Biometric Traits and Ecology of Sichel, Pelecus cultratus (Linnaeus, 1758) with Notes on its Recent Status in the Middle Flow of the Danube River Tributaries (Slovenia and Croatia)
Biometric Traits and Ecology of Sichel, Pelecus cultratus (Linnaeus, 1758) with Notes on its Recent Status in the Middle Flow of the Danube River Tributaries (Slovenia and Croatia)
Merica Slišković1, Meta Povž2, Marina Piria3,*, Goran Jakšić4, Ana Gracanin5 and Gorana Jelić Mrčelić1
1University of Split, Faculty of Maritime Studies, Ruđera Boškovića 37, 21000 Split, Croatia
2Umbra, Institute of Natural Sciences, Ulica bratov Ucakar 108, 1000 Ljubljana, Slovenia
3University of Zagreb, Faculty of Agriculture, Department of Fisheries, Beekeeping, Game Management and Special Zoology, Svetošimunska 25, 10000 Zagreb, Croatia
4AQUATIKA–Freshwater Aquarium Karlovac, Branka Čavlovića Čavleka 1a, 47000 Karlovac, Croatia
5University of Wollongong, Centre for Sustainable Ecosystem Solutions, School of Biology, Northfields Ave. Wollongong, NSW 2522, Australia
ABSTRACT
Available knowledge on the sichel, Pelecus cultratus, from the middle Danube River and its tributaries is insufficient. This study aims to provide new data on the morphology and ecology of sichel from the Mur River, and its distribution in Croatia and Slovenia. In 2009, the schooling of sichel were observed at a high-water level in the Mur River of Slovenia. In total, 14 specimens were sampled by fishermen using sport fishing techniques. The age, condition, length-weight relationship (LWR), 20 morphometric and four meristic traits, were analysed. Fulton condition coefficient and LWR value indicated that sichel specimens’ were well adapted to the environmental conditions. Positive allometry in both males and females were observed (W = 0.004 L3.119). This represents the first LWR dataset for sichel. The morphometric and meristic traits values from this research add to the limited data that is presently available. By comparing historical and recent data on sichel distribution, we observed a dramatically declining trend. Therefore, further research and targeted protection of the sichel in the middle Danube River tributaries are urgently required.
Article Information
Received 15 May 2017
Revised 02 February 2018
Accepted 18 April 2018
Available online 19 November 2018
Authors’ Contribution
MP and MP designed the study and prepared the manuscript. MS, GJ and AG analyzed and interpreted the data and finalized the article. GJM supervised the work.
Key words
Freshwater fish, Danube basin, Distribution, Morphometric and meristic traits, Conservation.
DOI: http://dx.doi.org/10.17582/journal.pjz/2019.51.1.117.125
* Corresponding author: [email protected]
0030-9923/2019/0001-0117 $ 9.00/0
Copyright 2019 Zoological Society of Pakistan
Introduction
The sichel, Pelecus cultratus (Linnaeus, 1758), is unique among European Cyprinids of the family Leuciscinae (Kottelat and Freyhof, 2007), by inhabiting open waters of large rivers and lakes, estuaries and salt-rich seas (e.g. Baltic Sea) (Riede, 2004; Kottelat and Freyhof, 2007). It has a long, slender, highly compressed body with a sharp keel from throat to anus (Freyhof and Kottelat, 2008). The length of life for the sichel ranges between 9 to 13 years (Kottelat and Freyhof, 2007; Gaigalas, 2001). The maximum total length recorded for the species was 60.0 cm, with a weight of 2.0 kg (Muus and Dahlström, 1968). First spawning occurs between the ages of 3 and 5 during spring (Kottelat and Freyhof, 2007). Spawning sichel produce floating eggs, which occurs in the midstream (Vostradovsky, 1973) at temperatures above 12°C (Kottelat and Freyhof, 2007). The optimal water temperature for sichel ranges from 10°C to 20°C (Baensch and Riehl, 1991). In rivers, eggs drift with the current and hatch after three to four days (Freyhof and Kottelat, 2008). In their first summer, juveniles may potentially migrate towards estuaries (Kottelat and Freyhof, 2007). Immediately after spawning, this semi-anadromous adult fish returns to estuaries where it feeds on zooplankton, terrestrial invertebrates and small fish (Kottelat and Freyhof, 2007).
The main distribution area of the sichel belongs to the Ponto-Caspian region which is made up of the Black Sea, Caspian Sea, Azov Sea and the Aral Sea. In addition, it is common in the basins of the Baltic Sea, from the Odra River to the Neva River (Raczyński et al., 2011; Froese and Pauly, 2017). The western limit of its distribution occurs in Austria and Bavaria (Ratschan, 2015). The sichel also occurs in southern Sweden and Finland, in the Ladoga Lake and the Onega Lake, and occasionally on the Baltic coast west of Vistula, as well as on the Finnish coast north of 61°N (Kottelat and Freyhof, 2007).
