Proximate Composition and Mineral Content of Selected Chickpea Cultivars
Research Article
Proximate Composition and Mineral Content of Selected Chickpea Cultivars
Arshad Mehmood Khattak1, Saleem Ullah1*, Farida Anjum2, Hamid U. Shah1 and Sahib Alam1
1Department of Agricultural Chemistry and Biochemistry, Faculty of Nutrition Sciences, The University of Agriculture, Peshawar, Khyber Pakhtunkhwa, Pakistan; 2Soil and Plant Nutrition Department, Agriculture Research Institute Tarnab, Peshawar, Khyber Pakhtunkhwa, Pakistan.
Abstract | Chickpea (Cicer arietinum) are an important source of nutrient and can be eaten either as salad or cooked as leafy green and their grain can be consumed as curries. Samples of tender leaves, green pods, green grains, Mature pods and Mature grains of seven different Chickpea cultivars KK-1, KK-2, KK-3, Chattan, Lawaghir, Sheenghar, Fakhar-e-Thal, were obtained from Karak Research Station Ahmad Wala and evaluated for proximate composition and mineral contents. The data related to proximate composition and mineral profile showed significant difference (P<0.05) by comparing cultivars and edible parts. Proximate composition (%) of the cultivars showed that Moisture (80.84) and Ash (3.50) were high in Lawaghar, Crude fiber (6.60) in KK-2, Crude protein in Chattan (7.87) while Crude fat (2.22) was high in KK-1. Among the edible parts, Moisture (85.54) and Ash (3.54) were high in Green grains while Crude fiber (6.38), Crude protein (8.29) and Crude fat (1.99) were maximum in Tender leaves. Mineral (mgKg-1) content of the cultivars showed that Na (38.3), Pb (0.30) and Cu (0.09) were high in Sheenghar, Cr (0.36), Ni (0.92) in KK-2, Fe (0.29) in KK-3, P (0.28) in Chattan while Mg (0.90) was high in Fakhar e Thal. In parts Na (40.2), P (0.24), Pb (0.32) and Mg (0.34) were high in Green grains, K (84.57), Cu (0.09) and Fe (0.22) in Tender leaves, Cr (0.36) in Green pods while Ni (0.69) and Zn (0.18) were maximum in mature pods. Chlorophyll was high (52.85) in Tender leaves. It was concluded from the data that cultivars varied in it nutritional composition and also the parts contained an appreciable amount of nutrients which along with grains may be utilized in daily diets or any other food formulation.
Received | August 02, 2018; Accepted | March 15, 2021; Published | June 04,2021
*Correspondence | Saleem Ullah, Department of Agricultural Chemistry, Faculty of Nutrition Sciences, The University of Agriculture, Peshawar, Khyber Pakhtunkhwa, Pakistan; Email: saleemagrichemist@gmail.com
Citation | Khattak, A.M., S. Ullah, F. Anjum, H.U. Shah and S. Alam. 2021. Proximate composition and mineral content of selected chickpea cultivars. Sarhad Journal of Agriculture, 37(2): 683-689.
DOI | http://dx.doi.org/10.17582/journal.sja/2021/37.2.683.689
Keywords | Chickpea cultivar, Proximate composition, Mineral, Chlorophyll
Introduction
Chickpea, also known as Garbanzo bean or Bengal gram (Lev-Yadun et al., 2000) cultivated in many parts of the world. Chickpea does not require more labour work and its production is more efficient than other crops (Shiferaw et al., 2007). Most of the people in the world used chickpea as a source of food, mostly for their protein content (FAO, 2008).
Among the major types of chickpea, the two important ones are Kabuli chickpea that is found in Asia and USA. The seeds of this type are larger in size and having white color. The 2nd one is known as desi chickpea; the seeds of this type are small in size and the color is light brown. Desi type is cultivated mainly in India and East Africa (Rincon et al., 1998).
