The Effects of Storage and Maturity Index on the Quality of Virgin Olive Oil from “Pendolino” Cultivar Produced in Nowshera, Pakistan
Research Article
The Effects of Storage and Maturity Index on the Quality of Virgin Olive Oil from “Pendolino” Cultivar Produced in Nowshera, Pakistan
Ata Ullah1,2, Tariq Masood1*, Zafar Ali Shah1, Azmat Ali Awan3, Sahib Alam1, Nasiruddin4, Muhammad Arif5 and Muhammad Arabi Awan6
1Department of Agricultural Chemistry and Biochemistry, The University of Agriculture Peshawar, Peshawar, Khyber Pakhtunkhwa, Pakistan; 2Agricultural Research Institute, Mingora, Swat, Khyber Pakhtunkhwa, Pakistan; 3Senior Scientific Officer, Pakistan Oilseed Department, Pakistan; 4Agricultural Research Institute, Tarnab, Peshawar, Khyber Pakhtunkhwa, Pakistan; 5Department of Human Nutrition, The University of Agriculture Peshawar, Peshawar, Khyber Pakhtunkhwa, Pakistan; 6Department of Entomology, The University of Agriculture Peshawar, Pakistan.
Abstract | Olives of the Pendolino cultivar were harvested at three different levels of maturity index (0, 3, and 6) from an olive orchard located in the district Nowshera, Pakistan. The harvested olives were crushed using a stone mill and the oil was extracted through a traditional mechanical pressing system. The influence of fruit ripening degree and storage time on peroxide value, iodine value, free fatty acids content, chlorophyll contents, carotenoids and fatty acid profile were examined. Key quality parameters such as peroxide value, iodine value, chlorophyll contents, carotenoid contents, and oleic acid percentage decreased significant during ripening. Conversely, linolenic acid and free fatty acid content exhibited an upward trend as ripening progressed. Total phenolic content showed a negative correlation with the maturity index. Significant changes in fatty acid composition were observed during ripening. Free fatty acid content and peroxide value increased significantly during storage, while iodine value, chlorophyll content, carotenoid, and total phenolic substances showed significant reduction. Nonetheless, throughout the entire ripening and storage period examined, all quality and purity parameters evaluated remained within the acceptable limits established by the International Olive Council (IOC) in 2010 for the best category of virgin olive oil.
Received | June 21, 2024; Accepted | September 16, 2024; Published | January 12, 2025
*Correspondence | Tariq Masood, Department of Agricultural Chemistry and Biochemistry, The University of Agriculture Peshawar, Peshawar, Khyber Pakhtunkhwa, Pakistan; Email: tariqafridi@aup.edu.pk
Citation | Ullah, A., T. Masood, Z.A. Shah, A.A. Awan, S. Alam, Nasiruddin, M. Arif and M.A. Awan. 2025. The effects of storage and maturity index on the quality of virgin olive oil from “Pendolino” cultivar produced in Nowshera, Pakistan. Sarhad Journal of Agriculture, 41(1): 88-99.
DOI | https://dx.doi.org/10.17582/journal.sja/2025/41.1.88.99
Keywords | Fatty acid profile, Maturity index, Pendolino cultivar, Storage time, quality, Virgin olive oil
Copyright: 2025 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Virgin olive oils (VOO) are defined by the COI/T.15/NC No 3 as Olive oil that is derived from the fruit of the olive tree (Olea europaea L.) through mechanical or physical methods without any chemical alterations. It is only subjected to processes such as washing, decantation, centrifugation, and filtration. From ancient times olive oil has been used in the Mediterranean areas as an important part of their diet and for its healthy effects. It is now used throughout the world for its nourishing, sensory and healthy effects (Cinquanta et al., 1997). Its dietary importance has been associated to its content of monounsaturated and polyunsaturated fatty acids, especially oleic acid (55–83%), the favorable ratio of unsaturated/saturated fatty acids and overall, for its antioxidant compounds content. These latter are important compounds able to act as free radical scavengers and are useful in the prevention of many cardiovascular diseases (Mraicha et al., 2010). The presence of minor components, such as phytosterols, carotenoids, tocopherols and pentacyclic triterpenes are also important in olive oil (Franco et al., 2014; Longobardi et al., 2012). VOO quality is the result of the application of good practices in all steps of production chain from the field to the shelf (Koski et al., 2002). Virgin olive oil quality depends on various factors, such as the variety, ecological conditions, cultural practices, harvest techniques, storage conditions and processing methods (Pristouri et al., 2010; Kandylis et al., 2011; Flamini et al., 2003; Vichi et al., 2003).
The total domestic consumption of edible oil in Pakistan is around 1.9 million tones, out of which 1.3 million tones is being imported from abroad. As many as Rs. 28 billion are being spent on the import of edible oil every year. Thus, compared to 30% indigenous output, 70% of oil is imported. To save valuable foreign currency, the government is particularly focusing on expanding the country’s canola, olive, and palm oil crops. Olive oil is produced from autumn to winter (it depends on the geographical area of production) and its shelf life is affected by many factors like storage conditions and packing (Mendez and Falque, 2007; Blekas et al., 2002). The oxidation process is the most important process that leads to the deterioration of olive oil organoleptic and nutritional characteristics. This phenomenon is affected by many factors i.e., O2 exposure, light and heat exposure of olive oil during storage etc. The result of the oxidation process is the production, in the oil, of a complex mixture of organic compounds like ketone, aldehydes, organic alcohol and esterified compounds, which are responsible for the quality loss and the appearance of the off-flavor of rancid (Tomaino et al., 2005). Many theories are formulated to explain the oxidation phenomenon in oils: Photo-oxidation, metal-induced oxidation and enzyme catalyzed oxidation (Choe and Min, 2005, 2006). The VOO stability, and then its shelf-life, are also greatly affected by its anti-oxidants content (polyphenols and tocopherols) and fatty acids composition (Khan and Shahidi, 1999).
Cultivating olive trees in arid and semi-arid regions of Pakistan, such as Chakwal, Fateh Jang, Zhob, Loralai, Killa Saifullah, North Waziristan, Bajaur Agency, Mohmand Agency, Khurram Agency, Hangu, Khyber Agency, South Waziristan, Malakand, Charsadda, and Haripur, would not only expand agricultural land but also enhance the production of edible oil in the country (Mohsin and Ashraf, 2015). Moreover, this would result in the creation of fresh olive nurseries, production of medicinal products derived from olives, cultivation of olive orchards, establishment of oil extraction mills, development of pickle industries, employment opportunities for daily wage workers and technical personnel, as well as fruit picking and marketing prospects throughout Pakistan. These initiatives would provide a means of generating income in the underprivileged regions. Olive planting has the potential to be a lucrative venture for exporting olive oil and generating foreign exchange revenue.
