Temporal Geospatial Mapping of Foot and Mouth Disease Outbreak in Aceh, Indonesia
Research Article
Temporal Geospatial Mapping of Foot and Mouth Disease Outbreak in Aceh, Indonesia
M. Daud AK1,2*, Mahdi Abrar2*, Teuku Reza Ferasyi3,4, Harapan Harapan5,6,7,8
1Graduate School of Mathematics and Applied Sciences, Universitas Syiah Kuala, Banda Aceh 23311, Indonesia; 2Department of Microbiology, Faculty of Veterinary Medicine, Universitas Syiah Kuala, Banda Aceh 23311, Indonesia; 3Department of Veterinary Public Health, Faculty of Veterinary Medicine, Universitas Syiah Kuala, Banda Aceh 23311, Indonesia; 4Center for Tropical Veterinary Studies-One Health Collaboration Center, Universitas Syiah Kuala, Banda Aceh 23311, Indonesia; 5Department of Microbiology, School of Medicine, Universitas Syiah Kuala, Banda Aceh, 23111, Indonesia; 6Tropical Disease Centre, School of Medicine, Universitas Syiah Kuala, Banda Aceh, 23111, Indonesia; 7Medical Research Unit, School of Medicine, Universitas Syiah Kuala, Banda Aceh, 23111, Indonesia; 8Tsunami and Disaster Mitigation Research Center (TDMRC), Universitas Syiah Kuala, Banda Aceh, 23111, Indonesia.
Abstract | Aceh has become an endemic for foot and mouth disease (FMD). This study used a descriptive epidemiological approach and geospatial mapping to track, analyze, and map the distribution patterns and trends of FMD outbreaks in Aceh, Indonesia using data from 23 districts between March 2022 to August 2023. The FMD case were collected from the Crisis Centre of Foot and Mouth Disease, Aceh Department of Agriculture, and Ministry of Agriculture, Indonesia. The data were analyzed using ArcGIS software to determine the spatial and clustering pattern of FMD. The hotspot and autocorrelation analysis with Moran’s I statistic were conducted to validate the spatial patterns. In 2022, the month with the greatest number of instances was July, followed by June, May, August, September, October, and December. There have been no recorded cases of FMD from January to August 2023. The Aceh Besar district was notably recognized as a prominent area with a high incidence of FMD outbreak followed by Aceh Tamiang and Aceh Utara. The data suggested that the occurrences of FMD were sporadic and randomly dispersed, as indicated by a Z-Score of -0.927 according to Moran’s I analysis. This study highlights that geospatial analysis could be employed to make informed decisions on different strategies, such as district-based isolation and implementation of general control measures in highly susceptible communities, to effectively manage the spread of FMD during outbreaks. Additionally, selecting the appropriate timing and approach for conducting control efforts and preventative measures is beneficial in addressing future outbreaks of FMD and other recurring diseases.
Keywords | Foot and mouth disease, Geographic information system, Cloven-hoofed animals, Mapping, Disease outbreak
Received | January 12, 2024; Accepted | March 14, 2024; Published | May 07, 2024
*Correspondence | M. Daud AK and Mahdi Abrar, Department of Microbiology, Faculty of Veterinary Medicine, Universitas Syiah Kuala, Banda Aceh 23311, Indonesia; Email: [email protected], [email protected]
Citation | Daud MAK, Abrar M, Ferasyi TR, Harapan H (2024). Temporal geospatial mapping of foot and mouth disease outbreak in Aceh, Indonesia. Adv. Anim. Vet. Sci., 12(6):1148-1156.
DOI | https://dx.doi.org/10.17582/journal.aavs/2024/12.6.1148.1156
ISSN (Online) | 2307-8316
Copyright: 2024 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
INTRODUCTION
The Food and Agriculture Organization (FAO) estimated that a substantial portion of the global agricultural output and the livelihoods of over a billion people depend on livestock (FAO, 2009). However, this industry has faced persistent challenges and significant threats from infectious disease outbreaks like FMD. Caused by the FMD virus (FMDV), this highly contagious disease affects cloven-hoofed animals, leading to severe economic losses due to diminished productivity and trade restrictions (Chen et al., 2022). These losses can have cascading effects on the livelihoods of farmers and the availability of affordable and nutritious food for the local population. In endemic areas, FMD causes global losses ranging from 6.5 to 21 billion USD annually, encompassing visible production losses, vaccination expenses, and treatment costs. Additionally, non-endemic regions experience an annual loss of over 1.5 billion USD due to FMD outbreaks (Knight-Jones and Rushton, 2013). A study have shown that outbreaks can disrupt livestock markets significantly, affecting production and access to international markets (Feng et al., 2017). Furthermore, FMD outbreaks can lead to the shutdown of exports from the livestock industry for an indefinite period, as access to foreign markets depends heavily on the disease status of domestic livestock populations (Pudenz et al., 2021).
