Spatial Hotspots of Mosquitoes (Diptera: Culicidae) in Attock, Pothwar, Punjab, Pakistan
Research Article
Spatial Hotspots of Mosquitoes (Diptera: Culicidae) in Attock, Pothwar, Punjab, Pakistan
Arif Mehmood1*, Muhammad Asam Riaz1, Abu Bakar Muhammad Raza1, Waqas Raza2, Muhammad Zeeshan Majeed1
1Department of Entomology, College of Agriculture, University of Sargodha, Pakistan; 2Plant Pathology Department, College of Agriculture, University of Sargodha, Pakistan.
Abstract | Hotspot information leads to precision in the application of control strategies. Mosquitoes inhabit diverse types of habitats depending upon host preference, biotic and abiotic characteristics of the habitats. This study mainly targeted the collection, identification, and sorting of mosquitoes habitat-wise in the Attock district of Pothwar region. Collection of specimens was done from specified habitats during 2014-16. A total of fourteen species belonging to four genera were collected from eight different habitats. The most abundant habitats were recreational parks, followed by residential areas and forests, while the least abundant habitats were graveyards. Maximum Simpson index (0.90) and Shanon Index (2.45) was observed in residential area, while maximum evenness (0.97) was recorded in streams. Quantitative habitat web structures were used for the first time to present habitat distribution graphically. These webs and diversity indices provide hotspot information, and hotspot information is always an efficient tool in precise control of mosquitoes in the form of epidemic occurrence.
Received | May 13, 2022; Accepted | June 15, 2022; Published | November 17, 2022
*Correspondence | Arif Mehmood, Department of Entomology, College of Agriculture, University of Sargodha, Pakistan; Email: amsentomologist@gmail.com
Citation | Mehmood, A., M.A. Riaz, A.B.M. Raza, W. Raza, M.Z. Majeed. 2022. Spatial hotspots of mosquitoes (Diptera: Culicidae) in Attock, Pothwar, Punjab, Pakistan. Sarhad Journal of Agriculture, 38(5): 60-64.
DOI | https://dx.doi.org/10.17582/journal.sja/2022/38.5.60.64
Keywords | Habitat web, Quantitative habitat webs, Diversity, Diversity index, Mosquitoes, Mosquitoes of Attock
Copyright: 2022 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Mosquitoes (Diptera: Culicidae) cause annoyance and transmit lethal diseases in humans and animals. These flies are present all over the world, including tropical regions and temperate regions (Sami-ur-Rehman et al., 2017). Mosquitoes transfer many lethal diseases in humans, like malaria, dengue, yellow fever, Zika Virus, Eastern Equine Encephalitis Virus, etc. Malaria causes almost 400,000 deaths every year, while dengue causes almost 40,000 deaths each year (Batzer et al., 2020).
Several factors regulate the population of mosquitoes; ecological characteristics are the most important. These characteristics are mainly related to the habitat. Habitats also provide important information about the specific insect like preferred host of the insect (Carpenter and LaCasse, 1955). The population and diversity of mosquitoes are highly dependent on different biotic and abiotic characteristics of the habitat (Reisen, 2010; Mehmood et al., 2021). The human-loving mosquitoes usually prefer natural and undisturbed habitats (Burkett-Cadena et al., 2013).
Mosquitoes usually aggregate around the preferred host. It is not their habit to aggregate around the commonly available host. They like greater diversity of hosts (Burkett-Cadena et al., 2011). They also have the tendency to adopt different environments.
The microhabitats, where usually the problem of waste disposal, drainage, and poor water supply exists are the most abundant habitats of mosquitoes, as these find hiding and breeding places in these habitats frequently than clean and better drainage containing habitats (Opoku et al., 2007). Microhabitats which contain slow running water, hoof prints, and vegetation are preferred by mosquitoes (Dida et al., 2018).
Habitat distribution of mosquitoes provides a map of the mosquitoes in relation to their habitats, thus in case of any epidemic spread in an area, this map gives the information of hotspot of any mosquitoes in that region, so by associating emergence of vector borne diseases with the vector and their hotspots, this study make the application of control practices much easier and precise.
Materials and Methods
This study was conducted during 2014-16, which covered Attock district of Pothwar region. The microhabitats included graveyard, scrap yard, park, forest area, crop area, streams, houses and animal sheds. Surveys of these microhabitats were made on fortnightly basis.