Olive and olive oil production with such an incredible future in Pakistan, the present manuscript was designed to study the quality characteristics of olive oil obtained from the cultivar Pendolino, of Italian origin, grown in the region of Nowshera in Pakistan. The variability of fatty acid composition and quality were monitored during the full ripening period and after the production, for three months of storage.
Materials and Methods
Sampling
Olive oils were extracted from olive fruits of the Pendolino variety harvested at three different maturity stages (using the maturity index table) from an olive orchard located at district Nowshera during the year 2017. The olive plants were 12 years old with a proper irrigation system. The average minimum to maximum annual temperature of the area ranges from 10.4oC to 37.4oC with an average annual rainfall of 782 mm (Pakistan Meteorological Department, 2017).
Maturity index
The maturity index of olives was determined from 100 randomly selected olive fruits according to the method used by Uceda and Frias (1975). This approach relied on assessing the hues of both the skin and flesh of olives. The maturity index values varied between class 0, which represents skin that is 100% intensely green, and class 7, which represents flesh and skin that are 100% black. The olives were halved to expose their inner meat and facilitate the process of grading.
0 = skin is a deep or dark green colour.
1 = skin is a yellow or yellowish-green colour.
2 = skin is a yellowish color with reddish spots.
3 = skin is a reddish or light violet colour.
4 = skin is black and the flesh is completely green.
5 = skin is black and the flesh is a violet colour halfway through.
6 = skin is black and the flesh is a violet colour almost through to the stone.
7 = skin is black and the flesh is completely dark.
The total number of olives in each category were counted and recorded. The following equation was applied to compute the maturity index:
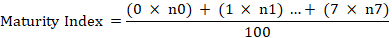
Where n is the number of fruits in each category.
Oil extraction
Only healthy olive fruits were selected and transferred to Pakistan Council of Scientific and Industrials Research (PSCIR) Lab Peshawar, Pakistan for the extraction of oil and further chemical analysis. Leaves were removed and olive fruits were crushed by a mechanical presser. The resultant paste was kneaded for 45 minutes and centrifuged in a three-phase vertical decanter by automated discharge centrifuge. The oily paste was separated into water and oil. The collected samples of virgin olive oil along with control were stored in amber bottles at controlled room temperature (20-25°C) and in dark for consecutively three months. All the samples were analyzed at one-month intervals up to three months for different quality parameters in triplicate.
Chemical analysis
Free fatty acid value and peroxide value: Free fatty acids content expressed as oleic acid percentage and peroxide value in meq of active O2 kg-1 of oil were performed according to the ISO methods: ISO 660: 2009(E), 2009, and ISO3960: 2007(E), 2007, respectively.
Total phenolic content (TPC)
Spectrophotometric method was used for the determination of the total phenol contents as described by (Wrolstad et al., 2004). Briefly oil sample (02 grams) was mixed with 10 ml methanol/water solution (80:20 V/V). One to two drops of tween-20 were added to the solution and mixed. Solution was centrifuged at 5000 rpm for ten minutes and the process was repeated three times. Two immiscible phases were formed. The supernatant part (methanolic phase) of the sample was collected in another tube with pipette and concentrated under vacuum at 30 oC. At the end of the extraction, the collected volume was recorded as the total volume of the olive oil sample. One ml of the aqueous methanolic solution (80:20 V/V) and 1 ml supernatant extracted part of the sample were mixed and was diluted by adding 5 ml deionized water. Two ml of sodium carbonate (15%) and 0.5 ml folin ciocalteu reagent was added respectively to the solution. The solution was diluted by adding 1.5 ml distilled water. The mixture was homogenized for 30 sec by vortex and kept in dark for two hours. The same procedure was repeated for blank. The absorbance reading was measure on spectrophotometer (Shimadzu Uv-2450 UV-visible, Japan) at 765 nm. Gallic acid calibration curve (Y= 10.231X; R2= 0.9957) was used for the determination of the total phenolic content in mg Gallic acid equivalent Kg of olive oil.
β- carotene value
β-Carotene is a precursor for vitamin A. The carotene content of Olive Oil was evaluated by the method given by (PORIM Test Method, 1993).
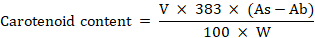
Where; V = volume used for analysis; 383 = Coefficient for carotenoids; As = Absorbance value for the sample; Ab = Cuvette error; W = Weight of sample (g).
Iodine value
The unsaturation of fat and oil is directly related to the iodine value. The Wijs method was used for the determination of the iodine value. Briefly oil sample (0.1 ml) was taken into 500 ml flask and 20 ml of cyclohexane and acetic acid with equal volume (1:1) was added. Another flask with solvent and reagent except sample was used as blank. Wijs reagent (25ml) was added to the sample and left for an hour in the dark. Then potassium iodide solution (20ml) and 150 ml distilled water were added into the sample. The yellow color of the solution due to iodine almost disappeared after titration with sodium thiosulphate solution (O.1 M). Titration was completed with addition of few drops of the freshly prepared starch indicator.
Following formula was used to calculate the iodine value in olive oil samples:
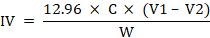
Where; C = Extract concentration (mol L-1); V1 = Volume (ml) of the sodium thiosulphate solution used for the blank; V2 = Volume (ml) of the sodium thiosulphate solution used for the oil sample; W = weight of sample (g).
Chlorophyll content
Chlorophyll content was determined in oil samples by using spectrophotometric method used by Minquez-Mosquera et al. (1991). Oil sample (7.5g) was dissolved in cyclohexane and volume was made to 25 ml. The absorption of the sample was recorded at 630 nm, 670 nm and 710 nm. The chlorophyll content was measured as mg Kg-1 of oil.
Chlorophyll pigments were expressed as:
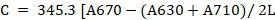
Where; C = Concentration of Chlorophyll pigment; L = sample holding cell thickness (10 mm); A = Absorbance in nm.
