Proximate Composition and Minerals Content of Wheat (Triticum aestivum L.) Under Boric Acid Stress via Energy-Dispersive X-Ray (EDX) Fluorescence Based Analysis
Research Article
Proximate Composition and Minerals Content of Wheat (Triticum aestivum L.) Under Boric Acid Stress via Energy-Dispersive X-Ray (EDX) Fluorescence Based Analysis
Muhammad Adnan1*, Muhammad Nauman Khan2,3, Barkat Ullah2, Faisal Zaman2, Alevcan Kaplan4, Hubert O. Dossou-Yovo5, Sajid Ali Khan Bangash6, Sana Wahab7, Mehreen Ghazal8 and Muhammad Hassan Sarfraz9
1Iqbal Ahamd Qarshi Laboratory, Qarshi Brands Departnment at Qarshi industries (Pvt.), Ltd. Hattar Haripur, Pakistan; 2Department of Botany, Islamia College Peshawar, 25120, Pakistan; 3Biology Laboratory, University Public School (UPS), University of Peshawar, 25120, Pakistan; 4Department of Crop and Animal Production, Sason Vocational School, Batman University, Batman 72060, Turkiye; 5Laboratory of Applied Ecology, Faculty of Agronomic Sciences, University of Abomey-Calavi, Benin, P.O Box 1974 Godomey, Benin; 6Institute of Biotechnology and Genetic Engineering, The University of Agriculture, 25130 Peshawar, Pakistan; 7Department of Plant Sciences, Faculty of Biological Sciences, Quaid-i-Azam University, Islamabad 45320, Pakistan; 8Centre of Plant Biodiversity, University of Peshawar, Peshawar 25120, Pakistan; 9Botnar Institute of Musculoskeletal Sciences, Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Sciences, University of Oxford, OX3 7LD, United Kingdom.
Abstract | Wheat (Triticum aestivum L.) is one of the first domesticated crop plants and is the most widely grown crop in the world, both in terms of cultivated area and yield, and wheat is known to be consumed by about two-thirds of the world’s population. The study was carried out to investigate the elemental and proximate composition of wheat against boron induced stress. Greenhouse experiments were conducted in October 2019 under natural light conditions to assess the chemical status of plants under various treatments such as the stress of inorganic boric acid. In the experiment, the pots were filled with sandy loam soil (1 kg). The pots were pre-treated with boron as boric acid (H3BO3) only once before sowing, at the doses of 0 mg/kg (control), 3 mg/kg, 6 mg/kg, 9 mg/kg, 12 mg/kg, 15 mg/kg and 18 mg/kg soil. The different concentrations of Boron (B) were prepared separately by taking a respective amount of boric acid. Pots without supplemented B constituted the control. No additional supplements were applied to the experimental soil. Each pot was irrigated with 100 mL of distilled water with intermittent intervals of 48 hours. All the treatments were replicated three times considering each pot as one replicate. Through EDX analysis, it was noticed that the boron stress treatment exhibited the maximum number of elements detected in (T2, T5, and T6) wheat crops followed by (T0, T1, and T3). A total of 17 elements were detected and observed in wheat including Ca, S, K, Si, Na, Mg, Fe, Al, Cu, Cr, Cl, Mn, B, C, N, Co, and Ni in different treatments of B. It has been determined that the amount of each element varies according to the amount of B applied. Thus, the study performed under B stress helps understand elemental and proximate content in various treatments in wheat plants.
Received | July 24, 2023; Accepted | March 19, 2024; Published | March 24, 2024
*Correspondence | Muhammad Adnan, Iqbal Ahamd Qarshi Laboratory, Qarshi Brands Departnment at Qarshi industries (Pvt.), Ltd. Hattar Haripur, Pakistan; Email: muhammadadnan112255@gmail.com
Citation | Adnan, M., M.N. Khan, B. Ullah, F. Zaman, A. Kaplan, H.O. Dossou-Yovo, S.A.K. Bangash, S. Wahab, M. Ghazal and M.H. Sarfraz. 2024. Proximate composition and minerals content of wheat (Triticum aestivum L.) under boric acid stress via energy-dispersive x-ray (EDX) fluorescence based analysis. Pakistan Journal of Weed Science Research, 30(1): 07-17.
DOI | https://dx.doi.org/10.17582/journal.PJWSR/2024/30.1.7.17
Keywords | Boron stress, EDX analysis, Elemental analysis, Human health, Wheat, Crude fiber
Copyright: 2024 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Wheat is the most widely crop in Pakistan and is cultivated from plains to over 4000 m (Ali and Nasir, 1989; Nasir et al., 1972; Stewart, 1958, 1982; Stewart et al., 1972). Triticum aestivum L. belongs to the family Poaceae (Gramineae), one of the largest families of flowering plants. It consists of 620 genera and 8000 species. Plants are annuals, leaf-blades are flattened, inflorescence may have a spike, spikelets solitary at the nodes of the tough or fragile rachis, laterally compressed, 2-6(-9)-flowered (Cope, 1982; Heun et al., 1997). It is widely distributed in all the regions of the world (Anjum and Muhammad, 2012). The reason why it is highly preferred is the adaptability of the wheat plant and its ability to produce high-yielding products. Wheat is also rich in essential amino acids, minerals, vitamins, and phytochemicals (Shewry, 2009). Wheat is listed among the ‘big eight’ food allergens which together account for about 90 % of all allergic responses (Poole et al., 2006). Several wheat proteins have been reported to be responsible for allergic responses to the ingestion of wheat products but only one syndrome has been studied in detail (Battais et al., 2005; Tatham and Shewry, 2008). Cereals are an important source of micronutrients such as minerals and vitamins which are essential in a balanced diet and for a healthy life (Akin et al., 2020).