Furthermore, the sichel was observed in the Mur River, the tributary of the Danube River, as a very rare and sporadic species (Mrakovčić et al., 2006). Although this species was recorded in several tributaries of the Mur River basin in north-eastern Slovenia (Munda, 1927; Glowacki, 1896), Sava River and its tributaries in Croatia, and Drava River (Mojsisovics, 1883; Brusina, 1892; Sabioncello, 1967), recent research did not confirm its presence (Jelić et al., 2012; Simonović et al., 2017).
The sichel is included in Annex II of the International Association for the Protection of Species of the European Commonwealth of the Baltic States, Fauna Flora Habitat (FFH) Directive (Balzer et al., 2004). It has been classified as Least Concern (LC) by the global and regional IUCN Red list of threatened species (Freyhof Kottelet, 2008). The sichel is not recorded in the Slovenian Red list of fish, but has been classified as a Data Deficient (DD) species by the Croatian Red list (Mrakovčić et al., 2006).
Several studies have focused on the species’ biology, nutrition, reproductive ecology (Herzig and Winkler, 1983; Herzig et al., 1994; Auer, 1995; Tátrai and Herzig, 1995; Liu and Herzig, 1996), morphology (Adamicka, 1984; Wais, 1995), ecology, systematics (Unger, 1926; Balon, 1956; Harsanyi, 1986; Kottelat and Freyhof, 2007), migration behaviour (Heckel and Knerr, 1858; Siebold, 1863; Jäckel, 1864; Seeley, 1886; Glowatcki, 1896; Munda, 1927) and distribution (Jurajda et al., 1992; Lusk and Jurajda, 1995). However contemporary knowledge is limited due to a lack of recent research.
Thus, the aim of this research was to (i) provide new data on the morphology and ecology of sichel, and (ii) investigate its historical and current distribution in the tributaries of the Danube River in Slovenia and Croatia. Furthermore, a critical discussion on present and future status of this species in inland waters of Slovenia and Croatia is also provided, with habitat conservation needs proposed.
Material and Methods
Study area and fish sampling
In Slovenia and Croatia, the main tributaries of the Danube River basin are the Sava and Drava Rivers. One of the Drava River tributaries is the Mur, a river in central Europe, rising from Hohe Tauern national park of the Central Eastern Alps in Austria. The Mur’s total length is around 480 kilometres, 330 km are within the interior of Austria; 95 km flow in and around Slovenia (67 km along the borders with Austria and Croatia, 28 km inside Slovenia), and the rest forms the border between Croatia and Hungary (Fig. 1). On the river, no dams have been built between the border of Austria and Slovenia to its outlet.
In early September 2009, shoals of sichel were detected in the Mur section between the towns of Ceršak and Veržej, at the state border between Slovenia and Austria. Fourteen specimens of sichels were caught by an angler within two days. The catch site was located on the Slovenian side, downstream the Ceršak town impassable weir. Identification of specimens was based according to Povž and Sket (1990) and Kottelat and Freyhof (2007).
Age, condition and length-weight relationship
Each individual was measured for weight (W, g) by an electronic scale (the accuracy of 1 g), and for the total length (L, cm). To determine age, 10-20 scales were taken from each specimen, below the lateral line and above the insertion of the pectoral fin. The scales were examined by microscope (magnification of 20-30 times). Annuli were identified following the standard criteria proposed by Ricker (1968), and the sex of each specimen was defined after the body was opened.
Fulton’s equation (Ricker, 1975) was used to calculate the condition coefficient (K) by the following equation:
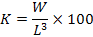
Where, L is the total length (cm) and W is wet weight (g). The K mean values for each age group, and for males and females, were calculated. The relationship between the total length L and condition coefficient K was calculated as K = a + b × L.
The equation W = a × L was applied in order to establish the length-weight relationship (LWR), where W is the weight in grams, L is the total length in cm, and a and b are the constants (Ricker, 1975).
Morphometric and meristic traits
All individuals were measured for 20 morphometric and four meristic (the number of hard and soft rays in dorsal, pectoral, ventral and anal fins) traits according to the Brylinska (1986) (Fig. 2). The relative values of all 20 measured morphometric traits were calculated to the standard body length (2) as well as the relative values of four measured morphometric traits (4, 5, 6 and 7), to head length (3).