Indonesia, an archipelagic nation in Southeast Asia, is home to a diverse and substantial livestock industry, making it a vital contributor to the nation’s economy and food security. FMD outbreaks have been reported previously in Indonesia resulting significant losses for livestock population and leading to challenges posed by the movement of animals across the country’s vast and varied landscape (Susila et al., 2023). Between 2022–2023, the FMD outbreak occurred in Indonesia and this outbreak drew significant attention from the agricultural and veterinary public health sectors. The first cases of FMD during 2022–2023 outbreak were reported in four districts in East Java in May 2022 and two districts in Aceh province on August 2022 before they were spread other provinces in Indonesia (DREF, 2023). By July 2022, there were 233,370 FMD active cases in 246 sub-districts across 22 provinces in Indonesia (Sutawi et al., 2023). The outbreak has affected many livestock, including cattle, buffaloes, sheep, and goats. The Indonesian government then declared the status of specific emergencies for FMD and was aiming to get the outbreak under control by the end of 2022. The investigation indicated that the current outbreak of FMD in Indonesia is due to the FMDV serotype O (O/ME-SA/Ind-2001e sub lineage) (Chen et al., 2022).
The 2022–2023 FMD outbreak presented a unique transmission since the first cases were also in Aceh, a province located in the westernmost of Indonesian archipelago located on the tip of Sumatra Island. Aceh is a unique and possesses distinct geographical and socio-economic characteristics and features. Aceh is known for its coastal areas and substantial livestock population of which livestock farming plays a crucial role in the livelihoods of Aceh population (Handayani and Safrida, 2023). It made Aceh particularly susceptible to the impacts of an FMD outbreak, warranting a detailed examination of its effects on the province’s economy and food security. The livestock sector in Aceh contributes significantly to the provincial economy and the well-being of its people (Sutawi et al., 2023). Therefore, understanding the incidence and outcomes of the outbreak of FMD in Aceh during FMD outbreak 2022-2023 is crucial for quantifying its economic impact on the region and developing strategies to mitigate these losses.
The aim of this study was to investigate the spatial and temporal dynamics of the FMD outbreak in Aceh in order to identify patterns that could inform more effective containment and mitigation strategies. The study hypotheses were that geospatial mapping can reveal the details of the outbreak’s spread and uncover the epidemic pattern, which aids in developing targeted interventions on transmission and control strategies. To achieve that aim, the geospatial mapping of the FMD outbreak in Aceh was created to provide valuable insights and tools for controlling the disease, reducing its impact on livestock and public health, and preventing its spread. It involved using geographic information systems (GIS) and spatial analysis techniques to track, analyze, and map the spread and distribution of the FMD cases to be able to understand better the spatial patterns and factors associated with the outbreak. The findings and recommendations from this study are anticipated to provide valuable guidance for policymakers, veterinarians, and stakeholders in strengthening disease control and preparedness efforts, ultimately benefiting both the livestock industry and the broader community in Aceh and beyond.
MATERIALS AND METHODS
Study area
Aceh is located between latitude 4.6951° N and longitude 96.7494 E on the island of Sumatra with a total area of 5.6839 km2. This province is mainly mountainous, except in the extreme north, with a reasonably broad coastal plain consisting of 23 districts. In addition, Aceh is bordered by North Sumatera in the south, the Indonesian Ocean in the west, the Malacca Strait in the east, and the Bay of Bengal in the north (Figure 1).
Source of data
The FMD data used in this study were compiled by the Crisis Centre of Foot and Mouth Disease, Aceh Department of Agriculture, and Ministry of Agriculture, Indonesia, every week at the district level for sixteen months (May 2022 to August 2023). This information was also available on the website https://siagapmk.crisis-center.id/index.php. The data were summarized and presented by month.
Geographic information system procedure
The spatial analyst tools available in ArcGIS software, version 10.1 (ESRI; Redlands, USA), which were used to analyze the spatial pattern of the disease. Prior to conducting the analysis, ethical considerations and approvals were obtained in accordance with protocols for research involving disease outbreaks and animal populations (Approval number: 291/KEPH/I/2024). The dataset on FMD cases was processed using ArcMap to visualize potential clustering in different districts in Aceh. The classification of the FMD dataset into three classes based on the number of reported cases per district and month was crucial for the analysis. The classes were defined as low (<3 cases), moderate (>9000 cases), and high (>12000 cases) (Samphutthanon et al., 2014). To identify clustering patterns, spatial join and autocorrelation analysis were conducted using ArcGIS tools. The Density-Based Spatial Clustering of Applications with Noise (DBSCAN) algorithm was employed to group spatial locations close to each other with a minimum number of FMD cases within a specified radius (Ester et al., 1996). This algorithm is effective in identifying clusters of disease incidence based on spatial proximity. Furthermore, spatial autocorrelation techniques like Moran’s I were utilized to assess the clustering of FMD cases in Aceh. Moran’s I statistic helps in determining whether there is clustering, dispersion, or randomness in the spatial distribution of the disease cases. To identify hot spots of FMD incidence, the Getis-Ord Gi* statistic was applied (Getis and Ord, 2010). This technique evaluates whether high or low values of a variable cluster spatially, highlighting areas with significantly high disease occurrence surrounded by neighboring districts with similarly high values.