Wheat plants, like many other plants, need more or less nutrients to survive. Micronutrients play a vital role in plant nutrition and plant production. Agricultural soils generally show a deficiency in micronutrients such as zinc, boron, iron, and copper. Such a deficiency may occur due to the low contents of these elements. Although micronutrients comprising zinc, copper, iron, manganese, boron, molybdenum, and chlorine are required for plant growth in much smaller amounts, they are as essential as the major nutrients such as nitrogen, phosphorus, potassium (Sharma and Agrawal, 2005; Khan et al., 2019c). All these elements have a positive effect on plant growth when they are present in very small amounts (Aller et al., 1990). Boron, which is one of the micronutrient elements, is a very interesting element from the agricultural point of view due to its benefit for the development of plants and its very low toxicity range (Blevins and Lukaszewski, 1994). The most important function of boron so far reported in higher plants is its capacity to form borate esters with rhamnogalacturonan II residues (Kobayashi et al., 1996). This complex formation is critical for cell wall structure and function as it allows significant control of cell wall porosity and tension in the plant (Ryden et al., 2003; O’Neill et al., 2004). The disruptions to be experienced in the formation of this complex adversely affect normal plant growth. Boron has long been recognized as an essential micronutrient for higher plants. B deficiency affects vegetative and reproductive stages of plant growth. In the vegetative stage, its deficiency leads to the inhibition of growth and the inhibition of the development of vascular bundles. During reproduction, B deficiency causes inhibition of, or defects in, flower, seed and fruit development (Asad et al., 2002; Mishra et al., 2009). B is such a nutrient for plants and its deficiency and toxicity may disrupt plant growth and development, resulting in decreased yield (Seth and Aery, 2017). Excess B shows typical toxicity symptoms in the plant. B toxicity is most intense, causing reactive oxygen species to be formed through photo oxidative stress and adversely affecting many cellular processes. Moreover, B toxicity causes changes in the cell wall structure and causes metabolic disruption (O’Neill et al., 2004). It is very important to determine the amount of some elements that take part in such reactions, which are of vital importance in the cell.
The term elemental analysis typically refers to the determination of the amount of an element in a given sample, usually a weight percent (Freedman, 2001). Various techniques such as atomic absorption spectrometry (AAS), Energy Dispersive X-Ray Analysis (EDX) voltammetry, inductively coupled plasma atomic emission spectrometry (ICP-AES) and instrumental neutron activation analysis (INAA) are routinely used to determine trace elements in herbs (Niamat et al., 2012; Fagbohun et al., 2020, 2021). Trace elements research has been part of this explosion of scientific knowledge. Such elements are required in very small quantities for plant life. Relatively high levels of essential elements have been demonstrated to influence the retention of toxic elements in animals and human beings (Hejna et al., 2018). Okem et al. (2014) mentioned that low antimicrobial activity was induced by high levels of heavy elements. Hlihor et al. (2022) highlighted the accumulation of such elements in human tissues.
Cereals are plants known as very sensitive to B elements. It is therefore essential to examine the toxic effect of boron stress on wheat, considering the cultivation of wheat in the regions where boron deposits are located. Thus, our study aims to focus on the determination of Ca, S, K, Si, Na, Mg, Fe, Al, Cu, Cr, Cl, Mn, B, C, N, Co, and Ni as they are nutrient elements in wheat plants under B stress with following objectives: (1) B application was applied at different doses, (2) investigate the number of different elements in T. aestivum under B stress (3) observe the effects of B on these elements in T. aestivum.
Materials and Methods
Study area
The greenhouse experiment was carried out at the Department of Chemistry, Bacha Khan University, Charsadda in Pakistan from 2019 to 2020. The district Charsadda is located between 34° 03ˊ and 34° 28ˊ North latitude and 71° 28ˊ to 71° 33ˊ East longitude. This district, being the geographic center, is about 282 m asl, and covers an area of 996 km2. The annual rainfall is 460 mm, with June (44°C) as the hottest month whereas the coldest is January (5°C to 10°C) and the wettest being February. As shown in Figure 1, Charsadda is surrounded by four districts and one tribal area, on the East is district Mardan, on the North is Malakand, on the South are Peshawar and Nowshera districts and Mohmand Agency on the West (Khan et al., 2017, 2018a, 2019b; Khan and Badshah, 2019; Zaman et al., 2019).
Experimental design
Greenhouse experiments were conducted during October under natural light conditions in 2019. Seeds of T. aestivum were sown at 2 cm depth in pots with a height of 30 cm and 25 cm diameter. The pots were filled with sandy loam soil (1 kg). Boron is retained in the soil in the form of boric acid (H3BO3). Thereof, before the sowing, all pots were pre-treated with B as H3BO3 one time. The applied doses were 0 mg/kg (No B), 3 mg/kg, 6 mg/kg, 9 mg/kg, 12 mg/kg, 15 mg/kg, and 18 mg/kg soil. The different concentrations of B were prepared separately by taking a respective amount of boric acid. Pots without supplemented B constituted the control. No additional supplements were applied to the experimental soil. Each pot was irrigated with 100 ml of distilled water with intermittent intervals of 48 h (Figure 2).
Sample treatments
The plant samples were cleaned with 2% phosphate-free detergent solution and quickly washed with flowing distilled water, the residual moisture evaporated at room temperature; the samples were chopped and oven dried in paper envelopes to constant mass for moisture and dry matter determinations. The loss in mass was taken as % moisture content. The dried samples were ground to a fine powder using agate mortar and pestle, and sieved to obtain particles less than 20 mesh sieve and stored in airtight containers for further analyses through the procedure of (Effiong et al., 2009).
Sample preparation
The plant samples were washed three times with tap water and rinsed with distilled water to remove soil and airborne pollutants. The surface water was absorbed through Whatman filter paper no. 42. The plant tissues were separated and oven-dried at 70°C for 24 h to remove moisture content (Jha and Dubey, 2004). Moreover, through an electric grinder, the plant tissues were ground into a fine powder. All the samples were stored in labeled clean polythene bags for multi-elemental analysis.