Distribution data collection
Data on the distribution of the sichel were compiled for the catchment areas in Slovenia and Croatia from the available published papers and project reports, annual reports on catches of individual freshwater fish species, grey literature, and from the archives of fisheries societies of the Slovenian Anglers Union. The recent distribution of sichel includes data from the past ten years.
Statistical analysis
Minimum, maximum, mean value (x), standard deviation (S), standard error (m), and variation coefficient (v) were calculated using data analysis software system Statistica 12 (Dell Inc., Tulsa, OK, USA). LWR power regression was calculated by SPSS version 12 for Windows.
Results
The sichel specimens from the Mur River consisted of 11 males and 3 females belonging to the age groups from 3+ to 7+, with the majority of individuals in the 4+ age group. The value of total body length ranged from 24 to 33 cm, and weighed from 70 to 208 g. The minimum total length of females was 26 cm at the age 4+, and the maximum was 32 cm at the age 6+. Individuals with the total length 29, 30 and 31 cm were not found either in the males or in the females (Table I).
Both males and females had similar value of Fulton condition coefficient (K), 0.518 and 0.526, respectively (Table I). The relationship between the total length (L) and the condition factor (K) indicates that sichel specimens’ condition is better with older age (K=0.0023L+0.4547) (Fig. 3).
According to the value b of LWR, in males (b=3.212) a positive allometric growth was observed, and in females negative (b=2.765) (Table I). Positive allometry in both males and females (b= 3.119) was recorded, which represents the first given LWR data for sichel (Fig. 4).
Table I.- Age groups, number of specimens (n), weight (W), total length (L), minimum (Min), maximum (Max), mean±standard deviation (͞x±S), condition coefficient (K), and parameters of LWR (a, b) with confidence limits (CL) of the sichel from the Mur River.
|
Sex |
Age |
n |
W (g) |
L (cm) |
K |
LWR |
|||||||
|
Min |
Max |
͞x±S |
Min |
Max |
͞x±S |
a |
b |
r2 |
95% CL of b |
||||
|
Males (M) |
3+ |
2 |
70 |
76 |
73±4.24 |
24 |
25 |
24.5 ±0.70 |
0.496 |
||||
|
4+ |
5 |
82 |
112 |
101.6± 11.78 |
25 |
28 |
26± 1.20 |
0.532 |
|||||
|
5+ |
2 |
76 |
80 |
78±2.83 |
25 |
25 |
25±0 |
0.499 |
|||||
|
7+ |
2 |
176 |
208 |
192± 22.63 |
32.5 |
33 |
32.75 ±0.35 |
0.545 |
|||||
|
Total males |
11 |
70 |
208 |
139± 97.58 |
24 |
33 |
28.5± 6.36 |
0.518 |
0.003 |
3.212 |
0.959 |
2.77± 3.64 |
|
|
Fema-les (F) |
4+ |
1 |
98 |
98 |
98± 0.00 |
26.0 |
26.0 |
26±00 |
0.557 |
||||
|
6+ |
2 |
108 |
172 |
140± 45.25 |
28.5 |
32.0 |
30.25± 2.47 |
0.495 |
|||||
|
Total females |
3 |
98 |
172 |
135± 52.33 |
26 |
32 |
29± 4.24 |
0.526 |
0.011 |
2.765 |
0.916 |
1.12± 4.409 |
|
|
Total M+F |
14 |
70 |
208 |
139± 97.58 |
24 |
33 |
28.5± 6.36 |
0.522 |
0.004 |
3.119 |
0.952 |
2.73± 3.513 |
|
Table II.- Morphometric and meristic characters of the sichel from the Mur River (mean±standard deviation (͞x±S); minimum (Min); maximum (Max); standard error (M) and variation coefficient (V)).