The area under pulses cultivation in Pakistan is 1-1.7 M ha where major portion of the area is covered by chickpea that is 70% while lentil, mung, mash is 27% and minor pulses covered an area by 3%. The total area under pulse cultivation in Khyber Pakhtunkhwa is 10.2 M ha where about 37600 ha are covered by chickpea (gram) with net production of 20,000 tonnes and average yield of 531.9 kg ha-1 (ASP, 2010-11). Chickpea is able to fix atmospheric Nitrogen, so there is no need of (N) addition to the crop and in rotation, it enhances N content of soil and increases yield of subsequent cereal crops (Aslam et al., 1997). The chickpea has the property of fertility restoration i.e. water and nutrients of the soil (Ben et al., 2008). As chickpea is very nutritive and soil helping crop like other legumes . The present project was design to study the nutritional components of different edible parts during growing stages of plants of different cultivars with objectives keeping in mind such as to compare different cultivars of chickpea for their nutrients i.e. minerals and proximate composition. Comparing nutritional composition of different parts of chickpea cultivars used for eating purpose during plant growing stages. And to highlight the importance of different parts like leaves and green pods of chickpea along with seeds, which is used as Chola or Kabuli channa in daily cookings.
Materials and Methods
Samples of leaves, green pods and mature seeds were collected from Agricultural Research Station Ahmad Wala Karak. The samples of different parts at different growing stages were collected and brought to the Laboratory of Agricultural Chemistry and was analyzed for different parameters, particularly for proximate composition and mineral content.
Sample collection
The samples of seven chickpea cultivars namely Karak-1(KK1), Karak-2 (KK2), Karak-3 (KK3), Sheenghar, Lawaghar, Chattan, Fakhar-e-Thal were collected from Agriculture Research Station Ahmad Wala Karak. All the samples were collected in one kg paper bags and were brought to the laboratory, where they were washed and then air dried. After air drying the sample was tested for chlorophyll content. A part of the sample was used for moisture determination. The rest of the samples were placed at 70 oC. The oven temperature was increased up to 100 oC for complete drying. The samples were then ground with laboratory grinder and stored in papers bags for further analysis.
Proximate composition
Samples of leaves, green pods and pulses were analyzed for crude protein, crude fiber, crude fat, ash and moisture by standard methods of AOAC (2000).
Moisture content
Oven drying method was used for moisture determination. Petri dish was placed in oven and the oven temperature was kept at 105°C ±1°C. The weight (wt.) loss in percent was calculated as follows.
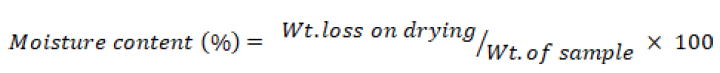
Ash content
Direct ignition method was used for ash content determination. One gram of each sample was placed in cleaned, dried pre-weighted crucibles. The samples were charred and kept in furnace at 600 °C. Percent Ash was calculated as follows.
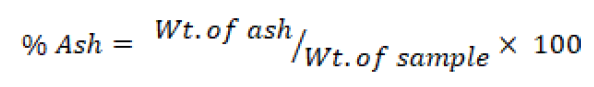
Crude fat
Crude Fat was determined by Soxhlets methods using Soxhlets apparatus. Crude fat was extracted with petroleum ether and calculated by the following formula.
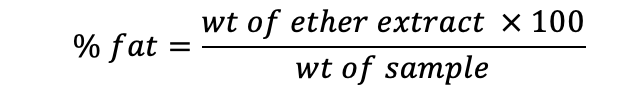
Crude protein
Kjeldahl method was used for crude protein determination. In the presence of digestion mixture, the sample was digested by heating with concentrated sulphuric acid. The mixture was then made alkaline. The ammonia so released was collected in (4%) boric acid solution and titrated against standard normal HCl. Total crude protein was calculated by multiplying the amount of nitrogen with the appropriate factor.