Energy dispersive X-ray (EDX) fluorescence analysis for minerals
Prepared samples of plant tissues were transported to the Centralized Resource Laboratory (CRL), University of Peshawar. The samples were quantified using an energy dispersive X-ray fluorescence spectrometer (EDX-7000, Na-U, Shimadzu, Japan) with loose powder method, calibration with Al-Cu standard (Chai et al., 2017; Nyakuma et al., 2021). One-gram powder from the samples of plant in replication of three was placed over a thin film lined a 10 mL Polypropylene cup and then mounted inside the EDX-7000 spectrometer (Khan et al., 2021; Yousaf et al., 2017). The instrument is equipped with an X-ray tube using a Rhodium (Rh) target and a high-performance silicon drift detector (SDD), operated with a maximum of 50 kV and 1000 μA and a PCEDX-Navi software. The elemental composition of all samples was detected under an air- based atmosphere. The analytes were then assessed with a collimator of 10 mm in diameter with a live acquisition time of the 60s (Bilo et al., 2015).
Proximate analysis
The collected specimens were analyzed for proximate analysis of dry matter, moisture contents, ash content, crude protein, and crude fat (Khan et al., 2018b, 2020).
Moisture content (% MC)
Moisture content was determined by taking 1 g of well-mixed sample in a dried and weighted China dish. The sample was kept in oven at 105 oC until a constant weight was obtained (Khan et al., 2015). Moisture content was calculated as following formula:
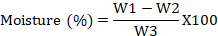
Where; W1= Initial weight of sample, W2= final weight of sample, and W= Weight of sample.
Dry matter (% DM)
Dry matter (DM) percentage of the forage plants was determined from oven-dried samples at 65 oC for 72 h following the AOAC method (Horwitz, 1975) by using the following formula.
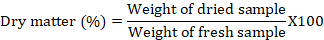
Ash content (%)
Ash content was determined using one to two grams of plant sample in a Muffle furnace at 550-600 oC, kept for 8 h according to the AOAC method (Horwitz, 1975), by using the following formula.
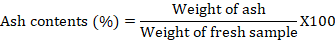
Crude protein (% CP)
Crude protein percentage was quantified following the Kjeldahl method (Bremner, 1960) by using the given formula.
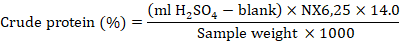
Crude fat (% CF)
Crude fats were determined from ether extract by using the reflux apparatus described (May and Galyean, 1996), by using the following formula.
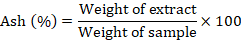
Results and Discussion
Energy dispersive X-ray (EDX) fluorescence analysis for minerals
Table 1 and Figures 3a-g reveal that the work under B stress exhibited the maximum number of elements detected in wheat crops. A total of 17 elements were detected and observed in wheat including Ca, S, K, Si, Na, Mg, Fe, Al, Cu, Cr, Cl, Mn, B, C, N, Co, and Ni in different treatments of B. Highest calcium content (0.65%) were detected in T5 followed by T4 (0.40 %),
Table 1: Various concentration of Boron on growth and mineral contents of T. aestivum.
|
S. No |
Treatments |
*Minerals composition (%) |
||||||||||||||||
|
Ca |
S |
K |
Si |
Na |
Mg |
Fe |
Al |
Cu |
Cr |
Cl |
Mn |
B |
C |
N |
Co |
Ni |
||
|
1 |
T0=0mg/kg |
0.24 |
- |
1.32 |
2.79 |
0.06 |
0.27 |
- |
0.61 |
- |
- |
0.17 |
0.10 |
0.60 |
42.06 |
0.86 |
- |
- |
|
2 |
T1=3mg/kg |
0.38 |
0.08 |
1.21 |
3.35 |
0.11 |
0.27 |
0.18 |
- |
0.80 |
- |
- |
- |
1.85 |
41.07 |
0.88 |
- |
- |
|
3 |
T2=6mg/kg |
0.18 |
0.03 |
0.80 |
3.91 |
0.17 |
0.10 |
0.18 |
0.32 |
- |
- |
0.24 |
0.01 |
0.59 |
41.42 |
- |
- |
- |
|
4 |
T3=9mg/kg |
0.29 |
0.08 |
1.11 |
2.39 |
- |
0.23 |
0.18 |
0.19 |
0.17 |
- |
0.27 |
- |
1.38 |
45.75 |
- |
- |
- |
|
5 |
T4=12mg/kg |
0.40 |
0.07 |
1.14 |
2.07 |
0.11 |
0.30 |
- |
0.41 |
- |
0.01 |
0.32 |
- |
- |
43.78 |
- |
- |
- |
|
6 |
T5=15mg/kg |
0.65 |
0.08 |
1.72 |
0.39 |
0.30 |
0.40 |
0.12 |
- |
- |
- |
0.42 |
0.02 |
1.59 |
40.55 |
0.95 |
- |
- |
|
7 |
T6=18mg/kg |
0.25 |
0.01 |
1.70 |
1.28 |
0.11 |
0.21 |
0.25 |
0.58 |
- |
- |
0.27 |
- |
- |
40.96 |
- |
0.10 |
0.02 |
*Ca: Calcium, S: Sulphur, K: Potassium, Si: Silicon, Na: Sodium, Mg: Magnesium, Fe: Ferric/Iron, Al: Aluminium, Cu: Copper, Cr: Chromium, Cl: Chlorine, Mn: Manganese, B: Boron, C: Carbon, N: Nitrogen, Co: Cobalt and Ni: Nickel.