Mosquitoes were collected with the help of aerial net, mouth operated aspirator and light traps in early morning and evening. Mosquito specimens collected from the said habitats were sorted quantitatively and qualitatively in PMAS Arid Agriculture University, Rawalpindi, Department of Entomology, Biosystematics laboratory. Identification was done under the Labomed microscope CZM6 using taxonomic keys, including Barraud (1936), Tyagi et al. (2015) and Qasim et al. (2014). The identified specimens were preserved in wooden boxes and deposited in the museum of Biosystematics Laboratory of Pir Mehr Ali Shah Arid Agriculture University.
The habitat distribution was represented by webs made in Coral Draw X6, while diversity indices were determined by PAST (4.0) software. To draw the webs, the scale used was determined by the following formula.

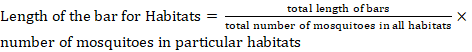
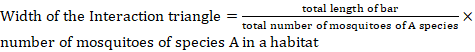
Results and Discussion
Pothwar region, Punjab, Pakistan covers four districts, including Attock, Jhelum, Rawalpindi and Chakwal. In this study, Attock district was chosen for exploration of mosquito fauna. Quantification of species-habitat linkage through a web structure is newly introduced; provide a more robust description of this linkage.
Simpson, Shanon, and evenness indices, which show richness, abundance, and evenness respectively, were recorded for the microhabitats in this area of Pothwar. The diversity information tell about the preferred niche of mosquito population (Leal-santos et al., 2017). Maximum Simpson index (0.90) was recorded in residential areas, while the least Simpson index (0.44) was recorded in graveyard, followed by crop area (0.67), animal shed (0.73), stream (0.73), scrapyard (0.78), forest area (0.87), and park (0.90) respectively. Maximum Shanon index (2.45) was recorded in residential area, while the least (0.63) was recorded in graveyard, followed by crop area (1.20), animal shed (1.35), stream (1.35), scrap yard (1.64), forest area (2.11), park (2.40) and residential area (2.45) respectively. Maximum evenness (0.97) was recorded in stream, while the least (0.83) in crop area, followed by scrap yard (0.85), residential area (0.89), forest area (0.91), graveyard (0.94), and animal shed (0.96) respectively (Table 1).
In this web, a total of 14 species comprising of 257 specimens belonging to genus Culex (8), Anopheles (2), Armigeres (2) and Aedes (2) in an interaction with different habitats was shown (Figure 1).
Table 1: Diversity Indices of mosquitoes found in Attock district during 2014-16.
|
Habitats |
Animal shed |
Scrap yard |
Graveyard |
Park |
Forest area |
Crop area |
Residential area |
Stream |
|
Diversity indices |
||||||||
|
Simpson Index |
0.73 |
0.78 |
0.44 |
0.90 |
0.87 |
0.67 |
0.90 |
0.73 |
|
Shannon Index |
1.35 |
1.64 |
0.63 |
2.40 |
2.11 |
1.20 |
2.45 |
1.35 |
|
Evenness |
0.96 |
0.85 |
0.94 |
0.92 |
0.91 |
0.83 |
0.89 |
0.97 |
Anopheles stephensi and Anopheles splendidus shared the same habitats, including animal sheds and residential area. High abundance of Anopheles stephensi and Anopheles splendidus was observed in animal sheds. We collected Anopheles splendidus from houses and animal sheds, while Ilahi and Salman (2013) had collected from rice fields. Our results are not in accordance with the findings of Ilahi and Salman (2013) it is because Anopheles splendidus like human and animal activity areas.
Culex cornotus was collected from four habitats, including animal sheds, crops, park and residential area. The highest abundance was found from animal sheds and the lowest abundance was recorded from residential area and crop area. Our findings are in partial accordance with Tyagi et al. (2015).
Culex nilgiricus was collected from the habitats, including scrap yard, park, forest area, crop area and residential area. Culex nilgiricus specimens were found in the same abundance from all the habitats. Our results are in concordance with Khan et al. (2015) and Tyagi et al. (2015). Culex theileri was collected from five habitats, including scrap yard, park, forest area, crop area and residential area. The abundances found from these habitats were the same. Banafshi et al. (2013), Ilahi and Salman (2013), collected Culex theileri from stagnant water, seepage pools, field crops. We have collected Culex theileri from these habitats as well as from scrapyard, park, forest area and houses. This shows that Culex theileri likes the places with high vegetation and human activity.
Culex vagans was collected from four habitats, including scrap yard, park, forest area and residential area. The abundances found from these habitats were the same. Culex vagans was found from scrap yard, park, crop area, forest area, houses, graveyard and animal shed. We collected Anopheles stephensi from houses, animal sheds and streams as was reported by Ali et al. (2013) and (2015).
Culex seniori and Culex tenuipalpis were found in the same habitats, including park and residential area. The abundances found from these habitats were the same. Culex pluvialis was collected from four habitats, including animal sheds, park, forest area and residential area. The abundances found from these habitats were the same. The findings of this study are in partial accordance with Tyagi et al. (2015).