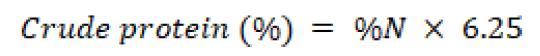
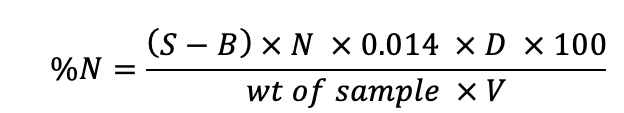
Crude fiber
Assay: Two gram chickpea sample was first acid digested with 200ml of 1.5% HCl, later with 200ml of 1.5% NaOH. Then the digest was oven dried weighed and ashed (550 oC for 3 hour’s) and calculated by the following formula.
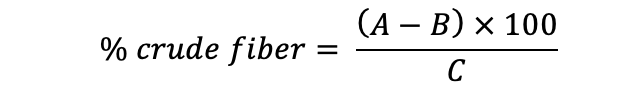
Where;
A= Crucible with dry residues (g); B= Weight of cruicible with ash (g); C= Weight of sample (g)
Phosphorus determination
Phosphorous was determined spectrophotometer method using SP3000+ spectrophotometer. Mixed reagent, ammonium molebdate and antimony potassium tartarate in one liter of 5N H2SO4 were used. The color developing reagent was prepared by adding 370 mg ascorbic acid to 70 ml of mixed reagents.
Standard phosphorous solution
Standard series (10, 20, 30 and 40 mg/L) prepared from K2 H2 PO2 , samples and blank were treated with coloring regent and blue color developed after 15 minutes were observed in spectrophotometer. The amount was calculated using the following formula.
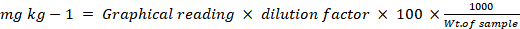
Flame photometer
Na and K were determined by flame photometer (USDA, 1954). Na standard prepared from NaCl and K standard prepared from KCl were read by the flame photometer after making zero with blank knobe. Samples reading was recorded and compared with their respective standards.
Atomic absorption spectroscopy
For the purpose of identification of trace elements i.e. copper (Cu), zinc (Zn), iron (Fe), magnesium (Mg), chromium (cr), nickel (Ni), lead (pb) Sandell (1959), O’Dell et al. (1972) and macro analysis levels with modification (Sattar and Chaudary, 1978) methods were employed.
Chlorophyll meter
The chlorophyll reading was taken on fresh leaf basis with the help of At leaf chlorophyll meter (Ahmad et al., 2012).
Statistical analysis
The data was statistically analyzed (STATISTIX) using two factorials completely randomized design (CRD). The Least Significant Difference (LSD) was performed at a significant level of 5% (P<0.05) for comparison of mean values (Steel et al., 1997).
Results and Discussion
Seven different cultivars experimentally plotted in Karak Research Station Ahmad Wala were sampled at different edible stages. Those samples were analyzed for various chemical parameter which were present as follow.
Table 1 shows proximate composition of cultivars. The data showed that the mean moisture content was higher in Lawaghar (84.20%) followed by Sheenghar (83.83%), while the lowest moisture content (78.67%) were found in KK-3. Crude fiber showed that highest mean value of crude fiber was found in KK-2 as 6.60% that was followed by Sheenghar having the value of 6.41%. The minimum value (5.14%) of crude fiber content was found in Chattan cultivar. Crude protein ranged from 5.78 to 7.87% in the cultivars. The maximum (7.87%) crude protein was found in Chattan followed by KK-2 (7.48%), while the minimum crude protein (5.78%) was detected in KK-3. Ash content ranged from 2.09 to 3.50%. The maximum ash content (3.50%) was recorded in KK-2 followed by Sheenghar (3.43%), while Chattan appeared having the lowest ash content (2.09%) in the selected cultivars. The highest crude fat (2.22%) was found in KK-1 followed by 1.88% both in Chattan and Lawaghar, while the lowest value (1.58%) was determined in Fakhar-e-Thal, all the constituents varied significantly among the selected cultivars.