Hotspot and autocorrelation analysis
To determine data that exhibits significant or insignificant spatial patterns, hotspot and autocorrelation analysis, commonly known as the Getis-Ord Gi* statistical analysis, was conducted. An analysis was conducted on the highest and lowest data values, known as hot and cold spots. This analysis was based on statistical spatial groups, considering correlations within a specific distance from the detected location. The distance was measured using the inverse distance squared method. Furthermore, hotspot analysis was conducted to investigate the spatial connection in developing particular clusters. The method used in this analysis followed to the methodology developed by (Ord and Getis, 1992).
The Moran’s I statistic was commonly used to estimate global spatial autocorrelation, which evaluates the extent of spatial dispersion or clustering of a variable across geographical areas. Moran’s I method measures the degree of similarity in values between adjacent regions in a spatial dataset. A positive Moran’s I coefficient indicates the presence of positive spatial autocorrelation, which means that similar values tend to be clustered together. On the other hand, a negative coefficient reveals the existence of negative spatial autocorrelation, showing that dissimilar values are close to each other. The analysis facilitates a more profound understanding of the fundamental spatial mechanisms and identifying regions where spatial clustering or dispersion may occur (Ma et al., 2017; Ord and Getis, 1995).
In ArcGIS, spatial autocorrelation was used to compute Moran’s I values, which are then represented as Z and P values. These values serve as indicators of importance, with 1.0 indicating positive spatial autocorrelation and -1.0 indicating negative spatial autocorrelation. Index values approaching 1.0 signify a high degree of clustering or random dispersion. According to Mitchell (1999), the formula for spatial autocorrelation, known as Moran’s I, is expressed:

With Zi representing the deviation of i from the mean (xi - X-), Zj representing the deviation of the variable j from the mean (xj - X-), Wi, j represents the spatial weights between i and j, n represent the total number of districts, and S0 represent the sum of all spatial weights.

and Z score formula:
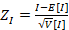
Where E[I]= -1/(n-1) and V[I] = E[I2] – E [I]2
RESULTS AND DISCUSSION
Based on the reported cases, FMD outbreaks started in May 2022 and peaked in July 2022 before disappeared in December 2022. In retrospect, July 2022 had the highest reported cases, followed by June, May, August, September, October, December, and November of the same year (Table 1). The number of reported cases showed fluctuations over the study period, with a first increase in May 2022, a decrease at the end of May, and an increase sharply in the middle of June to July then decreased from August to December 2022 (Figure 2). No cases were reported in early January to August 2023. A similar pattern was also identified in other regions like Ethiopia where the trend of FMD outbreaks significantly decreased from August to December in the period 1999 to 2016 (Aman et al., 2020) and a seasonal pattern from 2010 to 2019 (Woldemariyam et al., 2022). Both studies implied that seasonal trend in FMD outbreak in Ethiopia have potential link to environment condition in that country. Punyapornwithaya et al. (2022) also noted that a rising trend of FMD episodes in Thailand, particularly in areas bordering FMD-endemic countries, indicating the influence of socio-economic factors such as trade and animal movements. These findings suggest that certain environmental and ecological factors may contribute to the spatial distribution of FMD outbreaks in the regions (Guerrini et al., 2019).
FMD outbreak pattern trend occurred in Aceh was also similar with FMD outbreak reported from other countries. For instance, in Colombia, the cyclical and seasonal patterns of FMD outbreaks observed over two decades showed (Gallego et al., 2007). These can be driven by various factors, including the natural immunity cycle in livestock populations, the evolution of the virus, and changes in herd immunity (Bachanek-Bankowska et al., 2018; Di Nardo et al., 2021). India and Bangladesh, both countries, also had cyclical and seasonal patterns over ten years post-monsoon (Rahman et al., 2020; Sharma and Singh, 1993). Other South American countries, like Brazil and Argentina, have also experienced cyclical and seasonal patterns of FMD outbreaks despite significant improvements in disease control through vaccine campaigns and stringent biosecurity measures (Brito et al., 2016; Rivera et al., 2023). These findings indicate that although government efforts can lessen the impact of FMD, the fundamental ecological and biological factors still enable repeated and cyclical trends in outbreaks (Rweyemamu et al., 2008).
Environmental variables such as climate, vegetation cover, and geographical features can also influence the spread and persistence of the FMD virus. For instance, seasonal changes in temperature and humidity can create favorable conditions for the survival of the virus in the environment and increase the likelihood of transmission among livestock populations. Socio-economic factors such as livestock management practices, trade networks, and vaccination coverage can also play a significant role in shaping the dynamics of FMD outbreaks (Nazari et al., 2023). Therefore, a multifaceted approach that combines effective disease control strategies with a comprehensive understanding of environmental and biological influences is essential (Blacksell et al., 2019; Tesfaye, 2020).
