Study on the Extracting Technology for Antioxidant Oligopeptides from Donkey Meat by Two-Step Enzymatic Hydrolysis
Study on the Extracting Technology for Antioxidant Oligopeptides from Donkey Meat by Two-Step Enzymatic Hydrolysis
Lanjie Li1, Jingjing Zhang2, Ruiyan Zhang2, Ning Zhang2, Zixiang Wei2, Guiqin Liu1,*, Riaz Hussain Pasha3, Muhammad Akram Khan4 and Saif-Ur-Rehman5
1College of Agronomy, Liaocheng University, Liaocheng 252000, China
2Institute of Bio-pharmaceutical, Liaocheng University, Liaocheng 252000, China
3Department of Veterinary Biomedical Sciences (Histology), Faculty of Veterinary and Animal Sciences, PMAS-Arid Agriculture University, Rawalpindi, Pakistan
4Department of Veterinary Pathology, Faculty of Veterinary and Animal Sciences, PMAS-Arid Agriculture University, Rawalpindi, Pakistan
5Department of Parasitology and Microbiology, Faculty of Veterinary and Animal Sciences, PMAS-Arid Agriculture University, Rawalpindi, Pakistan
ABSTRACT
Donkey (Equus asinus L.) is an important source of medicinal material in China. Both the hide and meat of donkeys have a variety of physiological activities such as the hide is sufficiently employed for making Ejiao (Colla Corii Asini). The physiological efficacy value of donkey meat is not maximized due to lack of advanced extracting methodologies. In this study, an innovative extracting technology was established for the extraction of antioxidant oligopeptides from donkey meat by two-step enzymatic hydrolysis. The influensce of enzymolysis on the degree of hydrolysis (DH) and protein recovery (PR) were evaluated, and the chemical composition, antioxidant activity, and molecular weight distribution of oligopeptides in hydrolysates were studied. The optimum enzymatic hydrolysis conditions were identified at 5.96 pH, 0.7% enzyme-substrate ratio, 55ºC temperature and 3 h time. Under these optimum conditions, DH of 25.4%, PR of 95% was obtained. Moreover, hydrolysates were rich in oligopeptides, especially di- and tri-peptides and demonstrated good antioxidant activities. These results introduce a new methodology with the provision of fundamental data for the production of antioxidant oligopeptides from donkey meat.
Article Information
Received 06 May 2020
Revised 30 June 2020
Accepted 28 July 2020
Available online 05 May 2021
(early access)
Published 12 February 2022
Authors’ Contribution
LL and MAK wrote the manuscript. JZ and ZW performed the experiment. RZ and NZ contributed to analysis and manuscript preparation. GL presented the concept of the study. LL, MAK, RHP, MAK and SR analysd the data.
Key words
Donkey meat, Papain enzyme, Antioxidant properties, Extracting technology.
DOI: https://dx.doi.org/10.17582/journal.pjz/20200506020526
* Corresponding author: guiqinliu@lcu.edu.cn
0030-9923/2022/0003-1063 $ 9.00/0
Copyright 2022 Zoological Society of Pakistan
Introduction
Donkey (Equus asinus L.) is an important source of medicinal products in China (Smith and Pearson, 2005). Both the hide and meat of donkeys have been proved to have a variety of physiological activities. For example, donkey hide could enrich blood, enhance white blood cell, and is used in adjuvant chemotherapy. Donkey meat improves liver and kidney functions, promotes blood tonic, body immunity, anti-aging, profit lung, and eyesight effects (Li et al., 2018). Currently, the hide is extensively used to make raw material for Ejiao (Colla Corii Asini). However, the donkey meat is mostly consumed in the form of primary form (Tian et al., 2018) which does not truly reflect its physiological efficacy (Wang and Liu, 2019). Some studies (Yang et al., 2018; Juan et al., 2008) have shown that donkey meat has higher protein content (21.50%) and lower fat content (3.20%) than other livestock and poultry meat, such as the beef (19.90% protein and 4.20% fat) and the chicken (19.30% protein and 9.40% fat). Furthermore, the amount of eight essential amino acids in donkey meat accounts for 39.40% of the total amino acid, and the essential amino acid index (EAAI) is 65.48, higher than that of cattle, sheep, and pork. Such particular characteristics in donkey meat increase its functional activities as compared to other meat. To get maximum consumption of all beneficial ingredients, there is need to establish the deep processing technology for donkey meat. In this perspective, extraction of antioxidant oligopeptides from donkey meat by two-step enzymatic hydrolysis could be a promising strategy.
