Atef Mohamed El-Sagheer
Agricultural Zoology and Nematology Department, Faculty of Agriculture, Al-Azhar University, Assiut (71524), Egypt.
Abstract | The fields of banana (Musa sapientum L.) cultivations were surveyed for the prevalence of phytonematodes in Assiut governorate at middle of Upper Egypt. The encountered phytonematodes were identified during the survey belong to sixteen genera comprising sixteen genera belonging to nine families viz., Criconemella, Criconemoides, Ditylenchus, Helicotylenchus, Heterodera, Hoplolaimus, Longidorus, Meloidogyne, Paratylenchus, Pratylenchus, Radopholus, Rotylenchulus, Rotylenchus, Tylenchorhynchus, Tylenchus and Xiphinema. It was observed that there was variability in population density, frequency of occurrence and prominence value of some plant parasitic genera e.g., Meloidogyne in Abo-Teg and East Mangabad has the most prominence value (17.20 and 19.80) followed by Longidorus and Radopholus in Manfalot (17.20 and 15.36), respectively. The lowest prevalent genus was Heterodera which was absent in two surveyed localities viz., Abo-Teg and Assiut regions but present in Manfalot and East Mangabad with the prominence value 1.82 and 0.22 and population densities 43 and 11, respectively.
Received | August 21, 2020; Accepted | November 10, 2020; Published | December 21, 2020
*Correspondence | A.M. El-Sagheer, Agricultural Zoology and Nematology Department, Faculty of Agriculture, Al-Azhar University, Assiut (71524), Egypt; Email: [email protected]; [email protected]
Citation | El-Sagheer, A.M. 2020. Status of phytonematodes in a main commercial banana production of upper Egypt. Pakistan Journal of Nematology, 38(2): 156-160.
DOI | http://dx.doi.org/10.17582/journal.pjn/2020/.38.2.156.160
Keywords | Phytonematodes, Survey, Banana, Population density, Occurrence %, Prominence value, Upper Egypt
Introduction
Phytonematodes are the most destructive group of plant pathogens worldwide and their control is extremely challenging (Trudgill and Blok, 2001; Karssen, 2002). They parasitize a large variety of crops worldwide and their impacts on yield losses have been estimated to a billion of euros annually (Sikora and Fernandez, 2005; El-Sagheer, 2019).
Nematodes are a major pest of banana crop throughout the world; several phytonematodes attack banana and other crops in the subtropics causing economic damage that is a serious threat on the world crop production and to the world economy (Abd-Elgawad and Askary, 2015). Finally, there is the loss sustained by the world community, especially in those parts of the world that are struggling against food and raw material shortages, population growth and slow development (Oka, 2010). These losses are not confined to food crops. They affect also the cash crops which form a major source of revenue to the farmers and to the industry (Upadhyay et al., 1972; Sikora and Fernandez, 2005). This work aims to study the distribution and prevalence of phytonematode genera associated with banana (Musa sapientum L.) in middle of Upper Egypt as one of the important production areas of banana in Egypt.
Materials and Methods
Sampling sites and collection
A total number of 450 soil and root samples were collected form rhizosphere of banana plants cv. maghrabi, williams, assiuty and hindi at an age of 1-3 years from Assiut Governorate, near the coast of the Nile River and for a distance of 10 km which represented one of the largest commercial production areas for banana in Upper Egypt (Figure 1). The texture of the soils ranged between silt and heavy clay soils, during April to November, 2019. Samples were always taken within 30-90 cm distance from the pseudo-stem at a depth of 20-30 cm, kept in polyethylene bags and directly sent to the laboratory for nematode extraction.
