret
Marik, J.J., 2000. Antioxidants and male infertility. Fertil. Steril., 73: 1065- 1066. https://doi.org/10.1016/S0015-0282(99)00621-4
Mohanty, M., Adhikari, S., Mohanty, P., and Sarangi, N., 2009. Effect of waterborne zinc on survival, growth, and feed intake of Indian major carp, Cirrhinus mrigala (Hamilton). Water, Air Soil Pollut., 201: 3-7. https://doi.org/10.1007/s11270-008-9921-7
Mourente, G., Tocher, D.R., Diaz, E., Grau, A. and Pastor, E., 1999. Relationships between antioxidants, antioxidant enzyme activities and lipid peroxidation products during early development in Dentex dentex eggs and larvae. Aquaculture, 179: 309-324. https://doi.org/10.1016/S0044-8486(99)00167-2
Muchlisin, Z.A., Musman, M., Fadli, N. and Siti-Azizah, M.N., 2011. Fecundity and spawning frequency of Rasbora tawarensis (Pisces: Cyprinidae) an endemic species from Lake Laut Tawar, Aceh, Indonesia. AACL Bioflux, 4: 273-279. https://doi.org/10.1186/1477-7827-8-49
Nasr-Esfahani, M.H., and Johnson, M.H., 1992. Quantitative analysis of cellular glutathione in early preimplantation mouse embryos developing in vivo and in vitro. Hum. Reprod., 7: 1281-1290. https://doi.org/10.1093/oxfordjournals.humrep.a137843
Nelson, J.S., 2006. Fishes of the world. 4th edition. John Wiley and Sons, Nueva York.
Ogino, C., and Yang, G.Y., 1980. Requirements of carp and rainbow trout for dietary manganese and copper. Nippon Suisan Gakkaishi, 46: 455-458. https://doi.org/10.2331/suisan.46.455
Ogino, C., and Yang, G.Y., 1978. Requirement of rainbow trout for dietary zinc. Bull. Jpn. Sot. Sci. Fish., 44: 1015-1018. https://doi.org/10.2331/suisan.44.1015
Połatajko, A., Jakubowski, N., and Szpunar, J., 2006. State of the art report of selenium speciation in biological samples. J. Analyt. Atomic Spec., 21: 639-654. https://doi.org/10.1039/B605654G
Satoh, S., Izume, K., Takeuchi, T., and Watanabe, T., 1987a. Availability to rainbow trout of zinc contained in various types of fish meals. Nippon Suisan Gakkaishi, 53: 1861-1866. https://doi.org/10.2331/suisan.53.1861
Satoh, S., Tabata, K., Izume, K., Takeuchi, T. and Watanabe, T., 1987b. Effect of dietary tricalcium phosphate on availability of zinc to rainbow trout. Nippon Suisan Gakkaishi. 53: 1199-1205. https://doi.org/10.2331/suisan.53.1199
Satoh, S., Takeuchi, T. and Watanabe, T., 1987c. Effect of deletion of several trace elements from a mineral mixture in fish meal diets on mineral composition of gonads in rainbow trout and carp. Cell, 3: 3. https://doi.org/10.2331/suisan.53.281
Satoh, S., Takeuchi, T., and Watanabe, T., 1991. Availability of manganese and magnesium contained in white fishmeal to rainbow trout Oncorhynchus mykiss. Nippon Suisan Gakkaishi, 57: 99-104. https://doi.org/10.2331/suisan.57.99
Shaji, C.P., Easa, P.S., and Gopalakrishnan, A., 2000. Freshwater fish diversity of Western Ghats, In: Endemic fish diversity of Western Ghats (eds. A.G. Ponniah and A. Gopalakrishnan). NBFGR-NATP publication, National Bureau of Fish Genetic Resources, Lucknow, India. 347. pp. 35-35.
Singh, R., Pandey, N.N., Tiwari, V.K., Gupta, M., Ganie, P.A., Prakash, C., and Am, B.R., 2019. Histological assessment of gonadal cyclicity of Chagunius chagunio. J. Entomol. Zool. Stud., 7: 391-395.
Swain, S., Felix, S., Anthony, C., and Chhandaprajnadarsini, E.M., 2017. Exotic fish species introduced in India and its impacts. Sci. India, pp. 27-29.
Tacon, A.G.J., and De Silva, S.S., 1983. Mineral composition of some commercial fish feeds available in Europe. Aquaculture, 31: 11-20. https://doi.org/10.1016/0044-8486(83)90253-3
Underwood, E.J., 1963. Trace elements in human and animal nutrition. Soil Sci., 95: 287. https://doi.org/10.1097/00010694-196304000-00029
Wang, D., and Zhao, L., 1994. Requirement of fingerling grass carp (Ctenopharyngodon idellus) for manganese. J. Shanghai Fish. Univ., 3: 1-2.
Wang, J., Pei, X., Liu, H., and Zhou, D., 2018. Extraction and characterization of acid-soluble and pepsin-soluble collagen from skin of loach (Misgurnus anguillicaudatus). Int. J. biol. Macromol., 106: 544-550. https://doi.org/10.1016/j.ijbiomac.2017.08.046
Watanabe, K., and Hidaka, T., 1983. Feeding behaviour of the Japanese loach, Misgurnus anguillicaudatus (Cobitididae). J. Ethol., 1: 86-90. https://doi.org/10.1007/BF02347834
Watanabe, T., Kiron, V., and Satoh, S., 1997. Trace minerals in fish nutrition. Aquaculture, 151: 185–207. https://doi.org/10.1016/S0044-8486(96)01503-7
Watanabe, T., Takeuchi, T., and Ogino, C., 1980. Effect on rainbow trout and chum salmon of deletion of trace elements from fish meal diet. Bull. Jpn. Soc. Sci. Fish., 46: 1521-1525. https://doi.org/10.2331/suisan.46.1521
Watanabe, T.J.A.F.S., 1988. Availability of minerals in fish meal to fish. Asian Fish. Sci., 1: 75-195. https://doi.org/10.33997/j.afs.1987.1.2.008
You, L., Zhao, M., Regenstein, J.M., and Ren, J., 2011. In vitro antioxidant activity and in vivo anti-fatigue effect of loach (Misgurnus anguillicaudatus) peptides prepared by papain digestion. Fd. Chem., 124: 188-194. https://doi.org/10.1016/j.foodchem.2010.06.007
Zhang, Z., Bai, Q., Xu, X., and Zhang, X., 2021. Effects of the dominance hierarchy on social interactions, cortisol level, HPG-axis activities and reproductive success in the golden cuttlefish Sepia esculenta. Aquaculture, 533: 736059. https://doi.org/10.1016/j.aquaculture.2020.736059
Zuberbuehler, C.A., Messikommer, R.E., Arnold, M.M., Forrer, R.S., and Wenk, C., 2006. Effects of selenium depletion and selenium repletion by choice feeding on selenium status of young and old laying hens. Physiol. Behav., 87: 430-440. https://doi.org/10.1016/j.physbeh.2005.11.007
To share on other social networks, click on any share button. What are these?