Fatty acids composition
Fatty acid composition of different oil samples was determined by cold trans esterification with methanolic KOH, and using GC-MS QP 2010 Plus (Shimadzu Tokyo, Japan). A capillary column TRB-FFAP of 30 m length, 0.35 mm internal diameter and 0.25 µm thickness (Technorama) was employed for separation of analytes and helium was used as the carrier gas at a constant flow of 1mL/min. The separation was obtained by thermal gradient: start temperature 14°C, then increased to 240°C at 5°C/min, hold time at final temperature 10 min. Injector temperature 230°C, operating in split mode, split ratio 1:75. The source temperature and the transfer line were set at 250°C. The Ionization energy was 70eV. MS scanning was in the range of 85 to 380 m/z. The system was controlled and the data which were acquired using the GC-MS solutions software provided by the supplier. Compounds were identified by comparing the mass spectra obtained with those of standard from the NIST library (NIST 05).
Statistical analysis
Two ways ANOVA with 0.05 level of significance was used, followed by least significant difference for multiple comparison were performed by Statistic 8.1 (Steel and Torrie, 1980).
Results and Discussion
Peroxide value
Statistical analysis of peroxide values (PV) showed significant differences among samples harvested at different maturity indices (Table 1). This parameter showed a decreasing trend during ripening process from 8.16 meq O2 kg-1 at zero maturity index to 6.01 meq O2 kg-1 at six maturity index of olive fruit. These results agree with those of Matos et al. (2007) and Salvador et al. (2001). Youssef et al. (2010) attributed these low peroxide values to the decrease of lipoxygenase activities in the olive fruits at full maturity stage. A significant progressive increase of peroxide values was observed during storage with a percentage of increase ranging from 60 to 100% with respect to the initial value. All the olive oil samples have peroxide values much below 20 meq O2 kg-1, the maximum permissible limit for their classification as extra virgin olive oil set by the International Olive Council.
Table 1: Effect of storage time intervals and maturity indices on the peroxide value (meq O2 kg−1) of virgin olive oil.
|
Storage time (Months) |
Maturity index 0 |
Maturity index 3 |
Maturity index 6 |
Mean |
|
0 |
6.4± 0.16 g |
5.13 ± 0.16i |
3.73±0.24j |
5.08 d |
|
1 |
7.6± 0.32 e |
6.34 ±0.32g |
5.56±0.29h |
6.51 c |
|
2 |
8.44± 0.33 |
7.74± 0.32e |
6.83±0.04f |
7.67 b |
|
3 |
10.15±0.24a |
8.75±0.29b |
7.93±0.40d |
8.94 a |
|
Mean |
8.16 a |
6.99 b |
6.01 c |
Values are means of three determinations ± Standard deviation. Mean sharing common letters in the same column and row are non-significantly (p ≥ 0.05) from each other.
Free fatty acid value
Free fatty acid (FFA) values are reported in Table 2. This parameter significantly increased during ripening reaching the highest value at the maturity index six. This behavior could be explained considering the progressive increase of enzymatic activities of lipolytic enzymes (Salvador et al., 2001; Yildirim, 2009).
Table 2: Effect of storage time intervals and maturity indices on the free fatty acid value (%) of virgin olive oil.
|
Storage time (Months) |
Maturity index 0 |
Maturity index 3 |
Maturity index 6 |
Mean |
|
0 |
0.52 ± 0.13 f |
0.85 ± 0.27d |
0.94±0.22 a |
0.77 c |
|
1 |
0.75± 0.33 e |
0.87± 0.37cd |
0.89±0.36ad |
0.83 b |
|
2 |
0.85± 0.32 d |
0.89± 0.32ad |
0.93±0.20ab |
0.89 a |
|
3 |
0.88±0.28bd |
0.91±0.24ac |
0.94±0.24a |
0.91 a |
|
Mean |
0.75 c |
0.88 b |
0.93 a |
Values are means of three determinations ± Standard deviation. Mean sharing common letters in the same column and row are non-significantly (p ≥ 0.05) from each other.
Storage significantly affected the free fatty acid content too, leading to a slight increase during storage of samples obtained at zero level of maturity index. In olive oil the free fatty acids content increase is the effect of lipolysis, which starts in the fruit when it still is on the tree and continues, after harvesting, during processing and after the extraction especially if traces of water are still present in the oil (Pereira et al., 2002). It seems that free fatty acid could act as a catalyst in the hydrolytic reaction involving triglycerides (Ayton et al., 2012). The minimum value (0.52%) of FFA was observed at zero maturity index and zero-day storage, while maximum value (0.94%) was noticed at six maturity index after three months storage time. The interaction analysis of maturity versus storage time showed significant result for this parameter. In fact, the increase of FFA showed a different behavior in respect to the maturity index. Samples obtained at higher maturity index showed low level or no significant increase of FFA. It could be possible that the oil samples obtained at high ripening stages could contain a low level of dispersed water, in this way the hydrolytic reactions were inhibited. FFA increase in virgin olive oil during storage was also observed in the study carried out by Gutierrez et al. (2002).
Chlorophyll
Results regarding chlorophyll content of VOO are presented in Table 3. The greenish color of certain olive oil is due to chlorophyll pigments which also plays a role in the stability of olive oil. The highest mean value for chlorophyll contents (7.80 mg kg-1) was observed at zero maturity indexes while the lowest mean value (2.96 mg kg-1) at maturity index of six. Chlorophyll content has a decreasing trend during storage, reporting the highest value (6.91 mg kg-1) at the beginning of storage and reaching the lowest value (3.77 mg kg-1) after three months storage in the dark at room temperature. When olive oil is exposed to light and oxygen, chlorophyll acts as sensitizer and produces highly reactive oxygen. Reactive oxygen species are produced while chlorophyll molecules are progressively decomposed (Caponio et al., 2005). Interaction analyses for chlorophyll content were also significant at the three maturity indices over three months storage time. Present results were in close agreement with (Rigane et al., 2013) who recorded up to 70% reduction in chlorophyll contents of olive oil during six months of storage.
Table 3: Effect of storage time intervals and maturity indices on the chlorophyll contents (mg kg−1) of virgin olive oil.
|
Storage time (Months) |
Maturity index 0 |
Maturity index 3 |
Maturity index 6 |
Mean |
|
0 |
10.35±0.04 a |
6.91±0.049c |
3.46±0.05 f |
6.91a |
|
1 |
8.57b±0.043b |
5.39±0.035d |
3.07±0.043g |
5.67b |
|
2 |
6.91±0.057c |
4.26±0.045e |
2.86±0.040h |
4.68c |
|
3 |
5.38±0.029d |
3.48±0.024f |
2.45±0.01 i |
3.77d |
|
Mean |
7.80 a |
5.01 b |
2.96 c |
Values are means of three determinations ± Standard deviation. Mean sharing common letters in the same column and row are non-significantly (p ≥ 0.05) from each other.