Nematode extraction, numeration and identification
The soil samples (250 g) were processed by some different methods to extract various genera of phytonematodes; Cobb’s decanting and sieving methods (Cobb, 1918) to extract the phytonematodes with different shapes and sizes, Seinhorst wet method (Seinhorst, 1964) and Stirring method was used to extract the Heterodera (Moens and Viaene, 2003) from the samples (100 g), Automotive Zonal Centrifugation and floatation method (Hendrickx, 1995) was used for phytonematodes extraction from roots samples. The extracted phytonematodes were counted in Hawksly slide under stereomicroscope and identified up to genera under compound microscope according to Golden (1971) and Mai and Lyon (1975); root-knot nematodes were identified depending on morphological characteristics of female perennial patterns according to Eisenback et al. (1981). Phytonematodes were estimated by the following formula (Norton, 1987):
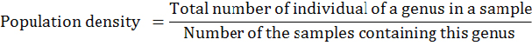
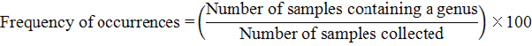

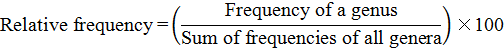
Results and Discussion
The distribution and population density of nematodes commonly in-habiting the rhizospheres of banana plants in different localities at Assiut Governorate were studied. Data presented in Table 1 revealed the presence of sixteen phytonematodes genera belonging to nine families from the rhizospheres of banana plantations at the examined localities. These genera were Criconemella (Criconematidae), Criconemoides (Criconematidae), Ditylenchus (Anguinidae), Helicotylenchus (Hoplolaimidae), Heterodera (Heteroderidae), Hoplolaimus (Hoplolaimidae), Longidorus (Longidoridae), Meloidogyne (Meloidogynidae), Pratylenchus (Pratylenchidae), Paratylenchus (Paratylenchidae), Radopholus (Pratylenchidae), Rotylenchulus (Hoplolaimidae), Rotylenchus (Hoplolaimidae), Tylenchorhynchus (Tylenchorhynchidae), Tylenchus (Tylenchidae) and Xiphinema (Longidoridae).
Generally, it was observed that the population density and frequency of occurrence of some genera was affected by the extent and type of agricultural treatments; weed removal or planting forage crops. Also, the study showed an absence of some genera, especially in heavy clay soils in the surveyed localities, as we move away from the Nile River coast to the valley soils, such as absence of Heterodera in Abo-Teg and Assiut districts and Ditylenchus in Abo-Teg. The population densities, frequencies of occurrence, absolute densities and prominence values of nematode genera in examined localities differed clearly according to locality and soil type. The genera Meloidogyne, Paratylenchus, Longidorus, Xiphinema, Tylenchorhynchus, Pratylenchus, Helicotylenchus, Hoplolaimus and Rotylenchulus were the most prevalent nematode genera with 100% occurrence as they were found in the all surveyed localities. In Manfalot the genera of Meloidogyne, Longidorus and Tylenchorhynchus occurred with prominence values 15.40, 17.20 and 15.30 and population densities of 154, 172 and 153, respectively. While in East Mangabad the second highest prominence values of 19.80, 11.60 and 16.90 with 198, 116 and 169 population densities were observed. In Abo Teg the prominence value rate of 17.20, 14.80 and 14.80 with population densities of 172, 148 and 148, correspondingly was noted. While in the East Assiut region the least occurrence of these genera was recorded.
Furthermore, the genera Criconemoides, Rotylenchus and Tylenchus showed moderate occurrence in most regions, the population densities of the genus Criconemoides ranged between 53 in Manfalot and 20 in East Assiut region. On the other hand, the genera Heterodera, Criconemella and Ditylenchus showed the lowest occurrence and prevalence value as compared to other genera; the genus Heterodera was absent in two surveyed localities viz., Abo-Teg and Assiut regions but occurred in Manfalot and East Mangabad with 1.82 and 0.22 prominence values with population densities 43 and 11, respectively. Also Ditylenchus was absent in East Assiut region, but it was found on a limited scale in Abo-Teg, East Assiut and Manfalot regions with prominence values 0.40, 2.39 and 2.92, respectively.
Generally, through this study the genus Radopholus is considered as the most dispersed in the distribution and was found in East Mangabad with 100% frequency of occurrence and prominence values 9.80, while in Abo-Teg region recorded 38 and 12.21, respectively as estimated previously.
The present study revealed the presence of sixteen phytonematode genera belonging to nine families associated with banana plantations in four locations of Assiut Governorates, Egypt. Out of these, 8-9 genera were found consistently with 100% frequency of occurrence in all surveyed sites. However, they differed in their prominence values and population densities which may be due to the soil type, cultivars of crop and other agronomic practices as reported earlier (Upadhyay et al., 1972; Naz et al., 2015) or the presence of a potential alternative hosts such as weeds (Kokalis and Rosskopf, 2012). Also, Ibrahim and Handoo (2016) reported that the genera Tylenchulus, Xiphinema, Hoplolaimus, Pratylenchus, Criconema, Meloidogyne, Helicotylenchus and Tylenchorhynchus, were the most frequently encountered nematode genera in Egyptian soils. Banana is the main fruit crop of the surveyed area which liesalong the coastal belt of Nile River. These crops are grown continuously for many years which may cause increase in the build-up of nematodes populations and may become potential threat to the banana plantations as these nematodes are major damaging pests of banana crop. Therefore, there is an urgent need to carry out further research on the biodiversity, prevalence, population dynamics and management of nematodes of this economically important crop.