|
Morphometric characters |
% Longitudo corporis |
|||||||||
|
͞x±S |
Min |
Max |
M |
V |
||||||
|
1 |
Longitudo totalis |
117.03±1.88 |
113.04 |
119.57 |
0.50 |
1.60 |
||||
|
3 |
Longitudo capitis lateraralis |
18.59±0.50 |
17.67 |
19.17 |
0.13 |
2.70 |
||||
|
4 |
Altitudo capitis |
14.15±0.85 |
12.84 |
16.28 |
0.23 |
5.98 |
||||
|
5 |
Spatium praeorbitale |
5.04±0.26 |
4.51 |
5.48 |
0.07 |
5.24 |
||||
|
6 |
Diameter oculi |
5.57±0.34 |
4.96 |
6.00 |
0.09 |
6.07 |
||||
|
7 |
Spatium postorbitale |
9.21±0.29 |
8.81 |
9.78 |
0.08 |
3.18 |
||||
|
8 |
Distantia praedorsalis |
68.27±1.56 |
66.51 |
71.74 |
0.42 |
2.29 |
||||
|
9 |
Distantia praeanalis |
65.25±2.18 |
60.98 |
68.78 |
0.58 |
3.34 |
||||
|
10 |
Altitudo corporis maxima |
20.73±0.55 |
20.00 |
21.74 |
0.15 |
2.66 |
||||
|
11 |
Longitudo pedenculi caudalis |
12.94±0.37 |
12.39 |
13.66 |
0.10 |
2.9 |
||||
|
12 |
Altitudo corporis minima |
6.78±0.29 |
6.19 |
7.5 |
0.08 |
4.34 |
||||
|
13 |
Longitudo basis D |
5.67±0.36 |
5.22 |
6.25 |
0.09 |
6.27 |
||||
|
14 |
Longitudo basis A |
23.48±0.62 |
22.66 |
24.65 |
0.17 |
2.65 |
||||
|
15 |
Longitudo P |
30.01±1.42 |
26.30 |
32.68 |
0.38 |
4.75 |
||||
|
16 |
Longitudo v |
13.31±0.26 |
12.84 |
13.7 |
0.07 |
1.96 |
||||
|
17 |
Altitudo D |
10.27±0.20 |
10.00 |
10.73 |
0.05 |
1.99 |
||||
|
18 |
Altitudo A |
12.9±0.52 |
12.23 |
13.70 |
0.14 |
4.05 |
||||
|
19 |
Distantia P-v |
27.31±0.55 |
26.3 |
28.42 |
0.15 |
2.00 |
||||
|
20 |
Distantia v-A |
16.08±0.57 |
15.35 |
17.30 |
0.15 |
3.54 |
||||
|
% Longitudo capitis lateralis |
||||||||||
|
4 |
Altitudo capitis |
76.21±5.54 |
70.00 |
92.11 |
1.48 |
7.28 |
||||
|
5 |
Spatium praeorbitale |
27.13±1.43 |
24.42 |
30.26 |
0.38 |
5.28 |
||||
|
6 |
Diameter oculi |
29.99±2.20 |
26.04 |
32.50 |
0.59 |
7.34 |
||||
|
7 |
Spatium postorbitale |
49.6±1.98 |
46.42 |
53.66 |
0.53 |
3.99 |
||||
|
Meristic characters |
||||||||||
|
D – Spines in dorsal fin |
2±0.00 |
2 |
2 |
0.00 |
0.00 |
|||||
|
D – number of soft rays in dorsal fin |
6.57±0.51 |
6 |
7 |
0.14 |
7.81 |
|||||
|
A – spines in anal fin |
2±0.00 |
2 |
2 |
0.00 |
0.00 |
|||||
|
A – number of soft rays in anal fin |
25.29±0.73 |
24 |
26 |
0.19 |
2.87 |
|||||
|
P – pines in pectoral fin |
1±0.00 |
1 |
1 |
0.00 |
0.00 |
|||||
|
P – number of soft rays in pectoral fin |
16±1.10 |
14 |
17 |
0.30 |
6.93 |
|||||
|
V – spines in ventral fin |
1±0.00 |
1 |
1 |
0.00 |
0.00 |
|||||
|
V – number of soft rays in ventral fin |
7±0.00 |
7 |
7 |
0.00 |
0.00 |
|||||
Relative morphometric and meristic characteristics for sichel were calculated and are presented in Table II. The morphometric characteristics of the head show greater variation (V between 3.99 and 7.34) than those of the body (V between 1.60 and 6.07). The lowest coefficient of variation (lower than 2) was obtained for the total body length (1), length V (16), and height of the dorsal fin (17). Using the same principles as Raczyński et al. (2011) the meristic characters for the sichel in the Mur can be presented as follows: D II 6-7, A II 24-26, P I 14-17, V I 7 (Table II).
The recent distribution of sichel in the past 10 years in Slovenia has been limited to only occasional appearance in the Mur River and to only sporadic occurrence in the Drava and Danube Rivers in Croatia.
Discussion
Sichel specimens presented in this paper belong to mature individuals, similarly to previous research from Vistula Bay, Poland (Gasowska, 1962; Stolarski, 1995; Raczynski et al., 2011). However, contrary to the caught specimens from Vistula bay, the majority of individuals from the Mur River were males (78%). Although sichel from this study belongs to age groups from 3+ to 7+ (24-33 cm L), the absence of individuals of 29, 30 and 31 cm L was observed. The possible reason for the males’ predominance and the absence of a particular length may occur due to the selectivity of the angler sampling method (Lewin et al., 2006; Arlinghaus et al., 2009).