Carotene pigments
Results of carotene content are reported in Table 4. A decreasing trend was observed for carotenoids content both during storage and ripening period. During storage, carotenoids act as antiradical against reactive oxygen species, breaking down and leading to a progressive reduction of their content in the oil (Capino et al., 2005). During ripening, carotenoids content decreases due to the reduced photosynthetic activity of chloroplasts (Roca and Mínguez-Mosquera, 2000). Interaction analyses of ripening stage versus storage time for carotene content were also significant. In fact, the progressive reduction of carotene content of olive oil during storage seemed to be faster in the oil obtained at the beginning of ripening. Rigane et al. (2013) reported up to 70% reduction in carotene contents of olive oil during six months of storage duration which are almost in line with the present results.
Iodine value (IV)
Results of iodine value of VOO during storage and ripening had been given in Table 5. There was a decreasing trend in mean iodine value with the increase of storage time and ripening stage. The interaction analysis of maturity versus storage time showed significant results for iodine value. The oil extracted at zero level of maturity index had the highest mean iodine value (84.24%) at zero-day storage while the oil from six level of maturity index had the lowest iodine value (60.61%) after three months storage time. Yildirim (2009) reported a reduction in the iodine values of VOO during storage, attributing it to a progressive decrease in unsaturated fatty acids, which are likely involved in the oxidation process.
Table 4: Effect of storage time intervals and maturity indices on the carotene contents (mg kg−1) of virgin olive oil.
|
Storage time (Months) |
Maturity index 0 |
Maturity index 3 |
Maturity index 6 |
Mean |
|
0 |
1.94±0.024a |
1.57±0.033b |
1.49±0.048c |
1.66a |
|
1 |
1.53±0.044bc |
1.04±0.020de |
0.98±0.020e |
1.18b |
|
2 |
1.05±0.032d |
0.69±0.020g |
0.65±0.032g |
0.79c |
|
3 |
0.84±0.021f |
0.54±0.016h |
0.45±0.016i |
0.61d |
|
Mean |
1.34 a |
0.96 b |
0.89 c |
Values are means of three determinations ± Standard deviation. Mean sharing common letters in the same column and row are non-significantly (p ≥ 0.05) from each other.
Table 5: Effect of storage time intervals and maturity indices on Iodine value (%) of virgin olive oil.
|
Storage time (Months) |
Maturity index 0 |
Maturity index 3 |
Maturity index 6 |
Mean |
|
0 |
84.56±0.38a |
77.90±0.15b |
76.89±0.15c |
79.78a |
|
1 |
76.89±0.16c |
70.75±0.27e |
68.74±0.26f |
72.13b |
|
2 |
71.75±0.26d |
68.74±0.26f |
65.61±0.37g |
68.70c |
|
3 |
68.11±0.49f |
64.94±0.12g |
60.61±0.35h |
64.55d |
|
Mean |
75.33 a |
70.58 b |
67.96 c |
Values are means of three determinations ± Standard deviation. Mean sharing common letters in the same column and row are non-significantly (p ≥ 0.05) from each other.
Total phenolic content (TPC)
Results for total phenolic contents in VOO are reported in Table 6. Highly significant results were obtained for TPC in VOO extracted at three maturity indices kept under three months storage period at room temperature in dark conditions. The highest mean value (757.50 mg kg-1) for TPC was observed at zero maturity indexes whereas the lowest mean value (637.50 mg kg-1) was noticed at maturity index of six. A significant gradual decrease was recorded for the mean TPC of VOO during storage. At the start of storage, the highest mean TPC (760.11 mg kg-1) was recorded for VOO which gradually decreased to 632.56 mg kg-1 after three months storage time. Polyphenols are susceptible to temperature and light. The concentration of total phenol contents was reduced during and after storage duration due to the splitting of TTP to other compounds by exposure to heat and light (Lee et al., 2007).
Table 6: Effect of storage time intervals and maturity indices on total phenolic contents (mg kg-1) of virgin olive oil.
|
Storage time (Months) |
Maturity index 0 |
Maturity index 3 |
Maturity index 6 |
Mean |
|
0 |
821.33±1.24a |
751.67±1.23c |
707.33±0.47e |
760.11a |
|
1 |
773.33±1.25b |
702.33±1.24e |
661.67±0.94f |
712.44b |
|
2 |
732.67±1.24d |
652.00±0.81g |
610.00±0.81i |
664.89c |
|
3 |
702.67±1.23e |
624.00±0.82h |
571.00±0.83j |
632.56 |
|
Mean |
757.50 |
682.50 |
637.50 |
Values are means of three determinations ± Standard deviation. Mean sharing common letters in the same column and row are non-significantly (p ≥ 0.05) from each other.
Interaction analyses for TPC were also found significant at the three maturity indices over three months’ storage time. The maximum mean value (821.33 mg kg-1) of TPC for oil was recorded at zero maturity indexes at zero-day storage while minimum value (571 mg kg-1) was noticed at six maturity index after three months storage time. The results of the present research were parallel to the findings of (Cinquanta et al., 1997) who reported that storage for 18 months at room temperature in the dark affected the TPC of olive oil samples. The reduction in TPC was due to the oxidation and hydrolytic activities during storage time and such activities increased during storage.
Fatty acid profile of virgin olive oil
Effects of storage time and maturity indices were recorded for fatty acid composition of Virgin Olive Oil (VOO).
Oleic acid (C18:1)
Oleic acid contents of the VOO were significantly affected by the maturity indices (Table 7). The highest mean value for oleic acid (76.84%) was observed at zero maturity indexes while the lowest mean value (66.96%) was observed for the six-maturity index. A significant progressive increase was recorded for the mean oleic acid content of VOO during storage. At the start of storage, the lowest mean oleic acid content (69.25%) was calculated for VOO which steadily increased to 73.79% after three months of storage. The increase in oleic acid percentage during storage is the consequence of the decrease in linolenic acid and linoleic acid (Yildrim, 2009). Polyunsaturated fatty acids (linolenic acid and linoleic acid) oxidize more quickly than mono-saturated fatty acids (oleic acid) during storage (Morello et al., 2004). Interaction analyses for oleic acids were also found significant at three maturity indices over three months storage time. Maximum mean value (79.61%) of oleic acid for oil was recorded at zero maturity indexes after three months of storage while minimum mean value (64.99%) was noticed at sixth maturity index at zero-day storage time. Similar results for Oleic acid content of VOO under storage were reported by (Morello et al., 2004) who documented an increase from 70.1 to 76.8% in oleic acid content in commercial olive oil of Arbequina cultivars in 12 months of storage duration.