Armigeres obturbans was collected from three habitats, including parks, forest area and stream. The highest abundance was observed in parks and the lowest abundance was found in both forest area and stream. Our results are in accordance with Rajput and Kulkarni (1991), Rajput and Singh (1990), Ilahi and Salman (2013) and Mehmood et al. (2016). Our results are in partial accordance with Ali et al. (2015) as we have not found any Armigeres obturbans from houses. This may be due to the preference of high vegetation and humidity.
Armigeres kuchingensis was collected from four habitats, including parks, forest area, stream and residential area. The abundances found from these habitats were the same. Our results are in conformity with Tyagi et al. (2015).
Aedes aegypti and Aedes albopictus shared the same habitats, including scrap yard, graveyard, parks, residential area, forest area and streams. Aedes albopictus specimens were equally abundant in all the habitats while Aedes aegypti was present in high abundances in parks, forest area, residential area and streams and in the low abundance in scrap yard and graveyard. Aedes aegypti was recorded from six habitats, including stream, park, forest area, residential area, grave yard and scrap yard the abundance was found low in graveyard and scrap yard, where the humidity was low, vegetation was less and the human movement and activities were less. Fakoorziba and Vijayan (2008) had the same findings as ours, but we collected Aedes albopictus from residential areas and crops also, which he had not, this is because Aedes albopictus is much more adaptive in nature.
Acknowledgments
I am thankful to PSF and PMAS Arid Agriculture University Rawalpindi for funding this project and providing infrastructure.
Novelty Statement
To provide hotspot information, the quantitative web structures have been used in this study for the first time, these structures were in use to express food relations and tritrophic interactions. This is a novel approach in presenting spatial distribution.
Author’s Contribution
Arif Mehmood: Conducted the research, analysed the data and wrote the manuscript.
Muhammad Asam Riaz, Abu Bakar Muhammad Raza, Waqas Raza and Muhammad Zeeshan Majeed: Analysed the data and reviewed and edited the manuscript,
Conflict of Interest
There is no conflict of interest regarding the publication of this manuscript.
References
Ali, N., K. Khan and A. Kausar. 2013. Study on mosquitoes of Swat Ranizai sub division of Malakand. Pak. J. Zool., 45 (2): 503-510.
Ali, N., S. Noreen, K. Khan and S. Wahid. 2015. Population dynamics of mosquitoes and malaria vector incrimination in district Charsadda, Khyber Pakhtunkhwa (KP) Pakistan. Acta Tropica., 141: 25-31. https://doi.org/10.1016/j.actatropica.2014.08.020
Banafshi, O., M. Abai, H. Ladonni, H. Bakhshi, H. Karami and S. Azari-Hamidian. 2013. The fauna and ecology of mosquito larvae (Diptera: Culicidae) in Western Iran. Turk. J. Zool., 37: 298-307.
Barraud, P.J. 1934. The fauna of British India, including Ceylon and Burma. Diptera, Family Culicidae. Tribe Megarhinini and Culicini. Taylor and Francis, London, 455pp.
Barrera, R., M. Amador and A.J. MacKay. 2011. Population dynamics of Aedes aegypti and dengue as influenced by weather and human behavior in San Juan,Puerto Rico. Plos Neglected Trop. Dis., 5 (12):1-9. https://doi.org/10.1371/journal.pntd.0001378
Burkett-Cadena, N.D., C.J.W. McClure, R.A. Ligon, S.P. Graham, C. Guyer, G.E. Hill, S.S. Ditchkoff, M.D. Eubanks, H.K. Hassan and T.R. Unnasch. 2011. Host reproductive phenology drives seasonal patterns of host use in mosquitoes. Plos One, 6 (3): 1-7. https://doi.org/10.1371/journal.pone.0017681
Burkett-Cadena, N.D., C.J.W. McClure, L.K. Estup and M. D. Eubanks, 2013. Hosts or Habitats: What Drive the Spatial Distribution of Mosquitoes? Ecosphere. 4 (2): 1-16. https://doi.org/10.1890/ES13-00009.1
Batzer, D.P., Mehmood, A., Mead, D.G. and Champagne, D.E. 2020. Phenology of Coquillettidia perturbans and Culiseta melanura (Diptera: Culicidae) in East-Central Georgia, USA: Implications for the Ecology of Eastern Equine Encephalitis Virus. J. Entomol. Sci., 55(2): 156–162. https://doi.org/10.18474/0749-8004-55.2.156
Dida, G.O., Anyona, D.N., Abuom, P.O., Akoko, D., Adoka, S.O., Matano, A.S., Owuor, P.O. and Ouma, C. 2018. Spatial distribution and habitat characterization of mosquito species during the dry season along the Mara River and its tributaries, in Kenya and Tanzania. Infect. Dis. Poverty, 7(1): 2. https://doi.org/10.1186/s40249-017-0385-0
Carpenter, S.J. and W.J. Lacasee. 1955. Mosquitoes of North America. University of California Press Berkeley, Los Angeles, London, 1974: 361 pp.