Oligopeptides (the low molecular weight peptides) are produced chemically or enzymatically from the degradation of proteins into peptides of variable chain lengths (Adler-Nissen, 1986). In food industry, enzymatic hydrolysis remains excellent strategy as compared to other chemical methods (Wang and Shahidi, 2018). The meat muscle protein is dissolved by proteolytic enzymes into oligopeptides which are then hydrolyzed into soluble fraction (Kurozawa et al., 2008). The hydrolysates are rich in oligopeptides, especially di- and tri-peptides, and have high nutritional and therapeutic values (Jang et al., 2016). Extraction of oligopeptides by enzymatic hydrolysis have been extensively studied, such as from turkey meat (Kurozawa et al., 2008; Pasqualin et al., 2014), sandfish (Arctoscopus japonicus) (Jang et al., 2016), chicken (Kurozawa et al., 2009), mussel (Silva et al., 2010; Sidorova et al., 2014), abalone viscera (Weng et al., 2019), yellowfin tuna (Nurilmala et al., 2019), and swim bladders of miiuy croaker (Zhao et al., 2018). However, little is known regarding the application of enzymatic hydrolysis for donkey meat.
The enzymatic hydrolysis could provide excellent benefits since donkey meat is a good source of antioxidant peptides including essential amino acids, such as methylhistidine and hydroxymethyllysine. The well-known antioxidant peptides of carnosine and anserine exist endogenously in muscle tissue, acting as free radical scavengers and metal ion chelators. In addition, the large abundance of meat, such as turkey (Wang and Shahidi, 2018), abalone (Weng et al., 2019), carp (Xue et al., 2011), and duck (Lusha et al., 2015), have been utilized for preparing antioxidant peptides in meat industry.
The present study was aimed to establish an extraction technology by two-step enzymatic hydrolysis for antioxidant oligopeptides from donkey meat. The effects of pH value, enzyme-substrate ratio, temperature, and time on enzymatic hydrolysis of donkey meat were investigated, and the chemical composition, antioxidant activity, and molecular weight distribution of oligopeptides in hydrolysates were studied. Based on these results, we aim to provide fundamental data for the production of antioxidant oligopeptides from donkey meat.
Materials and methods
Materials
Frozen donkey meat from the legs of 2-year-old male Dezhou donkey and Ejiao oligopeptide were purchased from Dong-E-E-Jiao Co., Ltd. (Liaocheng, China) and immediately stored at -20ºC. 1,1-diphenyl-2-picrylhydrazyl (DPPH) and Pyrogallol were obtained from Sigma-Aldrich (St. Louis, MO, USA). Papain enzyme was purchased from GXdoing-Higher Bio-Tech Co., Ltd (Guangxi, China), with a declared activity of 100 U/mg. Reference compounds (N-Hippuryl-His-Leu hydrate, Bacitracin, Aprotinin Hydrochloride, Cytochrome C) were purchased from Shanghai yuanye Bio-Technology Co., Ltd (Shanghai, China). All other chemicals used were of analytical grade.