Table 7: Effect of storage time intervals and maturity indices on oleic acid (%) of virgin olive oil.
|
Storage time (Months) |
Maturity index 0 |
Maturity index 3 |
Maturity index 6 |
Mean |
|
0 |
74.71±0.42c |
68.05±0.67fg |
64.99±0.50h |
69.25d |
|
1 |
75.60±0.40c |
69.27±0.062ef |
66.66±0.33g |
70.51c |
|
2 |
77.45±0.46b |
70.62±0.44 e |
67.06±1.19g |
71.71b |
|
3 |
79.61±0.40a |
72.64±1.19d |
69.13±0.82f |
73.79a |
|
Mean |
76.84 a |
71.33 b |
66.96 c |
Values are means of three determinations ± Standard deviation. Mean sharing common letters in the same column and row are non-significantly (p ≥ 0.05) from each other.
Linoleic acid (C18:2)
Statistical analysis of the data obtained for linoleic acid of VOO extracted at three different maturity indices showed significant results (Table 8). Increase in linoleic acid value of oil was witnessed during ripening process. The highest mean value for linoleic acid (15.47%) was observed at sixth maturity index while lowest mean value (14.79%) was noticed for the zero maturity indexes. Similarly, a significant decrease was recorded for the mean linoleic acid values of VOO with the passage of time under storage. At the start of storage, the highest linoleic acid (15.86%) content had been recorded for VOO which was reduced to 12.59% after three months storage in the dark at room temperature. The decrease in linoleic acid (polyunsaturated) percentage in the olive oil during storage is due to the breakdown of double bonds of polyunsaturated fatty acids. Unsaturated fatty acids oxidized quickly to saturated fatty acids over time (Ayton et al., 2012). Interaction analyses for linoleic acids values were also found significant at three maturity indices over three months storage time. The maximum mean value (17.08%) of linoleic acid for oil was recorded at sixth maturity index at zero-day storage while minimum mean value (11.19%) was noticed at zero maturity indexes at the end of three months storage time.
Table 8: Effect of storage time intervals and maturity indices on linoleic acid (%) of virgin olive oil.
|
Storage time (Months) |
Maturity index 0 |
Maturity index 3 |
Maturity index 6 |
Mean |
|
0 |
13.13±0.24b |
17.37±0.22e |
17.08±0.22e |
15.86a |
|
1 |
12.07±0.06a |
16.37±0.41d |
16.37±0.41d |
14.93b |
|
2 |
11.8±0.70a |
15.26±0.12c |
15.15±0.12c |
14.07c |
|
3 |
11.9±0.37a |
13.29±0.36b |
13.29±0.36b |
12.59d |
|
Mean |
14.79 c |
15.57 b |
15.47 a |
Values are means of three determinations ± Standard deviation. Mean sharing common letters in the same column and row are non-significantly (p ≥ 0.05) from each other.
Similar results for Oleic acid content of VOO under storage were reported by (Morello et al., 2004) who documented an increase from 12.5 to 17.54% in linoleic acid content in olive oil of Arbequina cultivars in twelve months of storage duration.
Linolenic acid (C18:3)
Statistically significant results had been recorded for linolenic acid content of VOO extracted at three different maturity indices and kept under dark at room temperature for three months time (Table 9). The highest mean value for linolenic acid (0.44) was observed at sixth maturity index while the lowest mean value (0.36%) was noted for the zero maturity indexes. A significant progressive decrease was recorded for the mean linolenic acid value of VOO with time in storage. At the start of storage, the highest linolenic acid value (0.53%) had been recorded for VOO which was gradually decreased to (0.32%) after three months’ storage in the dark at room temperature. The decrease in linolenic acid (polyunsaturated) percentage in olive oil during storage is due to the breakdown of double bonds of polyunsaturated fatty acids. Unsaturated fatty acids oxidized quickly to saturated fatty acids over time (Ayton et al., 2012). Interaction analysis for linolenic acid was also significantly different at three maturity indices over three months’ storage time. The maximum linolenic acid (0.42%) was witnessed for zero maturity indexes at zero days’ storage while minimum mean value (0.31%) was noticed at zero maturity indexes after three months of storage duration. The results of the present study were strongly supported by (Gomez-Alonso et al., 2007) who also noticed reduction in linoleic and linolenic acid for the Spanish olive oils stored at room temperature for 21 months.
Table 9: Effect of storage time intervals and maturity indices on Linolenic acid (%) of virgin olive oil.
|
Storage time (Months) |
Maturity index 0 |
Maturity index 3 |
Maturity index 6 |
Mean |
|
0 |
0.42±0.008bc |
0.58±0.012a |
0.59±0.012a |
0.53 a |
|
1 |
0.38±0.020cd |
0.45±0.032b |
0.46±0.030b |
0.43 b |
|
2 |
0.32±0.32e |
0.35±0.033de |
0.39±0.028cd |
0.35 c |
|
3 |
0.31±0.31e |
0.34±0.016e |
0.32±0.028e |
0.32 d |
|
Mean |
0.36 b |
0.43 a |
0.44 a |
Values are means of three determinations ± Standard deviation. Mean sharing common letters in the same column and row are non-significantly (p ≥ 0.05) from each other.
Palmitic acid (C16:0)
Palmitic acid value for the olive oil extracted at three maturity indices and kept at three different storage time intervals had been given in Table 10. Olive oil extracted at three different maturity indices showed significant differences in palmitic acid content. The highest mean value for palmitic acid (10.53%) had been recorded for zero maturity indexes while lowest mean value (8.77%) was observed for maturity index six. Storage interval also significantly affected the palmitic acid content of oil extracted at all the three maturity indices. There was a slight increasing trend in mean palmitic acid value with the passage of time in storage. The lowest mean palmitic acid value (8.45%) was found in VOO after one month of storage time which is increasing with passage of storage time and found to be highest (10.87%) after three months storage. Interaction analyses for palmitic acid were also significantly different at the three maturity indices over three months’ storage time. The maximum linolenic acid (12.99%) was witnessed for zero maturity indexes at zero days storage while minimum mean value (7.78%) was noticed after two months of storage for zero maturity index. Present results are strongly supported by the results of (Morello et al., 2004) who reported an increase in palmitic acid contents (12.5 to 17.54%) in Olive oil of Arbequina cultivars in twelve months of storage period.