T1 (0.38 %), T3 (0.29 %), T6 (0.25 %) and T0 (0.24 %) in decreasing order. Sulfur contents were maximum in T5, T3, and T1 (0.08 %) each followed by T4 (0.07 %), T2 (0.03 %), T6 (0.01 %) and absent in T0. The highest Potassium content was recorded in T5 (1.72 %), followed by T6 (1.70 %), T0 (1.32 %), T1 (1.21 %), T4 (1.14 %), T3 (1.11 %) while T2 (0.80 %) showed the lowest concentration from the rest of others. Regarding the silicon content, the maximum value was found in T2 (3.91 %) followed by T1 (3.35 %) and the remaining treatments exhibited less than T1 and T2. Treatment T0 had 2.79 % content, T3 with (2.39 %), T4 showing (2.07 %), T6 had (1.28 %) and T5 showed 0.39 % contents. Similarly, B showed a negative effect on the sodium content of wheat in T3 because no sodium content was obtained in this treatment while the lowest content was observed in all remaining treatments except T5 which showed the total of 0.30 % and considered as the highest content of all followed by T2 (0.17%), T1, T4 and T6 (0.11 %, each), and the control (T0) showed 0.06 % contents. Magnesium and iron contents were also observed and revealed less positive effects under B stress, the highest magnesium amount was recorded in T5 (0.40 %) followed by T4 (0.30 %) while the remaining had less amount than T4. Iron content was also the highest in T6 (0.25 %) followed by T1, T2 and T3 (0.18 %, each). T5 shows less amount of iron (0.12 %) while iron content was absent only in the control (T0). Similarly, T4 showed the negative effect of B on the element contents of wheat. Aluminum was also detected in different amounts of all treatments but the leading treatment was T0 (0.61) which exhibited the highest amount followed by T6 (0.58 %). Copper was present only in T1 (0.80 %) and T3 (0.17%) and absent in all other treatments. Moreover, B showed negative effects on element contents of wheat in these treatments. Chromium was observed in only T4 (0.01 %) which was very low and the remaining treatments resulted from the absence of chromium under B treatments. B also had an adverse effect on chromium concentration. Therefore, cobalt and nickel contents were not detected in all treatments except T6 (0.10 %) and (0.02 %) with very low amount. Such findings can result in B having negative effects on these elements and treatments (T0, T2, T4, T5 and T6). Chlorine concentration also varied from one treatment to another. Therefore, the maximum percentage was observed in T5 (0.42 %) followed by T4 (0.32 %) while T3 and T6 had the same amount (0.27 %) followed by T2 (0.24 %). Similarly, the lowest percentage was recorded for T0 and no chlorine content in T1. Manganese content was detected in only T0, T2 and T5 (0.10 %, 0.01 %, 0.02 %) and absent in T1, T3, T4 and T6. B on soil affects the B distribution in the wheat plant as it was noticed that the highest amount in T1 (1.85 %) followed by T5 (1.59 %), T3 (1.38 %), T0 (0.60 %), and T2 (0.59 %) and they also showed a negative effect on T4 and T6 because it induced a damage in the available contents of B in the plant. Similarly in the overall experiment the amount of carbon was present in the highest range as it shows the highest amount in T3 (45.75 %) and was recorded as leading followed by T4 (43.78 %), T0 (42.06 %), T2 (41.42 %), T1 (41.07 %), T6 (40.96 %) and T5 (40.55 %). Thus % nitrogen content was present in only T5 (0.95 %), T1 (0.88 %), and T0 (0.86 %) in decreasing order while absent in T2, T3, T4 and T6. Oxygen content also shows a positive effect under B.
In overall experiment the level of boron T5=15 mg/kg boric acid is useful for wheat since it increases the level of nutrients in wheat plant. Thus, the study performed under B stress helps understand elemental distribution in various treatments in wheat plants. This study might help in the identification of certain elements in wheat and a novel record for plant chemists and plant biologists for future research.
Proximate concentration of wheat under boron stress
Results in Table 2 and Figure 4A-E revealed the proximate assessment of wheat crop under induced boric acid showing essential variation in moisture content, dry matter, crude fat, crude ash and crude protein contents. Overall, the moisture content was observed as highest in T4 (9.92%) followed by T5 (9.10%), and T1 (8.78%), while the lowest moisture content were observed in T0 (8.10%).
Dry matter analysis resulted in maximum values in T0 and T5 (91.90%) dry matter yield each followed by T2 (91.685%) and T3 (91.47%), T6 (91.30%), T1 (91.22%), whereas the lowest value was observed in T4 (90.08%).
Table 2: Proximate composition of Boron stress on T. aestivum.
|
S. No. |
Treatments |
% Moisture content |
% Dry matter |
On dry matter basis |
||
|
% Crude fat |
% Crude ash |
% Crude protein |
||||
|
1 |
T0=0mg/kg |
8.10 |
91.90 |
0.91 |
15.09 |
10.62 |
|
2 |
T1=3mg/kg |
8.78 |
91.22 |
1.45 |
10.47 |
9.95 |
|
3 |
T2=6mg/kg |
8.315 |
91.685 |
1.05 |
13.35 |
11.54 |
|
4 |
T3=9mg/kg |
8.53 |
91.47 |
1.03 |
7.88 |
10.94 |
|
5 |
T4=12mg/kg |
9.92 |
90.08 |
1.32 |
8.55 |
12.12 |
|
6 |
T5=15mg/kg |
9.10 |
90.90 |
0.77 |
7.80 |
10.48 |
|
7 |
T6=18mg/kg |
8.70 |
91.30 |
2.03 |
13.05 |
14.33 |
The crude fats content ranged from 2.03% in T6 to 0.77% in (T5). Crude ash content was found higher and usually maximum at T0 (15.09%) followed by T2 (13.35%), T6 (13.05%) and minimum at T5 (7.80%) and T3 (7.88%).
Variation has been observed in composition of crude protein as T6 (14.33%), T4 (12.12%), T2 (11.54%), T3 (10.94%), T0 (10.62%), T5 (10.48%) and T1 (9.95%) in decreasing order.