Instrumentations
Shimadzu high performance liquid chromatography (LC-2030, Shimadzu Corporation, Kyoto, Japan) equipped two binary pumps, a thermostated column compartment and UV detector. Phenomenex BioSep-SEC-S3000 column (7.8 mm×300 mm, 5 μm, Phenomenex Inc., CA, USA). Thermostat oscillator (HNY-1102C, Tianjin Honour instrument Co., Ltd, Tianjin, China). Kjeldahl Analyzer (K9840, Hanon Instruments Co., Ltd, Shanghai, China). Ultraviolet Spectrophotometry (U-3900H, Hitachi, Ltd, Tokyo, Japan). Vortex Oscillator (VORTEX-5, Kylin-bell Lab Instruments Co., Ltd, Jiangsu, China). Low speed bench centrifuge (TD5A, Hunan Herexi Instrument and Equipment Co., Ltd, Hunan, China).
Standard solutions
The single and mixed standard stock solutions were prepared by accurately weighing appropriate amounts of the four reference standards and dissolving with water at the concentration level of 0.10 mg/mL. All solutions were stored at 4ºC in refrigerator. All solutions were filtered with 0.22 μm membrane filters before injected into the HPLC system.
Two-step enzymatic hydrolysis
The hydrolysis experiments were performed in thermostat oscillator to control the temperature, time, and speed. Donkey meat was ground and mixed with distilled water at a ratio of 15% (w/w) and then boiled at 100ºC for 20 min to deactivate endogenous enzymes. The mixture was then adjusted to the desired temperature and pH with 1.0 mol/L NaOH or 1.0 mol/L HCl, and papain enzyme was added at an enzyme-substrate (E/S) ratio of 0.7%. The mixture was agitated at 150 rpm in thermostat oscillator at 55ºC for 2 h, and the hydrolytic process was terminated by boiling at 100ºC for 20 min, ensuring inactivation of the enzyme. The pH was adjusted to that of early by the addition of 1.0 mol/L NaOH after the hydrolytic. Then the resulting solution was cooled at room temperature and centrifuged for 5 min at 4000 rpm to obtain the supernatant 1 (S-1) and precipitate 1 (P-1). The P-1 was mixed with distilled water at a ratio of 15% (w/w) and hydrolyzed for 3 h again. The supernatant 2 (S-2) and precipitate 2 (P-2) were obtained after terminated, cooled, and centrifuged. The S-1 and S-2 were analyzed after filtered with 0.22 μm membrane filter, and the P-2 was analyzed after dried for 12 h at 70ºC.
Determination of the degree of hydrolysis
The degree of hydrolysis (DH) was obtained by the pH-stat method with a simple modification and defined as the percent ratio between the number of peptide bonds cleaved (h) and the total number of bonds available for proteolytic hydrolysis (htotal) (Adler-Nissen, 1986). It was calculated according to the following equation:
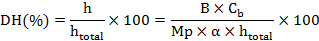
Where, B is the NaOH consumption (mL) to keep the same pH after the hydrolytic, Cb is the concentration of the NaOH (mol/L), Mp is the mass of the protein (g), which was determined by Kjeldahl Analyzer; htotal is the total number of peptide bonds in protein substrate (7.6 mmol/g in protein), and α is the degree of dissociation of the α-NH2 groups expressed as:
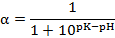
The pK value varies significantly with temperature and can be estimated as follows (Kristinsson and Rasco, 2000):
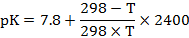
Where, T is the temperature (K).
Determination of the protein recovery
The protein recovery (PR) was the ratio of the percent protein in hydrolysates (MPs) to that in the original substrate (MP). It was calculated according to the following equation:
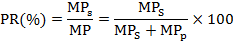
Where, MP is the mass of protein present in the original substrate (g), MPs is the mass of protein present in the supernatant (g), MPp is the mass of protein present in the precipitate (g), and MPs and MPp were determined by Kjeldahl Analyzer (Hayes et al., 2016; Yasemi et al., 2013).