The low condition factor (K) values (males, K=0.518; females, K=0.526) of sichel from the Mur River was related to the shape of the body (Kujawa et al., 2015), which is elongated, not very tall, and laterally strongly flattened. Also, K values could indicate the state of sexual maturity, degree of food source availability, age and sex of the species (Anibeze, 2000). Even K for sichel from the Mur River was similar in all age classes, while a slightly better condition was observed in the specimens of older age (Fig. 3) and in the males’ age 7 (K=0.545). However, K values of sichel reveal that specimens from the Mur River were also well adapted to the environmental conditions in comparison with the sichel reared in controlled conditions (Kujawa et al., 2015).
However, K between the females and males from this research was not significantly different; b value of LWR for female sichel shows negative allometry, which could occur due to post-spawning period (Treer et al., 2005) or the low number of analysed specimens (Froese, 2006). Apart from sex, b value could also be influenced by maturity season, habitat, diet, health, or preserving technique (Tesch, 1971; Hossain et al., 2017), which was not considered in this paper. Overall positive allometry of LWR for both males and females (b=3.119) was observed in sichel from the Mur River, which affirms suitable environmental conditions.
The results of morphometric measurements from this study were compared with the data given by Raczyński et al. (2011) for the specimens of sichel from Vistula bay, as the same calculation methods were used. Thus, for the sichel in the Mur, higher mean values were observed for the total body length (1), eye diameter (6), predorsal distance (8), preanal distance (9), length of base D (13), length P (15), height of annal fin (18), distance preorbital (5) and postorbital distance (7); lower mean values were observed for the head length (3), head height (4), postorbital distance (7), maximum body height (10), caudal peduncle length (11), minimum body height (12), length of base A (14), length V (16), height of the dorsal fin (17), distance P-V(19), distance V-A (20) and head height (4).
In this study, the variation coefficient of morphometric as well as meristic measures was below 10%, which can reveal poorly plastic features of the studied population of sichel (Raczyński et al., 2011). It was reported that greater differences were observed in the same species due to the ecological characteristics of the environment than to geographical distance (Nikolski, 1974; Norton et al., 1995). Previous studies on sichel reported a variation coefficient greater than 10% for head height (4), preorbital distance (5), eye diameter (6), postorbital distance (7), length of base D (13), and distance P-v (19) (Raczyński et al., 2011), but the specimens from this study belong to the population which could affect intrapopulation variation.
Meristic traits can provide more information on genotypes (Kozikowska, 1961), as they have much greater heritability than morphometric features (Tave, 1993), and are therefore very stable (Ivanković et al., 2011), as confirmed by this study. The comparison of meristic features of the sichel form the Mur River with those from Vistula Bay (Raczyński et al., 2011) revealed that only the mean number of soft rays in pectoral fin was higher (n=16), but was lower than in the sichel from the Dąbie Lake in Poland (n=17), as reported by Krzykawski and Więcaszek (1997). Most of the other meristic characteristic values from this research fit into the limited existing data (Vuković and Ivanović, 1971; Krzykawski and Więcaszek, 1997; Kottelat and Freyhof, 2007). However, the variation obtained in the number of branched anal rays (24-26) is much narrower than that (24-29) of Kottelat and Freyhof (2007) or Vuković and Ivanović (1971).
According to the historical data, sichel were present mostly in the Croatian inland waters and were most numerous in the Sava River and its tributaries (Kupa, Mrežnica, Una, Orljava), (Fig. 1), (Brusina, 1892; Sabioncello, 1967). Recent research and the new data of the Croatian ichthyofauna, have revealed the absence of this species from all tributaries of the Danube River, except for the Drava River (Opačak et al., 2010). According to Woschitz (2006), sichel historical distribution in the Hungarian-Croatian part of the Drava and in the Mur up to the Slovenian section indicates their occurrence, but with low abundance. In fact, this species was regularly recorded in the Austrian section of the Mur River and was considered as regular Styrian fish fauna (Kepka, 1971; Woschitz, 1996), but not in the Slovenian section of the same river (Povž, 2016). Occasionally, schools of sichel occurred in the Mur River of Slovenia, but this occured in the 1970s, again in 2009, and until today, they have not been reported again (M. Povž, personal communication, May 1, 2017).
According to the presented historical and recent data, the distribution area of sichel terribly decreased in the tributaries of the middle Danube River Basin, which particularly refers to Croatia. The main threat for this species is represented by the construction of dams across rivers (e.g. Iron Gate I and II Hydroelectric Power Stations), river regulation works (e.g. the Sava River), water regulation, excavation of stone and gravel, destruction of river habitats, sedimentation of organic substances, and pollution (Mrakovčić et al., 2006; Kujawa et al., 2016). Natural spawning grounds and migratory routes towards estuaries of the sichel larvae and adults after the spawning period were affected and consequently, species abundance rapidly declined. Recently, the importance of seasonal flooding for this species was observed (Górski et al., 2010). Sichel also possesses pelagic eggs that slowly mature, which could be an additional reason for its vulnerability (Mrakovčić et al., 2006). In the Croatian red book, sichel was included in the DD category (Mrakovčić et al., 2006). At present, there is still no research on any aspect of its distribution, ecology, or biology in this particular region of the Danube River Basin.