Table 1: Prevalence status of phytonematodes associated with banana plantations at Assiut Governorates, Egypt.
|
Phytonematode genera |
Abo-Teg n=100 |
Assiut n=65 |
East Mangabad n=110 |
Manfalot n=110 |
|||||||||||
|
PD |
FO% |
PV |
PD |
FO% |
PV |
PD |
FO% |
PV |
PD |
FO% |
PV |
||||
|
Criconemella |
18 |
46 |
1.22 |
11 |
69 |
0.91 |
34 |
61 |
2.66 |
31 |
59 |
2.38 |
|||
|
Criconemoides |
30 |
79 |
2.67 |
20 |
88 |
1.88 |
47 |
100 |
4.70 |
53 |
89 |
5.00 |
|||
|
Ditylenchus |
11 |
13 |
0.40 |
0 |
0 |
0.00 |
58 |
17 |
2.39 |
43 |
46 |
2.92 |
|||
|
Helicotylenchus |
122 |
100 |
12.20 |
103 |
100 |
10.30 |
217 |
100 |
21.70 |
149 |
18 |
6.32 |
|||
|
Heterodera |
0 |
0 |
0 |
0 |
0 |
0 |
11 |
4 |
0.22 |
43 |
18 |
1.82 |
|||
|
Hoplolaimus |
61 |
100 |
6.10 |
43 |
100 |
4.30 |
109 |
100 |
10.90 |
56 |
100 |
5.60 |
|||
|
Longidorus |
148 |
100 |
14.80 |
78 |
100 |
7.80 |
116 |
100 |
11.60 |
172 |
100 |
17.20 |
|||
|
Meloidogyne |
172 |
100 |
17.20 |
101 |
100 |
10.10 |
198 |
100 |
19.80 |
154 |
100 |
15.40 |
|||
|
Paratylenchus |
103 |
100 |
10.30 |
105 |
100 |
10.50 |
148 |
100 |
14.80 |
98 |
100 |
9.80 |
|||
|
Pratylenchus |
125 |
100 |
12.50 |
126 |
100 |
12.60 |
157 |
100 |
15.70 |
143 |
100 |
14.30 |
|||
|
Radopholus |
198 |
38 |
12.21 |
176 |
100 |
17.60 |
98 |
100 |
9.80 |
229 |
45 |
15.36 |
|||
|
Rotylenchus |
54 |
98 |
5.35 |
38 |
69 |
3.16 |
104 |
100 |
10.40 |
42 |
61 |
3.28 |
|||
|
Rotylenchulus |
39 |
100 |
3.90 |
45 |
100 |
4.50 |
47 |
72 |
3.98 |
54 |
100 |
5.40 |
|||
|
Tylenchorhynchus |
148 |
100 |
14.80 |
112 |
100 |
11.85 |
169 |
100 |
16.90 |
153 |
100 |
15.30 |
|||
|
Tylenchus |
57 |
48 |
3.95 |
38 |
45 |
2.34 |
61 |
56 |
4.56 |
72 |
100 |
7.20 |
|||
|
Xiphinema |
113 |
100 |
11.30 |
148 |
88 |
18.00 |
98 |
100 |
9.80 |
126 |
100 |
12.60 |
|||
|
Total PD |
1399 |
- |
- |
1144 |
- |
- |
1672 |
- |
- |
1618 |
|||||
n: Number of total samples collected from each district; PD: Population densities; FO: Frequency of occurrences (%); PV: Prominence value.
Novelty Statement
Consider the first report about phytonematodes pests as a major factor in limit of the productivity of the banana crop in an economic importance production area of Upper Egypt, and opening the way for future studies of this area.
Author’s Contribution
Atef Mohamed El-Sagheer did all work that associated with this manuscript.
Funding
This research did not receive any research funds.
Availability of data and materials
All data regarding this study are shown in the manuscript.
Conflict of interest
The authors have declared no conflict of interest.