Table 1: Proximate composition (%) of different chickpea cultivars.
|
Proximate composition |
Cultivars |
||||||
|
KK-1 |
KK-2 |
KK-3 |
Chattan |
Lawaghar |
Sheenghar |
Fakhar.e.Thal |
|
|
Moisture |
79.16d |
81.83b |
78.67d |
80.84c |
84.20a |
83.83a |
79.53d |
|
Crude fiber |
5.19d |
6.60a |
5.26cd |
5.14d |
5.69b |
6.41a |
5.55bc |
|
Crude Protein |
6.70bc |
7.48a |
5.78d |
7.87a |
6.45c |
6.49c |
6.97b |
|
Ash Content |
2.40bc |
2.09c |
2.83ab |
2.20bc |
3.50a |
3.33a |
3.43a |
|
Crude Fat |
2.22a |
1.80b |
1.79bc |
1.88b |
1.88b |
1.87b |
1.58c |
KK-1=karak-1 KK-2=Karak-2 KK-3=Karak-3.
The proximate composition of different parts of the cultivars were presented in Table 2. Proximate composition in different parts was varied significantly among different parts (P<0.05). The mean moisture content of different parts showed that immature grain contained higher moisture content (85.54%). The highest mean value of crude fiber content among the parts was found in leaves which were recorded as 6.38% and the lowest value (5.23%) was found in immature grain. Crude protein was maximum (8.29%) in leaves. The mean ash content was highest (3.54%) immature grain. Crude fat content showed that leaves consist of maximum crude fat content (1.99%), while the lower value (1.75%) was found in immature grain.
Table 2: Proximate composition (%) of different edible plant parts of chickpea cultivars.
|
Proximate composition |
Edible parts |
||||
|
Tender leaves |
Green pods |
Green grains |
Mature pods |
Mature grains |
|
|
Moisture |
78.80b |
79.12b |
85.54a |
_ |
_ |
|
Crude fiber |
6.38a |
5.39c |
5.23c |
5.94b |
5.52c |
|
Crude Protein |
8.29a |
5.71d |
6.63c |
6.49c |
6.98b |
|
Ash Content |
2.16 c |
2.79b |
3.54a |
_ |
_ |
|
Crude Fat |
1.99a |
1.81ab |
1.75b |
1.89ab |
1.86ab |
Table 3: Mineral and chlorophyll composition (mg kg-1) of different chickpea cultivars.
|
Mineral +Chlorophyll |
Cultivars |
||||||
|
KK-1 |
KK-2 |
KK-3 |
Chattan |
Lawaghar |
Sheenghar Fakhar-e-Thal |
||
|
Na |
31.81d |
33.00b |
32.21c |
32.62b |
31.31e |
38.3a |
31.45de |
|
K |
52.98a |
45.98d |
50.26b |
47.65c |
45.90e |
44.90g |
45.02f |
|
P |
0.18bc |
0.13cd |
0.14bc |
0.28a |
0.07d |
0.20b |
0.17bc |
|
Cr |
0.15g |
0.36a |
0.20f |
0.32b |
0.23e |
0.26d |
0.30c |
|
Pb |
0.18f |
0.24d |
0.21e |
0.29b |
0.17g |
0.30a |
0.26c |
|
Cu |
0.03c |
0.05b |
0.05b |
0.02d |
0.03c |
0.09a |
0.03c |
|
Ni |
0.56c |
0.92a |
0.51d |
0.45e |
0.45e |
0.62bc |
0.77ab |
|
Mg |
0.32e |
0.36b |
0.34c |
0.26f |
0.13g |
0.33d |
0.39a |
|
Zn |
0.17b |
0.11f |
0.12e |
0.10g |
0.20a |
0.13d |
0.14c |
|
Fe |
0.18b |
0.08d |
0.29a |
0.07e |
0.17c |
0.07e |
0.17c |
|
Chlorophyll |
41.90a 35.98ab |
0.21ab |
33.12b |
34.50ab |
23.57c |
33.05b |
|
KK-1=karak-1 KK-2=Karak-2 KK-3=Karak-3
Satelo et al. (1987) reported moisture content in chickpea cultivars, which was the same as the present study. Moisture content reported by Shad et al. (2009) was somewhat lower from the present study that might be due to variety difference or difference in location. These results were in agreement to those obtain by Guo et al. (2008) who reported crude fiber in the pressure cooked raw and roasted seeds of chickpea. Balojon and Fetuga (1986) and Hassan et al. (2005) also work in the same direction, supporting the present study. The results were agreed also to the findings of Khatoon and Parkash (2004) and also that of Sabinas et al. (2006). Tsialtas et al. (2002) determined ash content in vegetative parts of chickpea. Ash content (1.06%) of the whole chickpea flour was also reported by Sanchez-vioque et al. (1999). Alajaji and El-Adawy (2006) reported crude fat content during cooking experiment of chickpea which supported the present study. Smith et al. (1980) also reported crude fat of kabuli and desi type chickpea.