Factors such as animal movements and their product, importation policies, and transportation also significantly impact FMD spread (Rohma, 2022). For instance, the outbreak scenario in Aceh Tamiang district in Indonesia, reaching a peak of 3.878 cases on May 8, 2022 as seen in Figure 2, underscores the impact of importation policies on disease spread. These factors contribute to the rapid dissemination of FMD among livestock populations, resulting in economic implications such as reduced productivity and trade restrictions (Chen et al., 2022).
The spatial analysis of FMD outbreaks using the ArcMap tool, within ArcGIS software suite is essential for understanding the disease’s patterns and dynamics. It has been instrumental in analyzing the spatial distribution of FMD outbreaks at the district level (Souley et al., 2018). Previous studies by Guerrini et al. (2019) and Woldemariyam et al. (2022) have contributed insights into the temporal and spatial patterns of FMD outbreaks, aiding in the identification of high-risk areas for outbreaks and exhibit seasonal pattern, indicating non-random distribution and clustering in specific region. Moreover, retrospective spatial analysis, as demonstrated by Chen et al. (2020), has revealed clustered patterns in FMD outbreaks in China, mainly concentrated in the first half of each year, providing valuable information for decision-making and control strategies. Perry and Rich (2007) suggest that understanding the spatial distribution of FMD can aid in forecasting outbreak patterns, evaluating trade policies’ impact, and making informed decisions in public health to address the disease’s economic impact.
Furthermore, It is also employed to identify the origin of the virus in outbreak sources by overlaying spatial data and accurately locating the initial reported cases of FMD (Arjkumpa et al., 2020). According to Yang et al. (2022), FMDV prevalence is significantly influenced by spatiotemporal analysis, animal movements, and vaccine efficacy. Keeling et al. (2001) have proved that identifying the FMDV transmission mechanisms can be done with the help of spatial data and prevention strategies. Despite challenges related to data availability, data quality, and the complexity of FMD transmission mechanisms, spatial analysis remains a valuable tool in FMD research. Studies by González-Gordon et al. (2022) and have highlighted these limitations. Nevertheless, spatial analysis continues to play a crucial role in identifying outbreak sources, assessing disease clusters, and understanding transmission dynamics (Yang et al., 2022).
Based on the distribution and clustering cases of FMD, the highest number of cases was reported in Aceh Besar district, with 12.228 cases of which the first case was in May 2022. Furthermore, a clear pattern emerged regarding the distribution of FMD cases when analyzing spatial statistics through hotspot analysis (Figure 3). Aceh Besar district stands out prominently as a red hotspot, indicating a high concentration of FMD outbreaks with a 99% confidence level (p=0.001; Z-score= 3.196), notably concentrated around prominent cattle markets such as Seulimum, Sibreh, and Ulee Kareng (Negara, 2016). These markets, known for facilitating cattle mobility and trade, have been identified as significant contributors to FMD outbreaks, aligning with previous research on the transmission of FMD through livestock trade and religious festivals (Brusa et al., 2023; Parajuli et al., 2020; Silas, 2022; Toyyibi, 2022). Sansamur et al. (2020) also highlighted that the movement of manure transport vehicles and the circulation of manure bags among cattle farms were identified as potential risk factors for FMD outbreaks.
Aceh Tamiang with a 95% confidence level (p= 0.028; Z-score= 2.192) and Aceh Utara with a 95% confidence level (p= 0.028; Z-score= 2.192) districts also exhibit clustering of FMD cases, albeit to a lesser extent, indicating localized transmission dynamics. Conversely, Aceh Tengah and Simeulue districts report no cases, while Bener Meriah district registers only a few cases, likely due to their geographical isolation and potentially effective government policies such as vaccination programs, animal traffic restrictions, and three-zone biosecurity program (Sari and Abdullah, 2020; Tekleghiorghis et al., 2016) (Figure 3). However, Moran’s I analysis reveals unexpected negative spatial autocorrelation among FMD cases, suggesting a dispersed and random distribution rather than clustering (Z-score= -0.927; p= 0.353). This finding implies that FMD outbreaks occur sporadically and unpredictably across the region, challenging conventional assumptions about localized hotspots (Ma et al., 2017; Nazari et al., 2023). These finding challenges traditional assumptions about disease clustering and suggests that factors influencing FMD transmission may be more complex and varied than previously thought. While localized outbreaks around cattle markets and transportation hubs are evident, the absence of significant spatial clustering implies that FMD can also spread sporadically through other means, such as wildlife reservoirs or human-mediated transport.