Therefore, basic research for the establishment of new distribution area and targeted protection of the sichel in the middle Danube River tributaries are urgently required. This particularly applies to the aspects such as habitats of the early life stages (spawning and juvenile habitats), spread, and population dynamics. Further study on the weight, length and growth of the sichel throughout the years are recommended. Commercial fisheries activities targeting sichel should be suppressed so as to contribute towards successful conservation of the species.
Statement of conflict of interest
Authors have declared no conflict of interest.
References
Adamicka, P., 1984. On the construction of the Pontic cyprinid Pelecus cultratus (L.). Arch. Hydrobiol. J., 101: 9-19.
Anibeze, C.I.P., 2000. Length-weight relationship and relative condition of Heterobranchus longifilis (Valencienness) from Idodo River, Nigeria. Naga ICLARM Quart., 23: 34-35.
Arlinghaus, R., Matsumura, S. and Dieckmann, U., 2009. Quantifying selection differentials caused by recreational fishing: Development of modeling framework and application to reproductive investment in pike (Esox lucius). Evolut. Applic., 2: 335-355.
Auer, B., 1995. Freilanduntersuchung zur Biologie der Jungfische des Sichlings (Pelecus cultratus L.) im Neusiedler See [Dissertation]. Wien, Austria.
Baensch, H.A. and Riehl, R., 1991. Aquarien Atlas. Mergus Verlag für Natur-und Heimtierkunde, Melle, Germany.
Balon, E.K., 1956. Beitrag zur Systematik, Ökologie, Morphologie, Alter, Wachstum und Eizahl der Eier der Ziege (Pelecus cultratus (L.) in der Donau bei Medvedov. Biol. Prace, 2: 63-88.
Balzer, B., Schröder, E. and Ssymank, A., 2004. Ergänzung der Anhänge zur FFH-Richtlinie auf Grund der EUOsterweiterung. Natur Landschaft, 79: 145-151.
Brusina, S., 1892. Fauna fossile terziaria di Markusevec in Croazia. Con un elenco delle Dreissensidae della Dalmazia, Croazia e Slavonia. Glasnik Hrvatskog Naravoslovnog Društva, 7: 113-210.
Brylinska, M., 1986. Ryby Sladkovodne Polski. PWN, Warszawa.
Freyhof, J. and Kottelat, M., 2008. Pelecus cultratus. The IUCN Red list of threatened species 2008. https://doi.org/10.2305/IUCN.UK.2008.RLTS.T16494A5942384.en
Froese, R., 2006. Cube law, condition factor and weight-length relationships: History, meta-analysis and recommendations. J. appl. Ichthyol., 22: 241-253. https://doi.org/10.1111/j.1439-0426.2006.00805.x
Froese, R. and Pauly, D., 2017. Pelecus cultratus. Retrived April 30, 2017, from http://fishbase.org/summary/Pelecus-cultratus.html
Gaigalas, K., 2001. Fishes in the Curonian Bay basin, their resources and fishery. Klaipėda, Eglė.
Gąsowska, M., 1962. Klucze do oznaczania kręgowców Polski. I. Krągłouste i ryby. Cyclos-tomi et Pisces. PWN, Warszawa-Kraków.
Glowacki, J., 1885. Die Fische der Drau und ihres Gebietes. Untergymnasium zu Pettau, Pettau.
Glowacki, J., 1896. Die Fischfauna der Save und des Isonzo. Jahresberichte des k.k. Staats-Untergymnasium in Cilli, Cilli.
Gorski, K., Winter, H.V., De Leeuw, J.J., Minin, A.E. and Nagelkerke, L.A.J., 2010. Fish spawning in a large temperate floodplain: The role of flooding and temperature. Freshw. Biol., 55: 1509-1519. https://doi.org/10.1111/j.1365-2427.2009.02362.x
Harsanyi, A., 1986. Sichling, Pelecus cultratus in der Donau. Fisch. Teichw., 11: 330-331.
Heckel, J. and Kner, R., 1858. Die Susswasserfische der Ostreichischen Monarchie mit Rücksicht auf die angrenzenden Länder. Engelmann, Leipzig.
Herzig, A. and Winkler, H., 1983. Contribution to the biology of the razor-fish - Pelecus cultratus (L.). Öesterr. Fisch., 36: 113-128.