Fakoorziba, M.R. and A. Vijayan. 2008. Breeding habitats of Culex tritaeniorhynchus (Diptera: Culicidae), a Japanese encephalitis vector, and associated mosquitoes in Mysore, India. J. Entomol. Res. Soc., 10 (3): 1-9.
Ilahi, I. and M. Suleman. 2013. Species composition and relative abundance of mosquitoes in Swat, Pakistan. Int. J. Innov. Appl. Stud., 2 (4): 454-463.
Khan, I.A., M.M.U. Din, S. Hussain, R. Akbar, M. Saeed, A. Farid, W. Fayaz and R.A. Shah. 2015. A Study of Mosquito Fauna of District Upper Dir, Khyber Pakhtunkhwa-Pakistan. J. Entomol. Zool. Stud., 3(5): 455-458.
Leal-Santos, F.A., Jacobina, A.C.M., Oliveira, M.M. De, Arruda Santana, M.B., Serra, O.P., Gonçalves, A.V., Garcêz, A.R.S., Thies, S.F., Slhessarenko, R.D., Dantas, E.S. de O. and Leite-Jr, D.P. 2017. Species composition and fauna distribution of mosquitoes (Diptera: Culicidae) and its importance for vector-borne diseases in a rural area of Central Western -Mato Grosso, Brazil. Entomol. Brasilis, 10(2):94. https://doi.org/10.12741/ebrasilis.v10i2.687
Mehmood, A., M. Naeem and A. Mohsin. 2021.Seasonal Occurrence and Abundance of Mosquitoes in Pothwar Plateau. Pak. J. Zool., 1-7. https://doi.org/10.17582/journal.pjz/20180715070731
Mehmood, A., M. Naeem, I. Bodla and A. Mohsin. 2016. Systematics of Anopheles and Armigeres (Culidae: Diptera) in Pothwar Region, Punjab, Pakistan. Intl. J. Mosq. Res., 3(5): 5-10.
Poveda, G., N.E. Graham, P.R. Epstein,W. Rojas, I.D. Velez, M.L. Quinones and P. Martens. 1999. Climate and ENSOvariability associated to malaria and dengue fever in Colombia. Proc. Symp. Glob. Change Stud. Boston: Am. Meteorol. Soc., 10: 173-76.
Qasim, M., M. Naeem and I. Bodlah. 2014. Mosquito (Diptera: Culicidae) of Murree Hills, Punjab, Pakistan. Pak. J. Zool., 46 (2): 523-529.
Rajput, K.B. and T.K. Singh. 1990. Records of Anopheline mosquitoes collected from Manipur with Ecological Notes. Rec. Zool. Sur. Ind., 87 (3): 197-206.
Rajput, K.B., S.M. Kulkarni. 1990. Records of Culicine mosquitoes from Bastar district (Madhya Pradesh) India (Diptera: Culicidae), Part·I genus Toxorhynchites, Tripteroides, Uranotaenia and Orthopodomyia. Rec. Zool. Sur. Ind., 87 (1): 83-88.
Reisen, W.K. 2010. Landscape epidemiology of vector-borne diseases. Ann. Rev. Entomol., 55:461-483. https://doi.org/10.1146/annurev-ento-112408-085419
Sami-ur-rehman, M., Sami-ur-rehman, M., Nasir, M. F., Mehmood, A. and Mushtaq, M. 2017. Fauna of Culex (Culicidae ; Diptera ) mosquitoes in tehsil Pind Dadan Khan , Jhelum , Punjab , Pakistan. Intl. J. Mosq. Res., 4(5): 28–31.
Tyagi, B.K., A. Munirathinam and A. Venkatesh. 2015. A catalogue of Indian mosquitoes. Int. J. Mosq. Res., 2 (2): 50-97.
Opoku, A., O.D. Ansa-Asare and J. Amoako, 2007. The Occurrences and Habitat Characteristics of Mosquitoes in Accra, Ghana. West Afr. J. Appl. Ecol., 11 (1): 1-21. https://doi.org/10.4314/wajae.v11i1.45730
To share on other social networks, click on any share button. What are these?