Wheat is an important cereal crop and in the present context, loss in yield due to any of the biotic or abiotic stresses is unendurable. Cereals are an important source of micronutrients; such as minerals and vitamins which are essential for a healthy life (Akin et al., 2020). B has long been recognized as an essential micronutrient for higher plants and B stress (deficiency or toxicity) in crops including wheat is common and economically important. B deficiency affects vegetative and reproductive stages of plant growth (Asad et al., 2002; Mishra et al., 2009). B is said to be taken up by plants as undissociated boric acid. Within plants, B is relatively immobile. It is not readily relocated from old to young plant tissues (Wang et al., 1996). Wheat is known to respond to the application of several macro and micronutrients during its growing stages and results in enhanced output in terms of yield. Although micronutrients comprising zinc, copper, iron, manganese, boron, molybdenum and chlorine are required by plants in much smaller amounts, they are as essential as the major nutrients such as nitrogen, phosphorus, potassium etc. (Sharma and Agrawal, 2005; Khan et al., 2019a). Micro and macro elements and heavy metals have certain known risks (Ullah et al., 2017). Soil pH is also an important factor; when its regulating material effect is applied to grasses, usually as lime, the plant molybdenum level rises and the levels of Cu, Fe, Mn, and Zn decline as the pH is raised from the acid range towards neutrality (Kosta and Byrne, 1982). Therefore, various studies have contributed to the elemental study of plants using different techniques but results in the research corroborate those of Khan et al. (2012), who studied elemental analysis of some selected species of Ficus genus through atomic absorption spectrophotometry technique. Those authors had observed thirteen elements including sodium, potassium, copper, zinc, chromium, calcium, manganese, ferric, nickel, magnesium, cadmium, cobalt, and lead in the genus Ficus with species such as Ficus benghalensis L., Ficus religiosa L., Ficus microcarpa L.f., Ficus racemosa L., Ficus hispida L.f., Ficus carica L., and Ficus lacor Buch.-Ham. Similar to this, research of Seth and Aery (2017) is also in line with our study. In fact, they investigated B and its effects on biochemical constituents, enzymatic activities, and growth performance of wheat. They designed experiments as soils supplemented with different B concentrations and observed different growth parameters to determine the impact of B toxicity. They mainly found that B induced changes in the plant’s physiological parameters, leading to a subsequent reduction in growth, production of biomass, and yield characteristics. Therefore, Effiong et al. (2009) also studied Telfairia occidentalis Hookf (leaves and seeds) and Abelmoschus esculentus Moench (leaves and pod/seeds) for their mineral contents to assess their nutritive and energy values. The findings showed that fluted pumpkin leaves (86.29 ± 1.77 %) and seeds (74.29 ± 1.58 %) and okra leaves (78.53 ± 3.4 %) and pod/seeds (82.9 ± 0.75 %) had relatively high mean humidity content. There was a relatively high mineral content (potassium, phosphorus, iron, manganese, copper, zinc and vitamin C, suggesting that both vegetables could be good supplements of these nutrients. Similar to the present work Ullah et al. (2017) also determined the mineral composition of selected vegetables, commonly used as food in Pakistan. These are Amaranthus thunbergii Moq., Caralluma edulis (Edgew.) Meve & Liede, Allium atrosanguineum Schrenk, Rumex patientia L., and Portulaca oleracea L. collected from the arid region of South Waziristan Agency, Pakistan, and subjected to minerals analysis. Their findings have revealed that all these studied vegetables can provide human beings with vital nutrients. As a good source of proteins, fats and carbohydrates. A. thunbergii, C. edulis, and P. oleracea are capable of supplying energy to the user. Similar results were also made by Hussain et al. (2013) investigating elemental analysis of important leafy vegetables (Malva sylvestris L., Eruca sativa (L.) Cav., and Mentha sylvestris L.) and fleshy vegetables (Brassica rapa L., Brassica oleracea var. botyris L., and Raphanus sativus L.) which are commonly used in the rural areas of Usterzai, Pakistan. Their results concluded that R. sativus had the highest iron (13.03 ± 0.14), copper (9.96 ± 0.16), manganese (0.79 ± 0.01), cadmium (0.21± 0.01) and lead (0.44 ± 0.02) while E. sativa had the highest magnesium (25.65 ± 0.21) and M. sylvestris had the highest sodium (81.04 ± 0.17) concentrations. They concluded that B. oleracaea and R. sativus are active in terms of nutritional properties. Therefore, the amounts of radioactive elements such as cadmium and lead are small, making these vegetables healthier for local citizens. While outputs of Khan et al. (2019a), also corroborate our results. In fact, they also studied the effects of B on the growth and yield components of wheat from 2015 to 2016 during the winter season. Treatments included zinc (as zinc sulfate 25g/L), boron (as boric acid 20g/L), and zinc plots B (as zinc sulfate and boric acid 25 g/L and 20g/L, respectively). The recommended dose of NPK was applied at the rate of 60, 75, and 0 kg ha-1, respectively. Their study revealed that foliar application of zinc + boron in wheat showed significant variation for all of the parameters recorded study except days to emergence. In case of interaction, maximum plant height (103 cm), grains spike-1 (45), 1000 grains weight (37 g), grain yield (5966.67 kg ha-1), biological yield (19059 kg ha-1), and harvest index (31.30%) were recorded with foliar application of Zn+B. However, Grieve and Poss (2000) also studied wheat and their interactive effects of salinity and varying concentrations of B on growth, yield, and ion relations. While Qamar et al. (2020) also provided the same suggestion in their study and investigated the role of soil-applied B in improving the growth, yield, and fiber quality of the cotton crops. 5 different B doses (i.e., 0.00, 2.60, 5.52, 7.78, and 10.04 mg kg-1 of soil) and two cotton cultivars (i.e., CIM-600 and CIM-616) were included in the study. Soil applied B (2.60 mg kg-1) significantly improved growth, yield, physiological parameters, and fiber quality, while 10.04 mg kg-1 application improved B distribution in roots, seeds, leaves, and stalks. They noted significant improvement in plant height (12 %), leaf area (3 %), number of bolls (48 %), boll size (59 %), boll weight (52 %), seed cotton yield (52 %), photosynthesis (50 %), transpiration rate (10 %), stomatal conductance (37 %), and water use efficiency (44 %) of CIM-600 with 2.60 mg kg-1 compared to control treatment of CIM-616. Therefore, cultivar CIM-600 and 2.60 mg kg-1 soil B application recommended for higher yield and productivity.