Determination of the chemical composition
The changes of chemical composition in hydrolysates before and after enzymatic hydrolysis can be used to evaluate the enzymatic hydrolysis techniques. And the content of nitrogen, fat, moisture, and ash were determined. Total nitrogen content of hydrolysates was determined by using the Kjeldahl method. Proteins were estimated by multiplying total nitrogen content by the factor of 6.25. Fat was determined gravimetrically after Soxhlet extraction of dried samples with hexane. The moisture and ash content were determined according to National Food Safety Standards (GB 5009.3-2016 and GB 5009.4-2016), respectively. All measurements were performed in sextuplicate.
Measurement of antioxidant activity
The 1,1-diphenyl-2-picrylhydrazyl (DPPH), hydroxyl, and superoxide radical scavenging activity (RSA) assay are widely used for evaluating antioxidant activity of compounds and extracts (Zhang et al., 2015; Zhao et al., 2013).
DPPH radical scavenging activity assay
The DPPH radical scavenging activity was measured by the modified method of Gu et al. (2012). 1.0 mL of the supernatant was mixed with 0.5 mL of 0.15 mmol/L DPPH radical solution. Then the mixture was spun for 10 s using vortex oscillator and reacted for 30 min in dark at room temperature. Absorbance was measured at 517 nm using a spectrophotometer. The scavenging activity was calculated according to the following equation:
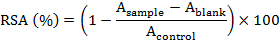
Where, Asample is the absorbance of the supernatant at 517 nm, Ablank is the absorbance of the blank (without supernatant or DPPH) at 517 nm, and Acontrol is the absorbance of the control (without supernatant, just DPPH) at 517 nm.
Hydroxyl radical scavenging activity assay
The hydroxyl radical scavenging activity was assayed using a modified version of the method of Bamdad and Chen (2013). 1.0 mL of 1.0 mmol/L FeSO4 was mixed with 1.0 mL of 1.5 mmol/L H2O2 solution. Then 1.0 mL of the supernatant was added and spun for 10 s using vortex oscillator. After 1 mL of 2.0 mmol/L sodium salicylate was added, the reaction system reacted for 30 min at 37ºC in water bath. Absorbance was measured at 510 nm using a spectrophotometer. The scavenging activity was calculated according to the following equation:
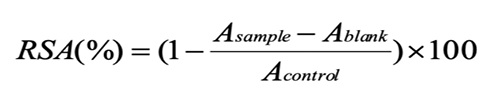
Where, Asample is the absorbance of the supernatant at 510 nm, and Acontrol is the absorbance of the control (water, instead of supernatant) at 510 nm.
Superoxide radical scavenging activity assay
The superoxide scavenging activity was determined by pyrogallol autoxidation systems with some modifications (Gao et al., 1998). 1.5 mL of 50 mmol/L Tris-HCl buffer (pH 8.2) and 1.0 mL of water were preincubated for 20 min at room temperature. Then 1.0 mL of the supernatant and 0.5 mL of 3.0 mmol/L pyrogallol were added. The rate of superoxide radical-induced polymerisation of pyrogallol (∆A/min) was measured as increased in absorbance at 420 nm for 3 min at room temperature. The scavenging activity was calculated according to the following equation:

Where, ∆A/minsample is the absorbance of the supernatant at 420 nm, and ∆A/mincontrol is the absorbance of the control (water, instead of supernatant) at 420 nm.
Determination of the molecular weight distribution
The molecular weight distribution (MWD) of hydrolysates and Ejiao Oligopeptide were determined by gel filtration chromatography. The system was composed of a Shimadzu LC-2030 chromatography equipped two binary pumps, a thermostated column compartment, a Phenomenex BioSep-SEC-S3000 column, and UV detector. The mobile phase was 0.1 moL/L phosphate buffer (pH 6.8). The flow rate of the mobile phase was adjusted at 1.0 mL/min. The injection volume of analytical solution was 10 μL. The column temperature was maintained at 25ºC and the detection wavelength was set at 220 nm (Yang et al., 2011; Jin et al., 2016).