Herzig, A., Mikschi, E., Auer, B., Hain, A., Wais, A. and Wolfram, G., 1994. Fischbiologische Untersuchung des Neusiedler Sees. Biol. Forschungs. Burgenland-Bericht, 81: 1-125.
Hossain, M.Y., , Hossen, M.A., Pramanik, M.N.U., Nawer, F., Rahman, M.M., Sharmin, S., Khatun, D., Bahkali, A.H., Elgorban, A.M. and Yahya, K., 2017. Life-history traits of the endangered carp Botia dario (Cyprinidae) from the Ganges River in Northwestern Bangladesh. Pakistan J. Zool., 49: 801-809. http://dx.doi.org/10.17582/journal.pjz/2017.49.3.801.809
Ivanković, P., Piria, M., Knezović, Z. and Treer, T., 2011. Meristic and morphometric characteristics of endemic Neretva chub, Squalius svallize from the Neretva river area, Bosnia and Herzegovina. J. appl. Ichthyol., 27: 1031-1032. https://doi.org/10.1111/j.1439-0426.2011.01741.x
Jäckel, A.J., 1864. Die Fische Bayerns, ein Beitrag zur Kenntniss der deutschen Süsswasserfische. Abh Zool-min Verein Regensburg, 9: 1-101.
Jelić, M., Jelić, D., Žutinić, P. and Ćaleta, M., 2012. Fish fauna of the lower reaches of the River Drava and surrounding marshland habitats near Donji Miholjac (eastern Croatia). Croatian J. Fish., 70: 153-167.
Jurajda, P., Gelnar, M. and Koubová, B., 1992. Occurence of ziege (Pelecus cultratus) in the River Morava with notes on its parasites. Folia Zool., 41: 187-189.
Kepka, O., 1971. Die Fauna der Steiermark. In: Die Steiermark: Land, Leute, Leistung. Styria, Graz, pp. 153-190.
Kottelat, M. and Freyhof, J., 2007. Handbook of European freshwater fishes. Publications Kottelat, Cornol and Freyhof, Berlin.
Kozikowska, Z., 1961. Wpływ środowiska na morfologię i biologię ryb. Sielawa, okoń; elementy wybrane. Ekol. Polska, 9: 542-678.
Krzykawski, S. and Więcaszek, B., 1997. Biometrics characteristics of sabrefish Pelecus cultratus (L.), Saithe pollachius virens (L.) and sea bass Dicentrarchus labrax (L.) from new localities in Polish waters. Acta Ichthyol. Piscat., 27: 3-15. https://doi.org/10.3750/AIP1997.27.2.01
Kujawa, R., Furgała-Selezniow, G., Mamcarz, A., Lach, M. and Kucharczyk, D., 2015. Influence of temperature on the growth and survivability of sichel larvae, Pelecus cultratus, reared under controlled conditions. Ichthyol. Res., 62: 163-170. https://doi.org/10.1007/s10228-014-0419-6
Kujawa, R., Kucharczyk, D., Furgała–Selezniow, G., Mamcarz, A., Ptaszkowski, M. and Biegaj, M., 2016. Substitution of natural food with artificial feed during rearing larvae of sichel Pelecus cultratus (L.) under controlled condtions. Turkish J. Fish. aquat. Sci., 16: 643-650.
Lewin, W.C., Arlinghaus, R. and Mehner, T., 2006. Documented and potential biological impacts of recreational fishing: insights for management and conservation. Rev. Fish. Sci., 14: 305-367. https://doi.org/10.1080/10641260600886455
Liu, Z., and Herzig, A., 1996. Food and feeding behaviour of a planktivorous cyprinid, Pelecus cultratus (L.), in a shallow eutrophic lake, Neusiedler See (Austria). Hydrobiologia, 333: 71-77. https://doi.org/10.1007/BF00017569
Lusk, S. and Jurajda, P., 1995. Record of Ziege (Pelecus cultratus) in the Dyje river. Folia Zool., 44: 284-287.
Mojsisovics, A., 1883. Zur Fauna von Belleye und Darda (II. Theil.). Mitthteil. Naturwissencshaft. Vereines Steiermark, 19: 122-170.
Mrakovčić, M., Brigić, A., Buj, I., Ćaleta, M., Mustafić, P. and Zanella, D., 2006. Red Book of freshwater fish of Croatia. Ministry of Culture, Zagreb.
Munda, A., 1927. Ribe v slovenskih vodah. Slovensko ribarsko društvo v Ljubljani, Ljubljana.
Muus, B.J. and Dahlström, P., 1968. Süßwasserfische. BLV Verlagsgesellschaft, München.