Table 10: Effect of storage time intervals and maturity indices on palmitic acid (%) of virgin olive oil.
|
Storage time (Months) |
Maturity index 0 |
Maturity index 3 |
Maturity index 6 |
Mean |
|
0 |
12.99±0.12a |
13.03±0.47a |
12.56±0.65a |
12.95c |
|
1 |
8.20±0.69de |
8.71±0.65ce |
9.78±0.0.50b |
8.89bc |
|
2 |
7.78±0.42e |
8.45±0.51de |
9.07±0.24bd |
8.43ab |
|
3 |
13.16±0.50a |
9.95±0.37b |
9.50±0.45bc |
10.8 a |
|
Mean |
10.53a |
10.03b |
10.22b |
Values are means of three determinations ± Standard deviation. Mean sharing common letters in the same column and row are non-significantly (p ≥ 0.05) from each other.
Palmitoleic acid (C16:1)
The results regarding palmitoleic acid are presented in Table 11. Highly significant results were obtained for both maturity indexes and storage duration. The highest mean value for palmitoleic acid (0.33%) was observed for maturity index six while the lowest mean value (0.29%) was noted for the maturity index zero. A significant gradual decrease was recorded in mean palmitoleic acid values of VOO with time under storage. At the start of storage, the highest palmitoleic acid value (0.34%) had been recorded for VOO which was slowly decreased to (0.28%) after three months storage in the dark at room temperature. Interaction analyses for palmitoleic acid were also found significant at three maturity indices over three months storage time. The maximum mean value (0.37%) of palmitoleic acid for oil was recorded for sixth maturity index at zero- day storage while minimum mean value (0.27%) was noticed at zero maturity indexes after three months storage time. The results of the present study were strongly in agreement with the results of (Gomez-Alonso et al., 2007) who found increase in palmitoleic acid for the Spanish olive oils stored at room temperature for 21 months.
Stearic acid (C18:0)
Statistical analysis of the data obtained for stearic acid of VOO extracted at three different maturity indices showed significant results as shown in Table 12. Very slightly Increase in stearic acid value of oil was witnessed during ripening process. The highest mean value for stearic acid (3.17%) was observed at sixth maturity index while lowest mean value (3.08%) was noted for zero maturity indexes. Similarly, a significant gradual decrease was recorded for the mean stearic acid value of VOO with the passage of time under storage. At the start of storage, the highest stearic acid content (3.38%) had been recorded for VOO which was gradually decreased to 2.94% after three months storage time in the dark at room temperature. Interaction analyses for the mean values of stearic acids were also found significant at the three maturity indices over three months storage time. The maximum mean value (3.56%) of stearic acid for VOO was recorded at sixth maturity index at zero-day storage while minimum mean value (2.91%) was noticed for sixth maturity index at the end of three months storage time. The results of the present study were supported by the findings of (Morello et al., 2004) who also reported decrease in stearic acid contents of olive oil of Arbequina cultivar under 12 months storage time.
Table 11: Effect of storage time intervals and maturity indices on Palmitoleic acid (%) of virgin olive oil.
|
Storage time (Months) |
Maturity index 0 |
Maturity index 3 |
Maturity index 6 |
Mean |
|
0 |
0.31±0.04cd |
0.34±0.04b |
0.37±0.08ef |
0.34a |
|
1 |
0.31±0.08b |
0.32±0.09c |
0.34±0.09cd |
0.32b |
|
2 |
0.29±0.08a |
0.30±0.12b |
0.32±0.08c |
0.30c |
|
3 |
0.27±0.12f |
0.28±0.12ef |
0.30±0.08de |
0.28d |
|
Mean |
0.29 c |
0.31 b |
0.33 a |
Values are means of three determinations ± Standard deviation. Mean sharing common letters in the same column and row are non-significantly (p ≥ 0.05) from each other.
Table 12: Effect of storage time intervals and maturity indices on Stearic acid (%) of virgin olive oil.
|
Storage time (Months) |
Maturity index 0 |
Maturity index 3 |
Maturity index 6 |
Mean |
|
0 |
3.23±0.16b |
3.36±0.12b |
3.56±0.12a |
3.38a |
|
1 |
3.11±0.16de |
3.07±0.61ef |
3.19±0.56cd |
3.12b |
|
2 |
3.00±0.46fgh |
3.06±0.52efg |
3.02±0.49fgh |
3.03c |
|
3 |
2.98±0.24ghi |
2.93±0.41 hi |
2.91±0.53i |
2.94d |
|
Mean |
3.08 b |
3.11 b |
3.17 a |
Values are means of three determinations ± Standard deviation. Mean sharing common letters in the same column and row are non-significantly (p ≥ 0.05) from each other.
Arachidic acid (C18:3)
Arachidic acid value for the olive oil extracted at three maturity indices while kept at three different storage time intervals had been given in Table 13. Olive oil extracted at three different maturity levels showed significant differences for the mean arachidic acid value. Highest mean arachidic acid content (0.43%) had been recorded for sixth maturity index while lowest mean value (0.31%) was observed for zero maturity indexes. Storage time interval also significantly affected the palmitic acid value of oil extracted at all the three maturity indices. There was a decreasing trend in mean arachidic acid value with the passage of time in storage. The highest mean arachidic acid value (0.43%) was found in VOO at zero day of storage time which was decreased with the passage of storage time and found to be lowest (0.28%) after three months storage. Interaction analyses for arachidic acid were also significantly different at the three maturity indices over three months storage time. The maximum mean arachidic acid (0.51%) was witnessed for maturity index six at zero days storage while minimum mean value (0.24%) was noticed after three months of storage for zero maturity indexes. The result of the present study was fairly similar to the results of (Gomez-Alonso et al., 2007) who also evaluated reduction in arachidic acid for the Spanish olive oils that were stored at room temperature for 21 months.