Conclusions and Recommendations
The current study concluded that the work under B stress resulted in the maximum number of elements detected in wheat crops. A total of 18 elements were detected and observed in wheat including Ca, S, K, Si, Na, Mg, Fe, Al, Cu, Cr, Cl, Mn, B, C, N, Co, and Ni in different treatments of B. The level of each element varies from one treatment to another. Thus, the study performed under B stress helps understand elemental distribution in various treatments in wheat plants. This study might help in the identification of certain elements in wheat and a novel record for plant chemists and plant biologists for future research.
Novelty Statement
This study presents novel insights into the proximate composition and mineral content of wheat (Triticum aestivum L.) subjected to boric acid stress, employing Energy-Dispersive X-Ray Fluorescence (EDX) analysis. By investigating the effects of boric acid stress on wheat, this research contributes valuable knowledge to understanding the plant’s responses to environmental stressors and its implications for agricultural sustainability and food security
Author’s Contribution
Muhammad Adnan: Conceptualization, formal analysis and writing – original draft.
Muhammad Nauman Khan: Data curation and methodology.
Barkat Ullah and Faisal Zaman: Formal analysis and investigation.
Alevcan Kaplan: Validation and writing– review and editing.
Hubert O. Dossou-Yovo: Validation and software.
Sajid Ali Khan Bangash: Writing– review and editing.
Sana Wahab: Formal analysis and validation.
Mehreen Ghazal: Resources and validation.
Muhammad Hassan Sarfraz: Software and writing– review and editing.
Conflict of interest
The authors have declared no conflict of interest.
References
Akin, P.A., Sezer, B., Sanal, T., Apaydin, H., Koksel, H. and Boyaci, İ.H., 2020. Multi-elemental analysis of flour types and breads by using laser induced breakdown spectroscopy. J. Cereal Sci. 92: 102920. https://doi.org/10.1016/j.jcs.2020.102920
Ali, S. and Nasir, Y., 1989. Flora of Pakistan-Islamabad, Karachi.
Aller, A.J., Bernal, J.L., Nozal, M.J.D. and Deban, L., 1990. Effects of selected trace elements on plant growth. J. Sci. Food Agric., 51: 447-479. https://doi.org/10.1002/jsfa.2740510404
Anjum, P. and Muhammad, Q., 2012. Pollen flora of Pakistan-LXIX. Poaceae. Pak. J. Bot., 44: 747-756.
Asad, A., Blamey, F. and Edwards, D., 2002. Dry matter production and boron concentrations of vegetative and reproductive tissues of canola and sunflower plants grown in nutrient solution. Plant Soil, 243: 243-252. https://doi.org/10.1023/A:1019909130031
Battais, F., Mothes, T., Moneret-Vautrin, D., Pineau, F., Kanny, G., Popineau, Y., Bodinier, M. and Denery-Papini, S., 2005. Identification of IgE-binding epitopes on gliadins for patients with food allergy to wheat. Allergy, 60: 815-821. https://doi.org/10.1111/j.1398-9995.2005.00795.x
Bilo, F., Lodolo, M., Borgese, L., Bosio, A., Benassi, L., Depero, L.E. and Bontempi, E., 2015. Evaluation of heavy metals contamination from environment to food matrix by TXRF: The case of rice and rice husk. J. Chem., 274340: 1-12. https://doi.org/10.1155/2015/274340
Blevins, D.G. and Lukaszewski, K.M., 1994. Proposed physiologic functions of boron in plants pertinent to animal and human metabolism. Environ. Health Perspect., 102: 31-33. https://doi.org/10.1289/ehp.94102s731
Bremner, J., 1960. Determination of nitrogen in soil by the Kjeldahl method. J. Agric. Sci., 55: 11-33. https://doi.org/10.1017/S0021859600021572
Chai, B., Liu, C., Wang, C., Yan, J. and Ren, Z., 2017. Photocatalytic hydrogen evolution activity over MoS2/ZnIn2S4 microspheres. Chinese J. Catal., 38: 2067-2075. https://doi.org/10.1016/S1872-2067(17)62981-4
Cope, T., 1982. Poaceae. Poaceae. pp. 461-462.
Effiong, G., Ogban, P., Ibia, T. and Adam, A., 2009. Evaluation of nutrientsupplying potentials of fluted pumpkin (Telfairia occidentalis, Hook, F.) and okra (Abelmoschus esculentus L.) Moench. Acad. J. Plant Sci., 2: 209-214.