Several factors may contribute to this dispersed pattern of FMD transmission. Environmental variables such as temperature, humidity, and landscape features can influence the survival and transmission of the FMD virus, potentially leading to diverse patterns of disease spread across different ecological niches (Colenutt et al., 2020; Klous et al., 2016). Additionally, human behavior, including movement patterns and trading practices, can facilitate the long-distance spread of FMD, particularly in regions with extensive livestock trade networks (Nazari et al., 2023). The interplay of these factors may result in a complex mosaic of FMD transmission dynamics, characterized by both localized outbreaks and sporadic cases dispersed across the landscape.
Understanding the implications of negative spatial autocorrelation is crucial for designing effective disease management strategies and allocating resources appropriately (Osbjer et al., 2015). While hotspot analysis can identify areas of high disease prevalence and prioritize intervention efforts, the absence of spatial clustering highlights the need for broader surveillance measures and targeted control strategies (Tsai et al., 2009). Additionally, public awareness campaigns and education initiatives such as biosecurity campaigns aimed at promoting responsible livestock management practices can contribute to reducing the risk of FMD transmission and enhancing community resilience to disease outbreaks (Paton et al., 2009). By focusing on high-risk areas identified through hotspot analysis and complementing it with spatially explicit surveillance methods, such as geostatistical modeling or remote sensing, authorities can enhance their ability to detect and respond to FMD outbreaks promptly (van Seventer and Hochberg, 2016). The combination of hotspot analysis and Moran’s I provides valuable insights into the spatial dynamics of FMD in Aceh province, informing strategic decision-making and guiding proactive measures to combat the spread of this economically significant livestock disease (González-Gordon et al., 2022).
CONCLUSIONs and Recommendations
The FMD outbreak in our data indicates that the FMD outbreak from 2022 to 2023 in Aceh started in May 2022 and subsided in December 2022 with a total number of 51.396 cases. Our analysis reveals a fluctuating trend in reported cases, with notable peaks in July, June, and May, correlating with a spatial concentration in certain districts, particularly Aceh Besar and Aceh Tamiang, offering new perspectives on its transmission patterns and underscores the critical importance of timely interventions and collaborative efforts in disease management. Analysis of the outbreak timeline and spatial distribution have revealed significant findings regarding the efficacy of various government policies, notably vaccination programs and biosecurity measures, in containing the spread of FMD in certain districts such as in remote or isolated areas such as Aceh Tengah, Simeulue, and Bener Meriah as low case district of FMD outbreak. However, challenges persist, as evidenced by the fluctuating trend and spatial concentration of reported cases in specific regions. Moran’s analysis indicates a degree of dispersion and random distribution, suggesting the need for targeted interventions to address localized outbreaks. The findings provide a couple of practical suggestions for policymakers and stakeholders. Firstly, we encourage the implementation of specific interventions designed especially for regions with a high risk, which were identified by spatial analysis. To effectively control the outbreak of FMD, it is necessary to implement a comprehensive strategy that includes vaccination campaigns, improvements in biosecurity, and surveillance approaches. Second, we emphasize the significance of continuous collaboration among government agencies, academic institutions, veterinary services, and local communities. Collaborative efforts are essential for effectively coordinating responses, sharing resources, and providing critical information to frontline responders. Finally, we stress the importance of isolating the virus at the district level and implementing strong control measures in locations vulnerable to FMD outbreaks. This proactive approach aims to handle future outbreaks before they occur. By implementing such recommendations, policymakers can effectively predict and reduce the effects of FMD outbreaks, protecting the livestock sector and public health. This study advances the current understanding of FMD management and provides actionable insights for policymakers to navigate future disease control and prevention challenges.
ACKNOWLEDGEMENTS
This study was funded by Directorate of Research, Technology and Community Service and Institute for Research, and Community Service Universitas Syiah Kuala Aceh Indonesia (Number: 0536/E5/PG.02.00/2023).
Novelty Statement
The novelty of this study lies in its application of a comprehensive temporal geospatial mapping approach to analyze the dynamics of Foot and Mouth Disease outbreaks specifically within the Aceh region of Indonesia. By integrating temporal and spatial data, this study offers a unique perspective on the spatiotemporal patterns of disease transmission, thus contributing to the advancement of disease surveillance and control strategies tailored to the local context.
AUTHOR’s CONTRIBUTION
All authors equally contributed for manuscript writing and correction.
Conflict of interest
There is no conflict of interests regarding the publication of this article.