Statistical analysis
The experimental data in this study were expressed as the mean and standard deviation (mean ± SD) of three or more iterations. The sample data collected were analyzed using analysis of variance (ANOVA), and Duncan’s multiple range test was used to determine the significant differences (p<0.05). All statistical analyses were performed using the SPSS 20.0 software (SPSS Inc., Chicago, IL, USA).
Results and Discussion
Single-factor experiment analysis
The antioxidant activity of enzymatic hydrolysates is affected by the degree of enzymatic hydrolysis. To obtain optimum hydrolysis conditions, the effects of experimental parameters, including the pH value, the enzyme: substrate ratio, the temperature, and the time, were investigated.
Effect of pH value
pH value of the system directly affects the activity of papain enzyme. Experiments were performed by changing the pH from 4.00 to 9.00 by adjusting with suitable hydrochloric acid and sodium hydroxide. Samples (from 6 individual batches) were hydrolyzed at various pHs, then the DH and PR of each hydrolysate were measured (Fig. 1A). There was no obvious difference of DH and PR from 5.00 to 7.00, which indicated that activity of papain enzyme was insensitive to pH value in this range.
So, the natural pH of system (pH 5.96) was selected. The results were consistent with the results of Baehaki’s experiments (Baehaki, 2015) on the antioxidant activity of skin and bone collagen hydrolyzed from striped catfish with papain enzyme.
Effect of enzyme concentration
The enzyme substrate ratio affects the efficiency of enzymatic hydrolysis reaction. In this part, 15 g donkey meat shredded was added into 85 mL water, and the effect of enzyme concentration was investigated in the range from 0.025 to 0.150 g (Fig. 1B). The DH was continuously increasing in the range of 0.025 to 0.150 g, which was suggestive that enzymatic hydrolysis reaction was accelerated with the increase of concentration. When the amount of enzyme was 0.100 g, the optimal PR was obtained. And the enzyme: substrate ratio was 0.7%. The results were basically consistent with the results of Lu’s experiments (Ling-Ling et al., 2013) on the extraction of Rana chensinensis skin collagen by papain and its antioxidant activity.
Effect of temperature
Appropriate temperature could promote hydrolysis by increasing the enzymatic activity. However, denaturation by heating may lead to disruption of the native peptide structure and loss of its biological activity. The effect of temperature on the efficiency of enzymatic hydrolysis in the range of 40 to 65ºC was evaluated shown in (Fig. 1C), the PR was invariant at temperature ranging from 50 to 60ºC. However, when the temperature was fixed to 55ºC, the DH reached the highest point. Therefore, 55ºC was selected for further reactions. The results of the study are inconsistent with Youle’s experiments (Youle et al., 2011) on the preparation and antioxidant properties of the hydrolysate and frations from Mytilus edulis by papain. In his study, the optimal hydrolysis conditions of the temperature 65ºC. The reason may be related to the enzyme activity of papain.
Effect of hydrolysis time
The hydrolysis time affects the DH and PR of hydrolysates, which further influences the antioxidant activity. To obtain the highest DPPH radical scavenging activity (DSA), experiments were performed by different lengths of time and the DH, PR, DSA of each hydrolysate were measured. As shown in (Fig. 1D), the radical scavenging activity of hydrolysates increased with time until 3 h, and then declined gradually. At this point, it had also good DH and PR. The results indicated that the radical scavenging activity was dependent on hydrolysis time. The results were basically consistent with the results of Youle’s experiments (Youle et al., 2011) on the preparation and antioxidant properties of the hydrolysate and frations from Mytilus edulis by papain.
Property characterization of peptides in hydrolysates obtained under the optimum conditions
The peptides present in hydrolysates obtained through the optimized conditions were characterized in terms of its chemical composition, antioxidant activity, and molecular weight distribution.