Mineral and chlorophyll content of cultivar were presented in Table 3. The mean value of Na in Sheenghar (38.36 mg kg-1) was highest followed by KK-2 (33.00 mg kg-1). Lawaghar cultivar contained lowest value of sodium content (31.31 mg kg-1). Potassium was highest (52.98 mg kg-1) in KK-1 followed by KK-3 that was recorded as 50.26 mg kg-1. The minimum value of potassium (44.90 mg kg-1) was found in Sheenghar cultivar. The phosphorus content ranged from 0.07 to 0.28 mg kg-1. Chattan cultivar appeared having the highest phosphorus (0.28 mg kg-1) followed by Sheenghar (0.20 mg kg-1), while the lowest phosphorus content (0.07 mg kg-1) was determined in Lawaghar. The chromium that ranged from 0.15 to 0.36 mg kg-1 having highest value KK-2 (0.36 mg kg-1) followed by Chattan (0.32 mg kg-1), while the minimum value was observed in KK-1 (0.15 mg kg-1). The mean value of lead in Sheenghar (0.30 mg kg-1) was highest followed by Chattan (0.29 mg kg-1). The lead appeared as having the minimum value (0.17 mg kg-1) in Lawaghar cultivar. Copper content in chickpea was highest (0.09 mg kg-1) in Sheenghar followed by 0.05 mg kg-1 both in KK-2 and KK-3. The copper appeared as having the minimum value (0.02 mg kg-1) in Chattan cultivar. The KK-2 contained highest value (0.92 mg kg-1) of nickel followed by Fakhar-e-Thal (0.77 mg kg-1) and the lowest mean content of nickel was found in Chattan and Lawaghar (0.45 mg kg-1). Magnesium content in chickpea cultivars showed that the highest mean value (0.39 mg kg-1) was found in Fakhar-e-Thal followed by KK-2 that was recorded as 0.36 mg kg-1. The minimum value (0.13 mg kg-1) of magnesium was recorded in Lawaghar cultivar. Zinc had maximum value in Lawaghar (0.20 mg kg-1) followed by KK-1 (0.17 mg kg-1), while Chattan contained lowest zinc content (0.10 mg kg-1). The iron maximum value (0.29 mg kg-1) was recorded in KK-3 followed by KK-1 (0.18 mg kg-1), While the lowest value was found in Chattan (0.07 mg kg-1) and also in Sheenghar (0.07 mg kg-1). The Table 3 also showed that it was highest in KK-1 (41.90 mg kg-1) followed by KK-3 where it recorded as 36.15 mg kg-1. Sheenghar cultivar appeared having the lowest chlorophyll content (23.57 mg kg-1).