REFERENCES
Aman E, Molla W, Gebreegizabher Z, Jemberu WT (2020). Spatial and temporal distribution of foot and mouth disease outbreaks in Amhara region of Ethiopia in the period 1999 to 2016. BMC Vet. Res., 16(1): 1–8. https://doi.org/10.1186/s12917-020-02411-6
Arjkumpa O, Sansamur C, Sutthipankul P, Inchaisri C, Na Lampang K, Charoenpanyanet A, Punyapornwithaya V (2020). Spatiotemporal analyses of foot and mouth disease outbreaks in cattle farms in Chiang Mai and Lamphun, Thailand. BMC Vet. Res., 16(1): 1–13. https://doi.org/10.1186/s12917-020-02392-6
Bachanek-Bankowska K, Di Nardo A, Wadsworth J, Mioulet V, Pezzoni G, Grazioli S, Brocchi E, Kafle SC, Hettiarachchi R, Kumarawadu PL, Eldaghayes IM, Dayhum AS, Meenowa D, Sghaier S, Madani H, Abouchoaib N, Hoang BH, Vu PP, Dukpa K, Knowles NJ (2018). Reconstructing the evolutionary history of pandemic foot-and-mouth disease viruses: The impact of recombination within the emerging O/ME-SA/Ind-2001 lineage. Sci. Rep., 8(1): 14693. https://doi.org/10.1038/s41598-018-32693-8
Blacksell SD, Siengsanan-Lamont J, Kamolsiripichaiporn S, Gleeson LJ, Windsor PA (2019). A history of FMD research and control programmes in Southeast Asia: Lessons from the past informing the future. Epidemiol. Infect., 147. https://doi.org/10.1017/S0950268819000578
Brito B, König G, Cabanne S, Beascoechea CP, Rodriguez L, Perez A (2016). Phylogeographic analysis of the 2000-2002 foot-and-mouth disease epidemic in Argentina. Infect. Genet. Evol., 41: 93–99. https://doi.org/10.1016/j.meegid.2016.03.026
Brusa V, Durrieu M, Van Gelderen CJ, Signorini ML, Schudel A (2023). Quantitative risk assessment of FMDV introduction in a FMD free country through bone-in beef and offal importation from a FMD free with vaccination country/zone. Prevent. Vet. Med., 218: 105995. https://doi.org/10.1016/j.prevetmed.2023.105995
Chen J, Wang J, Wang M, Liang R, Lu Y, Zhang Q, Chen Q, Niu B (2020). Retrospect and risk analysis of foot-and-mouth disease in china based on integrated surveillance and spatial analysis tools. Front. Vet. Sci., 6: 511. https://doi.org/10.3389/fvets.2019.00511
Chen R, Gardiner E, Quigley A (2022). Foot and mouth disease outbreak in Indonesia: Summary and implications. Glob. Biosecur., 4(1). https://doi.org/10.31646/gbio.175
Colenutt C, Brown E, Nelson N, Paton DJ, Eblé P, Dekker A, Gonzales JL, Gubbins S (2020). Quantifying the transmission of foot and mouth disease virus in cattle via a contaminated environment. MBio, 11(4): 1–13. https://doi.org/10.1128/mBio.00381-20
Di Nardo A, Ferretti L, Wadsworth J, Mioulet V, Gelman B, Karniely S, Scherbakov A, Ziay G, Özyörük F, Parlak Ü, Tuncer-Göktuna P, Hassanzadeh R, Khalaj M, Dastoor SM, Abdollahi D, Khan EUH, Afzal M, Hussain M, Knowles NJ, King DP (2021). Evolutionary and ecological drivers shape the emergence and extinction of foot and mouth disease virus lineages. Mol. Biol. Evol., 38(10): 4346–4361. https://doi.org/10.1093/molbev/msab172
DREF (2023). DREF operation-final report Indonesia. Foot and mouth disease outbreak. OCHA Service.
Ester M, Kriegel HP, Sander J, Xu X (1996). A density-based algorithm for discovering clusters in large spatial databases with noise. Kdd, 96(34): 226–231.
FAO (2009). Livestock in the balance. FAO.
Feng S, Patton M, Davis J (2017). Market impact of foot-and-mouth disease control strategies: A UK case study. Front. Vet. Sci., 4(Sep). https://doi.org/10.3389/fvets.2017.00129
Gallego ML, Perez AM, Thurmond MC (2007). Temporal and spatial distributions of foot-and-mouth disease under three different strategies of control and eradication in Colombia (1982-2003). Vet. Res. Commun., 31(7): 819–834. https://doi.org/10.1007/s11259-007-0125-1
Getis A, Ord JK (2010). The analysis of spatial association by use of distance statistics. Adv. Spatial Sci., 61(3): 127–145. https://doi.org/10.1007/978-3-642-01976-0_10
González-Gordon L, Porphyre T, Muhanguzi D, Muwonge A, Boden L, Bronsvoort BMDC (2022). A scoping review of foot-and-mouth disease risk, based on spatial and spatio-temporal analysis of outbreaks in endemic settings. Transbound. Emerg. Dis., 69(6): 3198–3215. https://doi.org/10.1111/tbed.14769
Guerrini L, Pfukenyi DM, Etter E, Bouyer J, Njagu C, Ndhlovu F, Bourgarel M, De Garine-Wichatitsky M, Foggin C, Grosbois V, Caron A (2019). Spatial and seasonal patterns of FMD primary outbreaks in cattle in Zimbabwe between 1931 and 2016. Vet. Res., 50(1): 1–12. https://doi.org/10.1186/s13567-019-0690-7
Handayani S, Safrida S (2023). Characteristics of cattle farm breeders in Aceh Province. IOP Conf. Ser. Earth Environ. Sci., 1183(1). https://doi.org/10.1088/1755-1315/1183/1/012037
Keeling MJ, Woolhouse MEJ, Shaw DJ, Matthews L, Chase-Topping M, Haydon DT, Cornell SJ, Kappey J, Wilesmith J, Grenfell BT (2001). Dynamics of the 2001 UK foot and mouth epidemic: Stochastic dispersal in a heterogeneous landscape. Science, 294(5543): 813–817. https://doi.org/10.1126/science.1065973
Klous G, Huss A, Heederik DJJ, Coutinho RA (2016). Human-livestock contacts and their relationship to transmission of zoonotic pathogens, a systematic review of literature. One Health, 2: 65–76. https://doi.org/10.1016/j.onehlt.2016.03.001
Knight-Jones TJD, Rushton J (2013). The economic impacts of foot and mouth disease - What are they, how big are they and where do they occur? Prevent. Vet. Med., 112(3–4): 161–173. https://doi.org/10.1016/j.prevetmed.2013.07.013
Ma J, Xiao J, Gao X, Liu B, Chen H, Wang H (2017). Spatial pattern of foot-and-mouth disease in animals in China, 2010-2016. PeerJ, 2017(12): e4193. https://doi.org/10.7717/peerj.4193
Mitchell A (1999). The ESRI guide to GIS analysis. Geogr. Patterns Relationships, 1: G 70.212. M57.