Nikolski, G.V., 1974. Ékologija ryb. Izd. Vysšaja škola, Moskva.
Norton, S.F., Luczkovich, J.J. and Motta, P.J., 1995. The role of ecomorphological studies in the comparative biology of fish. Environ. Biol. Fishes, 44: 287-304. https://doi.org/10.1007/BF00005921
Opačak, A., Jelkić, D. and Florijančić, T., 2010. Gospodarska osnova ŠRK Koprivnica (Management plan for Koprivnica fishery association). Poljoprivredni fakultet u Osijeku, Osijek, Hrvatska.
Povž, M., 2016. Daten aus der Natura 2000 Datenbank. Zavod Umbra, Ljubljana.
Povž, M. and Sket, B., 1990. Naše sladkovodne ribe. Založba Mladinska Knjiga, Ljubljana.
Raczyński, M., Krzemińska, K. and Czerniejewski, P., 2011. Morphological characterization of sichel, Pelecus culturatus from the Vistula Bay. Biol. Hodowli Zwierząt, 583: 281-292.
Ratschan, C., 2015. Der Sichling (Pelecus cultratus L. 1758) – eine bestandsbildende FFH-Art in Österreichischen Fließgewässern? Österr. Fisch., 69: 91-108.
Ricker, W.E., 1968. Methods for assessment of fish production in fresh waters. Blackwell Scientific Publication, London.
Ricker, W.E., 1975. Computation and interpretation of biological statistics of fish populations. Department of the Environment, Fisheries and Marine Service, Ottawa.
Riede, K., 2004. Global register of migratory species- from global to regional scales. Final Report of the R&D-Projekt 808 05 081. Federal Agency for Nature Conservation, Bonn.
Sabioncello, I., 1967. Sistematika slatkovodnih riba. In Z. Livojević, C. Bojčić (Ed.), Priručnik za slatkovodno ribarstvo. Posebna izdanja Agronomskog glasnika, Zagreb. pp. 21-90.
Siebold, C.T.E., 1863. Die Süßwasserfische von Mitteleuropa. Verlag von Willhelm Engelmann, Leipzig. https://doi.org/10.5962/bhl.title.61814
Simonović, P., Piria, M., Zuliani, T., Ilić, M., Marinković, N., Kračun-Kolarević, M. and Paunović, M., 2017. Characterization of sections of the Sava River based on fish community structure. Sci. Total Environ., 574: 64-271. https://doi.org/10.1016/j.scitotenv.2016.09.072
Stolarski, J., 1995. Sichel (Pelecus cultratus L.) from the Vistula Lagoon. Biulet. Morskiego Instyt. Rybackiego Gdynia, 135: 11-21.
Seeley, H.G., 1886. The freshwater fishes of Europe. Cassels & Company, London.
Tave, D., 1993. Genetics for fish hatchery managers. AVI, New York.
Tátrai, I. and Herzig, A., 1995. Effect of habitat structure on the feeding efficiency of young stages of razor fish (Pelecus cultratus L.): An experimental approach. Hydrobiologia, 299: 75-81. https://doi.org/10.1007/BF00016887
Tesch, F.W., 1971. Age and growth. In Methods for assessment of fish production in fresh waters (ed. W.E. Ricker). Blackwell Scientific Publications, London, pp. 98-103.
Treer, T., Aničić, I., Safner, R., Odak, T. and Piria, M., 2005. Post-spawning condition of endemic soft-muzzled trout Salmothymus obtusirostris in the Žrnovnica river. Ribarstvo, 63: 85-90.
Unger, E., 1926. Die Ziege (Pelecus cultratus L.) in Ungarn. Österr. Fisch. Zeit., 23: 61-63.
Vostradovsky, J., 1973. Freshwater fishes. The Hamlyn Publishing Group Limited, London.
Vuković, T. and Ivanović, B., 1971. Slatkovodne ribe Jugoslavije. Zemaljski muzej BIH, Sarajevo.
Vutskis, G., 1918. Amagyar Birodalom Allatvilaga/fauna Regni Hungariae. Anim. Hung., 1-2: 1-42.
Wais, A., 1995. Zur Morphologie des Sichlings Pelecus cultratus (Linnaeus) (Pisces: Cyprinidae). Annal. Naturhistor. Mus. Wien, 97B: 421-435.
Woschitz, G., 2006. Rote Liste gefährdeter Fische in der Steiermark [Brochure]. Graz, Austria.
Woschitz, G., 1996. Fischökologische Studie Mittlere Laßnitz. Studie im Auftrag des Grazer Sportanglervereins [Brochure]. Wien, Austria.
To share on other social networks, click on any share button. What are these?