Table 13: Effect of storage time intervals and maturity indices on Arachidic acid (%) of virgin olive oil.
|
Storage time (Months) |
Maturity index 0 |
Maturity index 3 |
Maturity index 6 |
Mean |
|
0 |
0.36±0.35d |
0.45±0.16bc |
0.51±0.12a |
0.44a |
|
1 |
0.35±0.32d |
0.43± 0.16c |
0.48±0.12ab |
0.42a |
|
2 |
0.32±0.32de |
0.34±0.35de |
0.42±0.29c |
0.36b |
|
3 |
0.24±0.16f |
0.29±0.16ef |
0.33±0.41de |
0.29c |
|
Mean |
0.32 c |
0.38 b |
0.44 a |
Values are means of three determinations ± Standard deviation. Mean sharing common letters in the same column and row are non-significantly (p ≥ 0.05) from each other.
Conclusions and Recommendations
Gradual reduction was noticed in chlorophyll, carotenes, total phenolic, oleic acid contents and slight variation in fatty acid profile while free fatty acid values were increased with the advancement in fruit maturity. However, throughout the maturity process, the quality parameters either increased or decreased were under the acceptable limits established by IOC (2010) for the best category of EVOO. The highest concentration of color pigments and total phenolic contents were found at zero and third maturity index compared to the six-maturity index. The value of free fatty acid, chlorophyll, carotenoids and total phenolic contents were significantly decreased for three months storage, but the values were found under acceptable limits established by IOC (2010) for the best category of VOO.
Acknowledgements
Authors are thankful to the Higher Education Pakistan for providing funds through “Access to Scientific Instrumentation Program” (ASIP) and PCSIR Peshawar, Pakistan for providing laboratory facilities.
Novelty Statement
The research reveals a complex relationship between storage length, maturity index, and qualitative attributes of virgin olive oil from Pendolino cultivar grown in district Nowshera Pakistani. Significant variations in free fatty acid content and peroxide value have been observed during different maturity indices and storage period. The data suggests that oil quality altered dynamically throughout curing process and storage time.
Author’s Contribution
Ata Ullah: Performed the formal analysis and wrote the original draft.
Tariq Masood and Zafar Ali Shah: Did conceptualization and supervision of the research work.
Azmat Ali Awan: Provided the olive fruit samples.
Sahib Alam and Muhammad Arif: Helped in the review and editing of the manucript.
Nasiruddin and Muhammad Arabi Awan: Applied the statistical analysis on the data obtained.
Conflict of interest
The authors have declared no conflict of interest.
References
Ayton, J., R.J. Mailer and K. Graham. 2012. The effect of storage conditions on extra virgin olive oil quality. Rural Ind. Res. Dev. Corp., 4(2): 231-241.
Blekas, G., E. Psomiadou, M. Tsimidou and D. Boskou. 2002. On the importance of total polar phenols to monitor the stability of Greek virgin olive oil. Eur. J. Lipid Sci. Technol., 104(6): 340-346. https://doi.org/10.1002/1438-9312(200206)104:6<340::AID-EJLT340>3.0.CO;2-L
Caponio, F., M.T. Bilancia, A. Pasqualone, E. Sikorska and T. Gomes. 2005. Influence of the exposure to light on extra virgin olive oil quality during storage. Eur. Food Res. Technol., 221(1-2): 92-98. https://doi.org/10.1007/s00217-004-1126-8
Choe, E., and D.B. Min. 2005. Chemistry and reactions of reactive oxygen species in foods. J. Food Sci., 70(9): R142-159. https://doi.org/10.1111/j.1365-2621.2005.tb08329.x
Choe, E., and D.B. Min. 2006. Mechanisms and factors for edible oil oxidation. Compr. Rev. Food Sci. Food Saf., 5(4): 169-186. https://doi.org/10.1111/j.1541-4337.2006.00009.x
Cinquanta, L., M. Esti and E.L. Notte. 1997. Evolution of phenolic compounds in virgin olive oil during storage. J. Am. Oil Chem. Soc., 74(10): 1259-1264. https://doi.org/10.1007/s11746-997-0054-8
Flamini, G., P.L. Cioni and I. Morelli. 2003. Volatiles from leaves, fruits, and virgin oil from Olea europaea Cv. Olivastra Seggianese from Italy. J. Agric. Food Chem., 51(5): 1382-1386. https://doi.org/10.1021/jf020854y
Franco, M.N., T. Galeano-Diaz, O. Lopez, J.G. Fernandez-Bolanos, J. Sánchez, C. De Miguel and D. Martin-Vertedor. 2014. Phenolic compounds and antioxidant capacity of virgin olive oil. Food Chem., 163: 289-298. https://doi.org/10.1016/j.foodchem.2014.04.091
Gomez-Alonso, S., V. Mancebo-Campos, M.D. Salvador and G. Fregapane. 2007. Evolution of major and minor components and oxidation indices of virgin olive oil during 21 months storage at room temperature. Food Chem., 100(1): 36-42. https://doi.org/10.1016/j.foodchem.2005.09.006
Gutierrez, F., and J.L. Fernandez. 2002. Determinant parameters and components in the storage of virgin olive oil. Prediction of storage time beyond which the oil is no longer of “extra” quality. J. Agric. Food Chem., 50(3): 571-577. https://doi.org/10.1021/jf0102158
ISO 3960:2007(E), 2007. Animal and vegetable fats and oils-determination of peroxide value-iodometric (Visual) endpoint determination, BSI Standards, London, UK.
ISO 660:2009 (E), 2009. Animal and Vegetable Fats and Oils-Determination of Acid Value and Acidity, Polish Committee for Standardization, Warsaw, Poland.