Chemical composition
The samples (from 6 individual batches) were hydrolyzed at the optimized conditions. As shown in Table I, the hydrolysate presented higher protein content (82.98%) and lower fat (1.07%) content than the waste (46.65%, and 32.17%). High protein content of hydrolysate was the result of protein dissolution in hydrolysis reaction, and the decrease in fat content of the protein hydrolysate contributed significantly to lipid oxidation stability. Given the small amount of waste obtained, the protein content of the waste (46.65%) presented a limited protein loss. The higher content of inorganic substance (7.02%) reported in hydrolysate was possibly due to the solubility of the water-soluble minerals.
Table I.- Chemical composition of the hydrolysate and waste (precipitate).
|
Content (wt. %) |
Hydrolysate |
Waste (precipitate) |
|
Proteins |
82.98±2.15 |
46.65±1.50 |
|
Fat |
1.07±0.05 |
32.17±1.14 |
|
Moisture |
8.64±0.55 |
14.72±0.82 |
|
Ash |
7.02±0.64 |
5.12±0.48 |
Data are presented as mean±SD (n=6).
Antioxidant activity of hydrolysates
The antioxidant activity of hydrolysates (from 6 individual batches) were determined by the scavenging ability of DPPH free radicals, hydroxyl free radicals, and superoxide free radicals. As shown in Figure 2, the hydrolysates had strong antioxidant capacity to scavenge DPPH, hydroxyl radicals, but revealed less powerful ability to scavenge superoxide radical. These results revealed that hydrolysates possibly contained peptides which could act as electron donors, react with free radicals to give rise to more stable products and terminate radical chain reactions.
Molecular weight distribution
The MWD of hydrolysates (from 6 individual batches) and Ejiao Oligopeptide were determined. The results (Fig. 3) indicated that several protein bands with molecular weights below 12.5 kDa were observed, and the hydrolysate was rich in low molecular weight peptides, especially diand tri-peptides. The hydrolysate had similar MWD to Ejiao Oligopeptide, which had been proved to exhibit a good antioxidant activity.
Conclusion
We developed the technology of enzymatic hydrolysis for antioxidant peptides derived from donkey meat. Little protein was determined in precipitate by Kjeldahl Analyzer, which revealed that the protein in meat was cleaved after the hydrolysis reaction. And the effects of experimental parameters, including the pH value, the enzyme: substrate ratio, the temperature, and the time, were investigated. At optimum conditions, a DH of 25.4%, PR of 95%, DSA of 69.75% were obtained and hydrolysates (3 h) had similar MWD to Ejiao Oligopeptide, which had been proved to have a good antioxidant activity. These results provide fundamental data for the application of donkey hydrolysates, such as protein supplementation in food systems.
Acknowledgments
The authors gratefully acknowledge the financial support of Shandong Donkey Industry Technology Collaborative Innovation Center (No. SDAIT-27), Doctoral Foundation of Liaocheng University (No. 318051636), Open Project of Shandong Collaborative Innovation Center for Antibody Drugs (No. CIC-AD1840 and No. CIC-AD1843).
Statement of conflict of interest
The authors have declared no conflict of interest.
References
Adler-Nissen, J., 1986. Enzymatic hydrolysis of food proteins. Elsevier Applied Science Publishers, Novo Industries, S/A, London.
Baehaki, A., 2015. Antioxidant activity of skin and bone collagen hydrolyzed from striped catfish (Pangasius pangasius) with papain enzyme. J. Chem. Pharm. Res., 7: 131-135.