Fagbohun, O.F., Joseph, J.S., Salami, O.A. and Msagati, T.A., 2021. Exploration of modern chromatographic methods coupled to mass spectrometric techniques for trace element and chemical composition analyses in the leaf extracts of Kigelia africana. Biol. Trace Element Res., 199: 1633-1648. https://doi.org/10.1007/s12011-020-02274-w
Fagbohun, O.F., Olawoye, B., Ademakinwa, A.N., Jolayemi, K.A. and Msagati, T.A., 2020. Metabolome modulatory effects of Kigelia africana (Lam.) Benth. fruit extracts on oxidative stress, hyperlipidaemic biomarkers in STZ-induced diabetic rats and antidiabetic effects in 3T3 L1 adipocytes. J. Pharm. Pharmacol., 72: 1798-1811. https://doi.org/10.1111/jphp.13362
Freedman, J.C., 2001. Biophysical chemistry of physiological solutions, Cell Physiology Source Book, Elsevier, pp. 3-15. https://doi.org/10.1016/B978-012656976-6/50093-7
Grieve, C.M. and Poss, J.A., 2000. Wheat response to interactive effects of boron and salinity. J. Plant Nutr., 23: 1217-1226. https://doi.org/10.1080/01904160009382095
Hejna, M., Gottardo, D., Baldi, A., Dell’Orto, V., Cheli, F., Zaninelli, M. and Rossi, L., 2018. Nutritional ecology of heavy metals. Animal, 12: 2156-2170. https://doi.org/10.1017/S175173111700355X
Heun, M., Schafer-Pregl, R., Klawan, D., Castagna, R., Accerbi, M., Borghi, B. and Salamini, F., 1997. Site of einkorn wheat domestication identified by DNA fingerprinting. Science, 278: 1312-1314. https://doi.org/10.1126/science.278.5341.1312
Hlihor, R.M., Roșca, M., Hagiu-Zaleschi, L., Simion, I.M., Daraban, G.M. and Stoleru, V., 2022. Medicinal plant growth in heavy metals contaminated soils: Responses to metal stress and induced risks to human health. Toxics, 10: 499. https://doi.org/10.3390/toxics10090499
Horwitz, W., 1975. Official methods of analysis. Association of Official Analytical Chemists Washington, DC.
Hussain, J., Rehman, N.U., Shinwari, Z.K., Ali, L., Al-Harrasi, A., Khan, A.L. and Mabood, F., 2013. Qualitative characteristics of the commonly used vegetables in Usterzai of Kohat region. Pak. J. Bot., 45: 2071-2074.
Jha, A. and Dubey, R., 2004. Carbohydrate metabolism in growing rice seedlings under arsenic toxicity. J. Plant Physiol., 161: 867-872. https://doi.org/10.1016/j.jplph.2004.01.004
Khan, A., Hayat, Z., Khan, A.A., Ahmad, J., Abbas, M.W., Nawaz, H., Ahmad, F. and Ahmad, K., 2019a. Effect of foliar application of zinc and boron on growth and yield components of wheat. Agric. Res. Technol., 21: 3-6. https://doi.org/10.19080/ARTOAJ.2019.21.556148
Khan, A., Khan, M.S., Hadi, F., Saddiq, G. and Khan, A.N., 2021. Energy-Dispersive X-ray (EDX) fluorescence based analysis of heavy metals in marble powder, paddy soil and rice (Oryza sativa L.) with potential health risks in District Malakand, Khyber Pakhtunkhwa, Pakistan. Environ. Pollut. Bioavailabil., 33: 301-316. https://doi.org/10.1080/26395940.2021.1986427
Khan, I.A., Habib, K., Rasheed Akbar, A.K., Saeed, M., Farid, A., Ali, I. and Alam, M., 2015. Proximate chemical composition of brinjal, Solanum melongena L. (Solanales: Solanaceae), genotypes and its correlation with the natural enemies in Peshawar. J. Entomol. Zool. Stud., 3: 07-11.
Khan, K.Y., Khan, M.A., Niamat, R., Shah, G.M., Fazal, H., Seema, N., Hussain, I., Ahmad, I., Inayat, H. and Jan, G., 2012. Elemental content of some anti-diabetic ethnomedicinal species of genus Ficus Linn. using atomic absorption spectrophotometry technique. J. Med. Plants Res., 6: 2136-2140. https://doi.org/10.5897/JMPR11.1276
Khan, M.N., Ali, S., Yaseen, T., Adnan, M., Ullah, S., Zaman, A., Iqbal, M., Shah, S.N., Ali, A. and Razzaq, A., 2020. Assessment of proximate and nutritional contents in selected weedy grasses for potential use as fodder in district Charsadda, KP. Proc. Pak. Acad. Sci. B. Life Environ. Sci., 57: 83-94.
Khan, M.N., Ali, S., Yaseen, T., Ullah, S., Zaman, A., Iqbal, M. and Shah, S., 2019b. Eco-taxonomic study of family Poaceae (Gramineae). RADS J. Biol. Res. Appl. Sci., 10: 63-75. https://doi.org/10.37962/jbas.v10i2.191
Khan, M.N. and Badshah, L., 2019. Floristic diversity and utility of flora of district Charsadda, Khyber Pakhtunkhwa. Acta Ecol. Sin., 39: 306-320. https://doi.org/10.1016/j.chnaes.2018.10.003
Khan, M.N., Hadi, F., Razaq, A. and Shah, S.M., 2017. Utilitarian aspects of weeds and their ecological characteristics in Ochawala valley, District Charsadda, Pakistan. ARPN J. Agric. Biol. Sci., 12: 182-189.
Khan, M.N., Razzaq, A., Hadi, F., Khan, N., Basit, A., Jan, F. and Khan, N., 2018a. Ethnobotanical profile of weed flora of district Charsadda, Khyber Pakhtunkhwa. RADS J. Biol. Res. Appl. Sci., 9: 14-23. https://doi.org/10.37962/jbas.v9i1.117
Khan, R., Khan, M.N., Ullah, H., Basit, A., Razzaq, A., Ahmad, M. and Ozdemir, F., 2018b. A comparative assessment of proximate and elemental composition six weedy grasses for their potential use as fodder. Prog. Nutr., 20: 182-190.