Kandylis, P., A.S. Vekiari, M. Kanellaki, N.G. Kamoun, M. Msallem and Y. Kourkoutas. 2011. Comparative study of extra virgin olive oil flavor profile of Koroneiki variety (Olea europaea var. Microcarpa alba) cultivated in Greece and Tunisia during one period of harvesting. LWT Food Sci. Technol. 44(5): 1333-1341. https://doi.org/10.1016/j.lwt.2010.12.021
Khan, M.A. and F. Shahidi. 1999. Rapid oxidation of commercial extra virgin olive oil stored under fluorescent light. J. Food Lipids, 6(4): 331-339. https://doi.org/10.1111/j.1745-4522.1999.tb00154.x
Koski, A., E. Psomiadou, M. Tsimidou, A. Hopia, P. Kefalas, K. Wähälä and M. Heinonen. 2002. Oxidative stability and minor constituents of virgin olive oil and cold-pressed rapeseed oil. Eur. J. Lipid Sci. Technol., 214(4): 294-298. https://doi.org/10.1007/s00217-001-0479-5
Lee, H., B.G. Stultz and D.A. Hursh. 2007. The Zic family member, odd-paired, regulates the Drosophila BMP, decapentaplegic, during adult head development. Development, 134(7): 1301-1310. https://doi.org/10.1242/dev.02807
Longobardi, F., A. Ventrella, G. Casiello, D. Sacco, M. Tasioula-Margari, A.K. Kiritsakis and M.G. Kontominas. 2012. Characterization of the geographical origin of Western Greek virgin olive oils based on instrumental and multivariate statistical analysis. Food Chem., 133(1): 169-175. https://doi.org/10.1016/j.foodchem.2011.09.130
Matos, L., J. Pereira, P. Andrade, R. Seabra and M. Oliveira. 2007. Evaluation of a numerical method to predict the polyphenols content in monovarietal olive oils. Food Chem., 102(3): 976-983. https://doi.org/10.1016/j.foodchem.2006.04.026
Mendez, A.I. and E. Falque. 2007. Effect of storage time and container type on the quality of extra-virgin olive oil. Food Contr., 18(5): 521-529. https://doi.org/10.1016/j.foodcont.2005.12.012
Mínguez-Mosquera, M.I., L. Rejano-Navarro, B. Gandul-Rojas, A.H. Sánchez-Gómez and J. Garrido-Fernández. 1991. Color–pigment correlation in virgin olive oil. J. Am. Oil Chem. Soc., 68: 332–336. https://doi.org/10.1007/BF02657688
Mohsin, A. and M.F. Ashraf. 2015. Olive cultivation potential in Pakistan. Technology Times. 6(26). Available from: http://technologytimes.pk/post.php?title=Olive+cultivation+potential+i.
Morelló, J.R., M.J. Motilva, M.J. Tovar and M.P. Romero. 2004. Changes in commercial virgin olive oil (cv. Arbequina) during storage, with special emphasis on the phenolic fraction. Food Chem., 85(3): 357-364. https://doi.org/10.1016/j.foodchem.2003.07.012
Mraicha, F., M. Ksantini, O. Zouch, M. Ayadi, S. Sayadi and M. Bouaziz. 2010. Effect of olive fruit fly infestation on the quality of olive oil from Chemlali cultivar during ripening. Food Chem. Toxicol., 48(11): 3235-3241. https://doi.org/10.1016/j.fct.2010.08.031
Pakistan Meteorological Department (PMD) - Climate Data, 2017.
Pereira, J.A., S. Casal, A. Bento and M.B.P.P. Oliveira. 2002. Influence of olive storage period on oil quality of three Portuguese cultivars of Olea europea, Cobrançosa, Madural, and Verdeal Transmontana. J. Agric. Food Chem., 50(22): 6335-6340. https://doi.org/10.1021/jf011661y
PORIM Test methods, 1993. Methods of test for palm oil and palm oil products. Section # 2, PORIM’s Publication: Kulampur, Malaysia.
Pristouri, G., A. Badeka and M.G. Kontominas. 2010. Effect of packaging material headspace, oxygen and light transmission, temperature and storage time on quality characteristics of extra virgin olive oil. Food Contr., 21(4): 412-418. https://doi.org/10.1016/j.foodcont.2009.06.019
Rigane, G., M. Boukhris, M. Bouaaziz, S. Sayadi and R.B. Salem. 2013. Analytical evaluation of two monovarietal virgin olive oils cultivated in the south of Tunisia: Jemri‐Bouchouka and Chemlali‐Tataouin cultivars. J. Sci. Food Agric., 93(5): 1242-1248. https://doi.org/10.1002/jsfa.6101
Roca, M. and M.I. Mínguez-Mosquera. 2000. Changes in chloroplast pigments of olive varieties during fruit ripening. J. Agric. Food Chem., 49(2): 832–839. https://doi.org/10.1021/jf001000l
Salvador, M.D., F. Aranda and G. Fregapane. 2001. Influence of fruit ripening on ‘Cornicabra’virgin olive oil quality a study of four successive crop seasons. Food Chem., 73(1): 45-53. https://doi.org/10.1016/S0308-8146(00)00276-4
Steel, R.G. and J.H. Torrie. 1980. Principles and procedures of statistics McGraw-Hill Book Co. Inc., New York. pp. 481.
Tomaino, A., F. Cimino, V. Zimbalatti, V. Venuti, V. Sulfaro, A. De Pasquale and A. Saija. 2005. Influence of heating on antioxidant activity and the chemical composition of some spice essential oils. Food Chem., 89(4): 549-554. https://doi.org/10.1016/j.foodchem.2004.03.011
Uceda, M. and L. Frias. 1975. Harvest dates. Evolution of the fruit oil content, oil composition and oil quality, in Proceedings of the II Seminario Ole´ıcola Internacional, International Olive Oil Council, Cordoba, Spain. pp. 125–130.
Vichi, S., A.I. Castellote, L. Pizzale, L.S. Conte, S. Buxaderas and E. Lopez-Tamames. 2003. Analysis of virgin olive oil volatile compounds by headspace solid-phase microextraction coupled to gas chromatography with mass spectrometric and flame ionization detection. J. Chromatogr. A. 983(1-2): 19-33. https://doi.org/10.1016/S0021-9673(02)01691-6
Wrolstad, R.E., T.E. Acree, E.A. Decker, M.H. Penner, D.S. Reid, S.J. Schwartz and P. Sporns. 2004. Pigments, colorants, flavors, texture and bioactive food components. Handbook of Food Analytical Chemistry. pp. 34-35. https://doi.org/10.1002/0471709085
Yıldırım, G., 2009. Effect of storage time on olive oil quality. Master’s thesis, İzmir Institute of Technology.
Youssef, N.B., W. Zarrouk, A. Carrasco‐Pancorbo, Y. Ouni, A. Segura‐Carretero, A. Fernandez‐Gutierrez and M. Zarrouk. 2010. Effect of olive ripeness on chemical properties and phenolic composition of chetoui virgin olive oil. J. Sci. Food Agric., 90(2): 199-204. https://doi.org/10.1002/jsfa.3784
To share on other social networks, click on any share button. What are these?