Bamdad, F. and Chen, L., 2013. Antioxidant capacities of fractionated barley hordein hydrolysates in relation to peptide structures. Mol. Nutr. Fd. Res., 57: 493-503. https://doi.org/10.1002/mnfr.201200252
Gao, R., Yuan, Z. and Gao, X., 1998. Mechanism of pyrogallol autoxidation and determination of superoxide dismutase enzyme activity. Bioelectrochem. Bioenerg., 45: 41-45. https://doi.org/10.1016/S0302-4598(98)00072-5
Gu, R.Z., Liu, W.Y. and Yi, W.X., 2012. Antioxidant and angiotensin i-converting enzyme inhibitory properties of oligopeptides derived from black-bone silky fowl (Gallus gallus domesticus Brisson) muscle. Fd. Res. Int., 49: 326-333. https://doi.org/10.1016/j.foodres.2012.07.009
Hayes, M., Mora, L. and Aluko, R.E., 2016. Boarfish protein recovery using the ph-shift process and generation of protein hydrolysates with ace-i and antihypertensive bioactivities in spontaneously hypertensive rats. Innov. Fd. Sci. Emerg., 37: 253-260. https://doi.org/10.1016/j.ifset.2016.03.014
Jang, H.L., Liceaga, A.M. and Yoon, K.Y., 2016. Purification, characterisation and stability of an antioxidant peptide derived from sandfish (Arctoscopus japonicus) protein hydrolysates. J. Funct. Fd., 20: 433-442. https://doi.org/10.1016/j.jff.2015.11.020
Jin, J., Ma, H. and He, R., 2016. Effects and mechanism of dual-frequency power ultrasound on the molecular weight distribution of corn gluten meal hydrolysates. Ultrason. Sonochem., 30: 44-51. https://doi.org/10.1016/j.ultsonch.2015.11.021
Juan, Y., Yong-Kang, L. and Yan-Chun, Z., 2008. Protein and amino acids composition of donkey meat and comparison between donkey meat and other livestock and poultry meat. Prod. Process., 12: 93-95.
Juan, Y., Yong-Kang, L., Yan-Chun, Z. and Zhe, Z., 2008. Nutrition composition of donkey meat and comparison with other livestock and poultry meat. Meat Res., 7: 20-22.
Kristinsson, H.G. and Rasco, B.A., 2000. Fish protein hydrolysates: Production, biochemical, and functional properties. Fd. Sci. Nutr., 40: 43-81. https://doi.org/10.1080/10408690091189266
Kurozawa, L.E., Park, K.J. and Hubinger, M.D. 2009. Influence of process conditions on enzymatic hydrolysis kinetics of chicken meat. J. Fd. Sci. Technol., 29: 557-566.
Kurozawa, L.E., Park, K.J. and Hubinger, M.D., 2008. Optimization of the enzymatic hydrolysis of chicken meat using response surface methodology. J. Fd. Sci., 73: 405-412. https://doi.org/10.1111/j.1750-3841.2008.00765.x
Li, J.F., Wang, Y. and Lu, D.L., 2018. The meat nutrition and medicinal value of donkey. Xinjiang Anim. Husband., 33: 11-16.
Ling-Ling, L., Xiang-Shu, J. and Su-Yun, S., 2013. Extraction of Rana chensinensis skin collagen by papain and its antioxidant activity. Fd. Res. Dev., 34: 20-23.
Lusha, W., Yulian, C. and Guanghong, Z., 2015. Antioxidant activities of protein hydrolysates from duck meat. J. Fd. Sci., 36: 146-151.
Nurilmala, M., Pertiwi, R.M. and Ochiai, Y., 2019. Characterization of collagen and its hydrolysate from yellowfin tuna Thunnus albacares skin and their potencies as antioxidant and antiglycation agents. Fish. Sci., 85: 591-599. https://doi.org/10.1007/s12562-019-01303-5
Pasqualin, C.C., Luisa, L.F. and Martins, F.L., 2014. Replacement of mechanically deboned chicken meat with its protein hydrolysate in mortadella-type sausages. J. Fd. Sci. Technol., 34: 478-484. https://doi.org/10.1590/1678-457x.6370
Sidorova, I.S., Seliaskin, K.E. and Mazo, V.K., 2014. Influence of enzymatic hydrolyzate of mussel meat on growth and some indicators of general adaptation syndrome in rats. Vopr. Pitan., 83: 22-28.