Mineral and chlorophyll content of different parts were presented in Table 4. Considered the sodium content in parts, the highest mean value (40.92 mg kg-1) was found in immature grain and the lowest value (13.00 mg kg-1) in leaves. The highest mean value of potassium was found in leave which were recorded as 84.57 mg kg-1 and the lowest value (30.70 mg kg-1) was found in mature grain. The mean phosphorus content of different parts showed that immature grain consist maximum value (0.24 mg kg-1). Chromium content was high (0.36 mg kg-1) in immature grain and pod. Lead was found high in mature grain (0.41 mg kg-1) and lowest value was found in mature grain plus pods that was found as 0.11 mg kg-1. Copper was found in leaves which were recorded as 0.09 mg kg-1 and rest of the part contained 0.02 mg kg-1 of copper. Nickel was found high (0.69 mg kg-1) in mature grain and the lowest value (0.53 mg kg-1) was found in immature grain. The highest mean value (0.34 mg kg-1) of magnesium was found both in leaves and immature grain. Zinc was high (0.18 mg kg-1) in mature grain. Leaves consist of maximum iron value (0.22 mg kg-1). Comparing the various parts, the highest mean value of chlorophyll was found in leaves which were recorded as 52.85 mg kg-1 and the lowest value was found in immature grain that was found as 15.22 mg kg-1.
The present study was supported by Khalil and Manan et al. (1990) who studied different chickpea cultivars for their chemical composition. These findings were associated with the study being reported by Thavarajah et al. (2009). Lelaboye and Pikuda (2009) also reported phosphorus content in chickpea cultivars, which showed the same range as the present study.
The study of Koch (1996) supported the present work who studied chromium in chickpea cultivar. Gajewska and Sklodowska (2005) studied chromium content in contaminated plant, which agreed to the present study.
Table 4: Mineral and chlorophyll (mg kg-1) composition of different edible parts of chickpea cultivars.
|
Minerals + Chlorophyll |
Edible parts |
||||
|
Tender leaves |
Green pods |
Green grains |
Mature pods |
Mature grains |
|
|
Na |
13.00e |
40.23b |
40.92a |
35.07d |
35.57c |
|
K |
84.57a |
45.21b |
43.67c |
30.70e |
33.48d |
|
P |
0.15c |
0.22b |
0.24a |
0.11d |
0.11d |
|
Cr |
0.07e |
0.36a |
0.20d |
0.32c |
0.35b |
|
Pb |
0.20c |
0.14d |
0.32b |
0.41a |
0.11e |
|
Cu |
0.09a |
0.02c |
0.04b |
0.04b |
0.02c |
|
Ni |
0.64b |
0.64b |
0.53d |
0.69a |
0.56c |
|
Mg |
0.34a |
0.26c |
0.34a |
0.26c |
0.31b |
|
Zn |
0.05e |
0.15c |
0.17b |
0.18a |
0.14d |
|
Fe |
0.22a |
0.17b |
0.10e |
0.14c |
0.12d |
|
chlorophyll |
52.85a |
_ |
15.22b |
_ |
_ |
Wierzbicka (1995) studied different chickpea cultivars for their chemical composition and reported lead in considerable amount. The same observation of heavy metal was also reported by Herrero et al. (2003) that agreed with the present study.
Conclusions and Recommendations
Chickpea cultivars were different with respect to its nutritional components, where Sheenghar showed most of the minerals in larger amount as compared to other ones. Leaves of chickpea, which is used as saag, contained higher values of different nutrients as compare to other parts of the chickpea plants. It is recommended that chickpea cultivars, like in the present study Sheenghar, preferably be used for particular nutritional formulations. Also, along with Chana (seeds of the chickpea plants) leaves and green pods should be used to utilized maximum nutritional benefits out of chickpea plants.
Novelty Statement
Novelty of this research study is to highlight importance of chickpea leaves in stem along-with grains that are usually used in curries.
Author’s Contribution
Arshad Mehmood Khattak: Principal author who conducted thesis research.
Saleem Ullah: Supervised the thesis work.
Farida Anjum: Helped in Lab. experiments.
Hamid Ullah Shah: Provided Lab. facilities.
Sahib Alam: Helped in Lab. experiments and thesis write up.
Conflict of interest
The authors have declared no conflict of interest.
References
Agri Statistic of Pakistan. 2010-2011.