References
Abd-Elgawad, M.M.M. and Askary, T.H., 2015. Impact of phytonematodes on agriculture economy. Biocontrol agents of phytonematodes/ Ed. by Askary, T.H. and Martinelli, P.R.P. CAB International, Wallingford, UK, pp. 3-49. https://doi.org/10.1079/9781780643755.0003
Cobb, N.A., 1918. Estimating the nema population of soil. Agric. Technol. Circle US Dept. Agric., 1: 48.
Eisenback, J.D., Hirschmann, H., Sasser, J.N. and Triantaphyllou, A.C., 1981. A guide to the four common species of root-knot nematodes (Meloidogyne spp.) with a pictorial key. A cooperative publication of department of plant pathology and genetics, North Carolina State University and USAID, Raleigh, North Carolina, pp. 48
El-Sagheer, A.M., 2019. Plant responses to phytonematodes infestations. Plant health under biotic stress. Vol. 2. Ed. by Ansari, R.A. and Mahmood, I. Singapore: Springer, pp. 161-175. https://doi.org/10.1007/978-981-13-6040-4_8
Golden, A.M., 1971. Classification of the genera and the higher categories of the order Tylenchida. Plant parasitic nematodes morphology, anatomy, taxonomy and ecology, Vol. 1. Ed. by Zuckerman, B.M., Mai, W.F. and Rhode, R.A. Academic Press, Inc. New York. pp. 191-230. https://doi.org/10.1016/B978-0-12-782201-3.50014-9
Hendrickx, G., 1995. An automatic apparatus for extracting free-living nematodes stages from soil. Nematologica, 41: 308.
Ibrahim, I.K.A. and Handoo, Z.A., 2016. Occurrence of phytoparasitic nematodes on some crop plants in northern Egypt. Pak. J. Nematol., 24: 163-169. https://doi.org/10.18681/pjn.v34.i02.p163
Karssen, G., 2002. The plant parasitic nematode genus Meloidogyne Göldi, 1892 (Tylenchida) in Europe. Brill, Leiden, Boston, pp. 257.
Kokalis-Burelle, N. and Rosskopf, E.N., 2012. Susceptibility of several common subtropical weeds to meloidogyne arenaria, M. incognita, and M. javanica. J. Nematol., 44: 142.
Mai, W.F. and Lyon, H.H., 1975. Pictorial key to genera of plant parasitic nematodes. 4th Ed., Ithaca and London, Cornell University Press, pp. 219.
Moens, M. and Viaene, N., 2003. General techniques used in nematology. International nematology course. University of Ghent, Gent, Belgium.
Naz, I., Palomares-Rius, J.E., Khan, S.M., Ali, S., Ahmad, M., Ali, A. and Khan, A., 2015. Control of Southern root knot nematode Meloidogyne incognita (Kofoid and White) Chitwood on tomato using green manure of Fumaria parviflora Lam (Fumariaceae). Crop Prot., 67; 121-129. https://doi.org/10.1016/j.cropro.2014.10.005
Norton, D.C., 1987. Plant nematode communities. Int. J. Parasitol., 17: 215-222. https://doi.org/10.1016/0020-7519(87)90044-0
Oka, Y., 2010. Mechanisms of nematode suppression by organic soil amendments. A review. Appl. Soil Ecol., 44(2): 101-115. https://doi.org/10.1016/j.apsoil.2009.11.003
Seinhorst, J.W., 1964. Methods for the extraction of Heterodera cysts from not previously dried soil. Nematologica, 10: 87-94. https://doi.org/10.1163/187529264X00664
Sikora, R.A. and Fernandez, E., 2005. Nematode parasites of vegetables. Plant parasitic nematodes in subtropical and tropical agriculture, 2nd Edition/ Ed. by Luc, M., Sikora, R. A. and Bridge, J., CABI Publishing, Wallingford, UK. pp. 319-392. https://doi.org/10.1079/9780851997278.0319
Trudgill, D.L. and Blok, V.C., 2001. Apomictic, polyphagous root-knot nematodes: Exceptionally successful and damaging biotrophic root pathogens. Annu. Rev. Phytopathol., 39: 53-77. https://doi.org/10.1146/annurev.phyto.39.1.53
Upadhyay, R.S., Oostenbrink, M. and Khan, A.M., 1972. The effect of different soil types on the density of nematode populations. Indian J. Nematol., 2: 42-53.
To share on other social networks, click on any share button. What are these?