Silva, V.M., Park, K.J. and Hubinger M.D., 2010. Optimization of the enzymatic hydrolysis of mussel meat. J. Fd. Sci. Technol., 75: 36-42. https://doi.org/10.1111/j.1750-3841.2009.01414.x
Smith, D.G. and Pearson, R.A., 2005. A review of the factors affecting the survival of donkeys in semi-arid regions of Sub-Saharan Africa. Trop. Anim. Hlth. Prod., 37: 1-19. https://doi.org/10.1007/s11250-005-9002-5
Tian, X., Dan, W. and Ning, Z., 2018. Identification of flavor-active compounds in spiced donkey meat by odor activity value(oav) calculation and gas chromatography-olfactometry-mass spectrometry. Fd. Sci., 8: 123-128.
Wang, D. and Shahidi, F., 2018. Protein hydrolysate from turkey meat and optimization of its antioxidant potential by response surface methodology. Poult. Sci., 97: 1824-1831. https://doi.org/10.3382/ps/pex457
Wang, R.H. and Liu, W.Q., 2019. The incidence and pathogens of donkey dermatoses in large scale donkey farms of liaocheng district donkey. Heilongjiang Anim. Sci. Vet. Med., 5: 75-78, 180-181.
Weng, W., Li, J. and Li, T., 2019. Antioxidant properties and arsenic speciation of ultrafiltration and nanofiltration derived abalone viscera hydrolysate fraction. J. Aquat. Fd. Prod. Technol., 7: 1-10. https://doi.org/10.1080/10498850.2018.1561570
Xue, S., Baohua, K. and Qian, L., 2011. Preparation carp protein antioxidant activity peptide using enzymatic hydrolysis. J. Northeast Agric. Univ., 42: 32-38.
Yang, B., Yang, H. and Jiang, Y., 2011. Amino acid composition, molecular weight distribution and antioxidant activity of protein hydrolysates of soy sauce lees. Fd. Chem., 124: 551-555. https://doi.org/10.1016/j.foodchem.2010.06.069
Yang, Y.X., Wu, X. and Wang, G.Y., 2018. China food composition tables, 6th edn. Peking University Medical Press, China.
Yasemi, M., Ghomi, M.R. and Darnahal, T., 2013. Yelid of protein recovery and degree of hydrolysis associated protein hydrolysates from Bighead Carp (Aristichthys nobilis) by using enzymes. Iran J. Fish. Sci., 22: 149-156.
Youle, Q., Yinfeng, X. and Bin, W., 2011. Preparation and antioxidant properties of the hydrolysate and frations from Mytilus edulis by papain: Antioxidant hydrolysate and frations from Mytilus edulis. In: 2011 International Conference on Remote Sensing, Environment and Transportation Engineering, Nanjing. Institute of Electrical and Electronics Engineers, Piscataway, New Jersey, United States, pp. 8403-8406. https://doi.org/10.1109/RSETE.2011.5964116
Zhang, Y., Sun, W. and Zhao, M., 2015. Improvement of the ace-inhibitory and DPPH radical scavenging activities of soya protein hydrolysates through pepsin pretreatment. Int. J. Fd. Sci. Tech., 50: 2175-2182. https://doi.org/10.1111/ijfs.12856
Zhao, J., Huang, G. and Jiang, J., 2013. Purification and characterization of a new DPPH radical scavenging peptide from shrimp processing by-products hydrolysate. J. aquat. Fd. Prod. Technol., 22: 281-289. https://doi.org/10.1080/10498850.2011.645125
Zhao, W.H., Luo, Q.B. and Pan, X., 2018. Preparation, identification, and activity evaluation of ten antioxidant peptides from protein hydrolysate of swim bladders of miiuy croaker (Miichthys miiuy). J. Funct. Fds., 47: 503-511. https://doi.org/10.1016/j.jff.2018.06.014
To share on other social networks, click on any share button. What are these?