Ahmad, B., T. Khalil, A. Ahmad, S.M.A. Basra, Z. Hussain and A. Ali. 2012. Effect of seed priming with ascorbic acid and hydrogen peroxide on emergence, regor and antioxidant activity of maize. Afr. Biotechnol., 11: 1127-1132. https://doi.org/10.5897/AJB11.2266
Akram, A.M. and R. Aleem. 2008. Detection of resistant sources for collar rot disease in chickpea germplasm. Pak. J. Bot., 40: 2211-2215.
Alajaji, A., Shaleh and A. EL-Adawy. 2006. Nutritional composition of chickpea (Cicer arietinum L.) as affected by microwave cooking and other traditional cooking methods. J. Food Composit. Anal., 19: 806-812. https://doi.org/10.1016/j.jfca.2006.03.015
Anonymous, 2004. Food and agriculture organization. hptt//faostat.fao.org.
AOAC. 2000. Official methods of analysis of AOAC. International 17th edition; Gaithersburg, MD, USA Association of Analytical Communities.
Aslam, M., Mahmood, I., Ahmad, S., Peoples, M.B., Herridge, D.F., 1997. Survey of chickpea N2 - fixation in the Potohar and Thal areas of Punjab Pakistan. In extending nitrogen fixation research to farmer’s fields. Int. Workshop Manage. Legume Nitrogen Fixation. pp. 353-360.
Balogun, A.M. and B.L. Fetuga. 1986. Chemical composition of some under-exploited leguminous crop seeds in Nigeria. J. Agric. Food Chem., 34: 189-192. https://doi.org/10.1021/jf00068a008
Ben, R.S., P. De-Lajudie and R. Mhamdi. 2008. Selection of high nitrogen-fixing rhizobia nodulating chickpea (Cicer arietinum) for semi-arid Tunisia. J. Agron. Crop Sci., 194: 431- 420.
Tharajah, D., P. Tharajah, A. Sarker and A. Vandenberg. 2009. A whole food for increased iron and zinc intake. J. Agric. Food Chem., 57: 5413-5419. https://doi.org/10.1021/jf900786e
Erler, F., F. Ceylan, F.B and C. Toke. 2013. Preliminary results on evaluation of chickpea, Cicer arietinum, genotypes for resistance to the pulse beetle, Callosobruchus maculates. J. Insect. Sci. 9: 58.
Gajewska, E., and M. Sklodowska. 2005. Antioxidative response and proline level in leaves and roots of pea plant subjected to nickel stress. Acta Physiol. Plant, 27: 329-339. https://doi.org/10.1007/s11738-005-0009-3
Gore, M., 2000. Spectrophotometer and spectrofluorrimetry. New York: Oxford University.
GOP, 2008-2009. Agricultural statistic of Pakistan. Federal bureau of statistics 5-SLIC building F-14. Blue area, Islamabad, Pakistan. pp. 22.
Guo, W., G. Tiwari, J. Tang and S. Wang. 2008. Frequency, moisture and temperature dependent dielectric properties of chickpea flour. Biosyst. Eng., 101: 217-224. https://doi.org/10.1016/j.biosystemseng.2008.07.002
Hassan, A., B. Gammaa, A. osman and E.E. Babiker, 2005. Effect of domestic processing on antinutrients and availability of protein and mineral of lupins seeds. J. Food Technol., 3: 263-268.
Herrero, E.M., A. Iopez-Gonzalvez, M.A. Ruiz, J.A. Lucas-Garcia and Barbas. 2003. Uptake and distribution of zinc, cadmium, lead and copper in brassica napus var. oleifera and helianthus annus grown in condamination soils-International. J. Phytoremed., 5: 153-167. https://doi.org/10.1080/713610177
Food and Agriculture Organization of United Nations. 2008. Economic and social department. Available from FAOSTAT statistical database agriculture. Rome, Italy.
Khalil, I.A. and F. Manan, 1990. Text Book of Chemistry. 2nd Edn., Taj Kutab Khana, Peshawar.