Khan, N., Ahmed, M.J. and Shah, S.Z.A., 2019c. Comparative analysis of mineral content and proximate composition from chilli pepper (Capsicum annuum L.) germplasm. Pure Appl. Biol., 8: 1338-1347. https://doi.org/10.19045/bspab.2019.80075
Kobayashi, M., Matoh, T. and Azuma, J., 1996. Two chains of rhamnogalacturonan II are cross-linked by borate-diol ester bonds in higher plant cell walls. Plant Physiol., 110: 1017-1020. https://doi.org/10.1104/pp.110.3.1017
Kosta, L. and Byrne, A.R., 1982. Analytical evaluation of comparative data on trace elements in biological materials. J. Radioanal. Chem., 69: 117-129. https://doi.org/10.1007/BF02515918
May, T. and Galyean, M., 1996. Laboratory procedures in animal nutrition research. New Mexico State University, Las Cruces.
Mishra, S., Heckathorn, S., Frantz, J., Yu, F. and Gray, J., 2009. Effects of boron deficiency on geranium grown under different nonphotoinhibitory light levels. J. Am. Soc. Hortic. Sci., 134: 183-193. https://doi.org/10.21273/JASHS.134.2.183
Nasir, E., Ali, S. and Stewart, R., 1972. Flora of west Pakistan.
Niamat, R., Khan, M.A., Khan, K.Y., Ahmad, M., Ali, B., Mazari, P., Mustafa, M. and Ahmad, H., 2012. Element content of some ethnomedicinal Ziziphus Linn. species using atomic absorption spectroscopy technique. J. Appl. Pharma. Sci., 2(3): 96-100.
Nyakuma, B.B., Oladokun, O., Wong, S.L. and Abdullah, T.A.T., 2021. Torrefaction of oil palm empty fruit bunch pellets: Product yield, distribution and fuel characterisation for enhanced energy recovery. Biomass Conv. Biorefin., pp. 1-21. https://doi.org/10.1007/s13399-020-01185-z
Okem, A., Southway, C., Stirk, W., Street, R., Finnie, J. and Van Staden, J., 2014. Heavy metal contamination in South African medicinal plants: A cause for concern. South Afr. J. Bot., 93: 125-130. https://doi.org/10.1016/j.sajb.2014.04.001
O’Neill, M.A., Ishii, T., Albersheim, P. and Darvill, A.G., 2004. Rhamnogalacturonan II: structure and function of a borate cross-linked cell wall pectic polysaccharide. Ann. Rev. Plant Biol., 55: 109-139. https://doi.org/10.1146/annurev.arplant.55.031903.141750
Poole, J.A., Barriga, K., Leung, D.Y., Hoffman, M., Eisenbarth, G.S., Rewers, M. and Norris, J.M., 2006. Timing of initial exposure to cereal grains and the risk of wheat allergy. Pediatrics, 117: 2175-2182. https://doi.org/10.1542/peds.2005-1803
Qamar, R., Hussain, A., Sardar, H., Sarwar, N., Javeed, H.M.R., Maqbool, A. and Hussain, M., 2020. Soil applied boron (B) improves growth, yield and fiber quality traits of cotton grown on calcareous saline soil. PLoS One, 15: e0231805. https://doi.org/10.1371/journal.pone.0231805
Ryden, P., Sugimoto-Shirasu, K., Smith, A.C., Findlay, K., Reiter, W.D. and McCann, M.C., 2003. Tensile properties of Arabidopsis cell walls depend on both a xyloglucan cross-linked microfibrillar network and rhamnogalacturonan II-borate complexes. Plant Physiol., 132: 1033-1040. https://doi.org/10.1104/pp.103.021873
Seth, K. and Aery, N.C., 2017. Boron induced changes in biochemical constituents, enzymatic activities, and growth performance of wheat. Acta Physiol. Plant., 39: 1-10. https://doi.org/10.1007/s11738-017-2541-3
Sharma, R.K. and Agrawal, M., 2005. Biological effects of heavy metals: An overview. J. Environ. Biol., 26: 301-313.
Shewry, P.R., 2009. Wheat. J. Exp. Bot., 60: 1537-1553. https://doi.org/10.1093/jxb/erp058
Stewart, R.R., 1958. The Flora of the Rawalpindi District, West Pakistan. Gordon College.
Stewart, R.R., 1982. History and exploration of plants in Pakistan and adjoining areas. The Flora of West Pakistan, 99-100.
Stewart, R.R., Nasir, E. and Ali, S., 1972. Flora of West Pakistan: An annotated catalogue of the vascular plants of West Pakistan and Kashmir. Fakhri Printing Press.
Tatham, A. and Shewry, P., 2008. Allergens to wheat and related cereals. Clin. Exp. Allergy, 38: 1712-1726. https://doi.org/10.1111/j.1365-2222.2008.03101.x
Ullah, I., Gul, S., Rehman, H.U., Ahmad, N., Ullah, I., Aziz-ud-Din, S.M.J. and Akbar, M., 2017. Analysis of nutrients and minerals of some wild edible plants. Int. J. Fauna Biol. Stud., 4: 35-39.
Wang, C.F., Duo, M.J., Chang, E. and Yang, J.Y., 1996. Essential and toxic trace elements in the Chinese medicine. J. Radioanal. Nucl. Chem., 211: 333-347. https://doi.org/10.1007/BF02039702
Yousaf, S., Ilyas, M., Khattak, A.K., Satti, S.Z. and Jan, I., 2017. Antimicrobial activities and mineral profile of selected wild plant Linum usitatissimum in Khyber Pakhtunkhwa, Pakistan. Soil Environ., 36(1): 45-50. https://doi.org/10.25252/SE/17/41156
Zaman, A., Asadullah, L.B., Muhammad, Z., Razzaq, A., Jelani, G., Ali, U., Raees, N. and Khan, M.N., 2019. Floristics of weeds in Triticum aestivum L. fields of Tehsil Shabqadar, District Charsadda, KP, Pakistan. Int. J. Bot. Stud., 4(5): 37-44.
To share on other social networks, click on any share button. What are these?