Nazari AM, Alesheikh AA, Neisani SZ, Lotfata A, Bayat S, Alipour S, Hoseini B (2023). Socioeconomic and environmental determinants of foot and mouth disease incidence: An ecological, cross-sectional study across Iran using spatial modeling. Sci. Rep., 13(1): 13526. https://doi.org/10.1038/s41598-023-40865-4
Negara ABW (2016). Perkiraan Nilai ekonomis akibat penyakit orf pada kambing di tiga pasar hewan kabupaten aceh besar (Estimation of the Economic Value Resulted by Orf Disease on Goat in Three Livestock Market in Aceh Besar District). J. Med. Vet., 8(1). https://doi.org/10.21157/j.med.vet..v10i2.4612
Ord JK, Getis A (1992). The analysis of spatial association. Geographical analysis, 24(3): 189–206. https://doi.org/10.1111/j.1538-4632.1992.tb00261.x
Ord JK, Getis A (1995). Local spatial autocorrelation statistics: Distributional issues and an application. Geogr. Anal., 27(4): 286–306. https://doi.org/10.1111/j.1538-4632.1995.tb00912.x
Osbjer K, Boqvist S, Sokerya S, Kannarath C, San S, Davun H, Magnusson U (2015). Household practices related to disease transmission between animals and humans in rural Cambodia Global health. BMC Publ. Health, 15(1). https://doi.org/10.1186/s12889-015-1811-5
Parajuli P, Pandit S, Kaphle K (2020). Foot and mouth disease (FMD) among animals in Nepal. Int. J. Appl. Sci. Biotechnol., 8(1): 7–13. https://doi.org/10.3126/ijasbt.v8i1.27785
Paton DJ, Sumption KJ, Charleston B (2009). Options for control of foot-and-mouth disease: Knowledge, capability and policy. Philos. Trans. R. Soc. B: Biol. Sci., 364(1530): 2657–2667. https://doi.org/10.1098/rstb.2009.0100
Perry BD, Rich KM (2007). Poverty impacts of foot-and-mouth disease and the poverty reduction implications of its control. Vet. Rec., 160(7): 238–241. https://doi.org/10.1136/vr.160.7.238
Pudenz CC, Mitchell JL, Schulz LL, Tonsor GT (2021). U.S. cattle producer adoption of secure beef supply plan enhanced biosecurity practices and foot-and-mouth disease preparedness. Front. Vet. Sci., 8. https://doi.org/10.3389/fvets.2021.660857
Punyapornwithaya V, Mishra P, Sansamur C, Pfeiffer D, Arjkumpa O, Prakotcheo R, Damrongwatanapokin T, Jampachaisri K (2022). Time-series analysis for the number of foot and mouth disease outbreak episodes in cattle farms in Thailand using data from 2010–2020. Viruses, 14(7). https://doi.org/10.3390/v14071367
Rahman AKMA, Islam SKS, Sufian MA, Talukder MH, Ward MP, Martínez-López B (2020). Foot-and-mouth disease space-time clusters and risk factors in cattle and buffalo in Bangladesh. Pathogens, 9(6): 423. https://doi.org/10.3390/pathogens9060423
Rivera AM, Sanchez-Vazquez MJ, Pituco EM, Buzanovsky LP, Martini M, Cosivi O (2023). Advances in the eradication of foot-and-mouth disease in South America: 2011–2020. Front. Vet. Sci., 9: 1024071. https://doi.org/10.3389/fvets.2022.1024071
Rohma MDC (2022). Kasus penyakit mulut dan kuku di Indonesia: Epidemiologi, diagnosis penyakit, angka kejadian, dampak penyakit, dan pengendalian. 3rd Natl. Conf. Appl. Anim. Sci., pp. 15–22. https://doi.org/10.25047/animpro.2022.331
Rweyemamu M, Roeder P, MacKay D, Sumption K, Brownlie J, Leforban Y, Valarcher JF, Knowles NJ, Saraiva V (2008). Epidemiological patterns of foot and mouth disease worldwide. Transbound. Emerg. Dis., 55(1): 57–72. https://doi.org/10.1111/j.1865-1682.2007.01013.x
Samphutthanon, R., Kumar Tripathi, N., Ninsawat, S., & Duboz, R. (2014). Spatio-temporal distribution and hotspots of hand, foot and mouth disease (HFMD) in northern Thailand. Int. J. Environ. Res. Public Health, 11(1): 312–336.