Khatoon, N. and J. Prakash. 2004. Nutritional quality of microwave-cooked and pressure-cooked legumes. Int. J. Food Sci. Nutr., 55: 441-448. https://doi.org/10.1080/09637480400009102
Koch, K.E.U., 1996. Carbohydrate modulated gene expression in plant. Ann. Rev. Plant Physiol. Plant Mol. Biol., 47: 509-540. https://doi.org/10.1146/annurev.arplant.47.1.509
Lelaboye, N.O.A. and O.O. Pikuda. 2009. Determination of minerals and antinutritional factor of some lesser-known crop seeds. Pak. J. Nutr., 8: 1652-1656. https://doi.org/10.3923/pjn.2009.1652.1656
Lev-Yadun, S., A. Gopher and S. Abbo. 2000. The cradle of agriculture science 288: 1062-1063. https://doi.org/10.1126/science.288.5471.1602
O’Dell, B.L., C.E. Burpo and J.E. Savage, 1972. Evaluation of zinc availability of foodstuffs of plant and animal origin. J. Nutr. 102: 653-660.
Rincon, F., M. Beatriz and M.V. Ibanez. 1998. Proximate composition and antinutritive substances in chickpea (Cicer arietinum L.) as affected by the biotype factor. J. Sci. Food Agric., 78: 382-388. https://doi.org/10.1002/(SICI)1097-0010(199811)78:3<382::AID-JSFA128>3.0.CO;2-J
Sabanis, D., E. Makri and G. Doxastakis. 2006. Effect of durum flour enrichment with chickpea flour on the characteristic of dough and lasagna. J. Sci. Food Agric., pp. 1938-1944. https://doi.org/10.1002/jsfa.2567
Sanchez-vioque, R., A. Clement, J. vioque, J. Bautista and F. Millan. 1999. Protein isolates form chickpea (Cicer arietinum). Chemical composition functional properties and protein characterization. Food Chem., 64: 237-243. https://doi.org/10.1016/S0308-8146(98)00133-2
Sandell, E.B., 1959. Coloumetric determination of traces of metal, 3rd. Int. Sci. New York. pp. 1032.
Sattar, A. and M. A. Chaudhary. 1978. Trace element content of food and their interrelationship with protein values in milled fractions of wheat and triticale. Pak. J. Biochem., 11: 48-54
Shad, M.A., H. Pervez, Zafar, M. Zia-ul-Haq and H. Nawaz. 2009. Evaluation of biochemical composition and physic-chemical parameter of oil from seed of desi chickpea varieties cultivated in arid zone of Pakistan. Pak. J. Bot., 41: 655-662.
Shiferaw, B., R. Jones, S. Silim, Tekelwold and E. Gwata. 2007. Analysis of production cost market opportunities and competitive eneses of desi and Kabuli chickpea in Ethiopan farmer project working paper 3, ILRI, Nairobi, Kenya. pp. 48.
Smith, C., W.E. Van Megan, L. Twaal-Hoven and H. Cook. 1980. Determination of trypsin inhibitor level in food stuffs. J. Sci. Food Agric., 31: 341-350. https://doi.org/10.1002/jsfa.2740310403
Sotelo, A., F. Flores and M. Hernandez. 1987. Chemical composition and nutritional value of mexican varieties of chickpea. Plant Food Hum. Nutr., 37: 299-306. https://doi.org/10.1007/BF01092205
Steel, R., J.H. Torrie and D.A. Dicky. 1997. Principles and procedure of statistics. A biometrical approach 3rd Ed. McGraw Hill, Book Co. New York: pp. 352-358.
Tsialtas, J.T., M. Kassioumi and D.S. veresoglou. 2002. Evaluating leaf ash content and potassium concentration as surrogates of carbon isotope diserimination in grassland species. J. Agron. Crop Sci., 188: 168-175. https://doi.org/10.1046/j.1439-037X.2002.00555.x
USDA, 1954. Diagnosis and improvement salino and alkali soins, U.S. salinity laboratory staff agriculture handbook. pp. 60.
Wierzbicka, M., 1995. How lead loses its toxicity to plant. Acta Soc. Bot. Poland, 54: 81-91.
To share on other social networks, click on any share button. What are these?