Sansamur C, Wiratsudakul A, Charoenpanyanet A, Punyapornwithaya V (2020). Cattle manure trade network analysis and the relevant spatial pathways in an endemic area of foot and mouth disease in Northern Thailand. Vet. Sci., 7(3). https://doi.org/10.3390/vetsci7030138
Sari EM, Abdullah MAN (2020). Sumber daya genetik ternak lokal kerbau simeulue. Syiah Kuala University Press. https://doi.org/10.52574/syiahkualauniversitypress.284
Sharma SK, Singh GR (1993). Cyclic behaviour of foot-and-mouth disease in India. Vet. Rec., 133(18): 448–450. https://doi.org/10.1136/vr.133.18.448
Silas LS (2022). Foot and mouth disease in cameroon: A systematic review to support its progressive control. J. Clin. Vet. Res., 2(1): 1–14. https://doi.org/10.54289/JCVR2200104
Souley KB, Thys E, Renault V, Abatih E, Marichatou H, Issa S, Saegerman C (2018). Spatio-temporal patterns of foot-and-mouth disease transmission in cattle between 2007 and 2015 and quantitative assessment of the economic impact of the disease in Niger. Transbound. Emerg. Dis., 65(4): 1049–1066. https://doi.org/10.1111/tbed.12845
Susila EB, Daulay RSD, Hidayati DN, Prasetyowati SRB, Wriningati AE, Irianingsih SH, Dibia IN, Faisal SA, Yupiana Y, Hidayat MM, Zainuddin N, Wibawa H (2023). Detection and identification of foot-and-mouth disease O/ME-SA/Ind-2001 virus lineage, Indonesia, 2022. J. Appl. Anim. Res., 51(1): 487–494. https://doi.org/10.1080/09712119.2023.2229414
Sutawi WA, Malik A, Suyatno HA, Rahayu ID, Hartatie ES (2023). Re-emergence of foot and mouth disease outbreak in Indonesia: A review. Adv. Anim. Vet. Sci., 11(2): 264–271. https://doi.org/10.17582/journal.aavs/2023/11.2.264.271
Tekleghiorghis T, Moormann RJM, Weerdmeester K, Dekker A (2016). Foot and mouth disease transmission in Africa: Implications for control. A review. Transbound. Emerg. Dis., 63(2): 136–151. https://doi.org/10.1111/tbed.12248
Tesfaye J (2020). Review on the epidemiology and economic impact of foot and mouth disease in Ethiopia. Agric. J., 14(5): 79–93. https://doi.org/10.36478/aj.2019.79.93
Toyyibi AM (2022). Religious life, economy when the plague of foot and mouth disease ahead of Eid Al-Adha Sharia maqashid perspective on Islamic Studies (ICIS) IAIN Ponorogo, 3: 99–107. https://prosiding.iainponorogo.ac.id/index.php/icis/article/view/787
Tsai PJ, Lin ML, Chu CM, Perng CH (2009). Spatial autocorrelation analysis of health care hotspots in Taiwan in 2006. BMC Publ. Health, 9. https://doi.org/10.1186/1471-2458-9-464
van Seventer JM, Hochberg NS (2016). Principles of infectious diseases: Transmission, diagnosis, prevention, and control. Int. Encycloped. Publ. Health, pp. 22–39. https://doi.org/10.1016/B978-0-12-803678-5.00516-6
Woldemariyam FT, Leta S, Assefa Z, Tekeba E, Gebrewold DS, Paeshuyse J (2022). Temporal and spatial patterns and a space–time cluster analysis of foot-and-mouth disease outbreaks in Ethiopia from 2010 to 2019. Viruses, 14(7): 1558. https://doi.org/10.3390/v14071558
Yang J, Wang X, Li K (2022). Temporal-spatial analysis of a foot-and-mouth disease model with spatial diffusion and vaccination. Front. Vet. Sci., 9: 952382. https://doi.org/10.3389/fvets.2022.952382
To share on other social networks, click on any share button. What are these?





