Productive Performance, Physiological Responses, Carcass Traits, and Meat Quality of Broiler Chickens Fed Quinoa Seeds
Research Article
Productive Performance, Physiological Responses, Carcass Traits, and Meat Quality of Broiler Chickens Fed Quinoa Seeds
Ahmed M. Elbaz*, Engy F. Zaki, Morsy A. S.
Desert Research Center, Cairo, Egypt.
Abstract | The objective of this study was to investigate the effect of feeding quinoa seeds as a source of energy and protein on performance, blood metabolite, and meat quality in broiler chickens. A total of four hundred and eighty 1-day-old Ross 308 chicks were randomly divided into four experimental groups: Control (CON), 5% of quinoa seeds (QS5), 10% of quinoa seeds (QS10), and 15% of quinoa seeds (QS15) groups. Results indicated that increasing inclusion levels of QS improved body weight gain, feed conversion ratio, and crude protein digestibility of broiler chickens. Including quinoa seeds significantly increased carcass dressing and decreased ( P < 0.05) abdominal fat content. Plasma triglyceride, LDL, creatinine, and uric acid levels were lower (P<0.05) in the birds fed QS supplementation, while HDL was significantly higher. Saturated fatty acid content and shear force value (more tender) were reduced (P<0.05) while increasing the levels of quinoa inclusion in broiler diets. It can be concluded that QS dietary supplementation would be recommended as a feasible approach to improve productive performance, nutrient digestibility, blood metabolite, and meat quality of broiler chickens.
Keywords | Quinoa, Broilers, Performance, Nutrient digestibility, Fatty acids, Meat quality
Received | October 07, 2021; Accepted | October 17, 2021; Published | January 10, 2022
*Correspondence | Ahmed M Elbaz, Desert Research Center, Cairo, Egypt; Email: dm.a.baz@gmail.com
Citation | Elbaz AM, Zaki EF, Morsy AS (2022). Productive performance, physiological responses, carcass traits, and meat quality of broiler chickens fed quinoa seeds. Adv. Anim. Vet. Sci. 10(2): 354-363.
DOI | http://dx.doi.org/10.17582/journal.aavs/2022/10.2.354.363
ISSN (Online) | 2307-8316
Introduction
The increased demand for animal protein and the rapid development in the poultry industry led to producing broiler chicks characterized by rapid growth that needs a balanced feed to meet all the bird’s requirements of energy, protein, and other elements (Choct, 2009). Also, the shortage and high prices of conventional ingredients feed such as corn and soybean, made nutritionists search for more feed ingredient sources that do not compete with human food, bear different climatic conditions, and are characterized by their high nutritional value to encourage its cultivation in Egypt. Quinoa was chosen as a plant that attracted the world’s attention in recent times due to its high nutritional and biological value and its tolerance to harsh environmental conditions. Quinoa (Chenopodium quinoa) is a stress-tolerant plant (salty/acid soils and cold/ hot climates), characterized by challenging different environmental conditions, also the seeds germinate very fast (Mujica et al., 2001: Yao et al., 2014). QS are distinguished from traditional feed ingredients by their high nutritional value and exceptional balance between fat, oil, and protein. In addition, QS has a high content of vitamins, minerals (working as cofactors in antioxidant enzymes), protein, and lipids (Filho et al., 2017), and an excellent balance of essential amino acids (high-quality protein). Quinoa protein contains high content of methionine (3.3 - 9.1 g/100 g protein) and lysine (2.4 - 7.8 g/100 g protein) which are the most important amino acids in poultry feeding, especially that most of the conventional cereals (maize) suffer from a lack of them (Dini et al., 2005). Besides, it’s found to contain numerous phytochemicals including phytosterols, phenolic, and bioactive peptides, which can be used as a natural antioxidant in poultry feed. The high nutritional value of quinoa improves the health of the birds (Dini et al., 2005). Adding to these previous quinoa features, the Food and Agriculture Organization of the United Nations (FAO, 2013) promoted quinoa production and consumption for humans and animals. However, QS contains some anti-nutrients such as saponins, tannins, and phytic acid (Ruales and Nair, 1993), which may hinder its use in poultry feed in large quantities. Anti-nutrition affects the utilization of some nutrients, which negatively affects the growth performance of broiler chickens. Therefore, it is necessary to treat quinoa seeds before adding them to poultry feed (such as soaking). Several studies have shown that soaking of quinoa seeds reduces anti-nutrients such as saponins, tannin, and phytate (Fadahunsi, 2009: Mazahib et al., 2013). This study aimed to evaluate quinoa seed as a feed additive in a chick’s diet and assess the effect on productive performance, blood metabolites, and meat quality of broiler chickens.
Material and Methods
Quinoa seed and handling
Seeds of quinoa (Chenopodium quinoa) were supplied by the following research project: “Climate Smart Agriculture Entrepreneurship Development of Quinoa Value in Egypt. The quinoa seeds were analyzed at the Central Food and Feed Laboratory in Egypt (Table 1). The seeds were soaked in distilled water for 48 h thereafter the soaked seeds were washed with distilled water several times in a row, according to the method described by Udensi et al. (2008). Samples were dried in a room with a temperature of 30 to 32ºC with stirring until complete drying (about 8 days).
Table 1: Chemical composition of quinoa (g 100 g−1 dried weight)
| Chemical composition | Quinoa |
| Protein (g) |
15.7 |
| Carbohydrate (g) | 67.3 |
| Fat (g) | 7.1 |
| Ash (g) | 3.4 |
| Fiber (g) | 2.9 |
Birds and experimental design
Four hundred and eighty chicks (Ross 308) were purchased from the commercial hatchery and the experiment was conducted for 42 days, divided into 4 equal groups, each group including 120 chicks (6 replicates/20 birds each). One group was fed with control feed (corn-soybean basal diet) while other groups were fed with a diet contained with quinoa seeds. QS5: diet contains 5% of quinoa seeds, QS10: diet contains 10% of quinoa seeds, and QS15: diet contains 15% of quinoa seeds. Feed, automatic nipple cup drinker, and light provided during the study period. The chemical analysis of the quinoa seeds was done to formulate the experimental diets as shown in Table (1). It was divided into two-phase diets: starter diet from 1-21 days old (iso-caloric = 3050, iso-nitrogenous = 23) followed by grower diet from 22-42 days old (iso-caloric = 3150, iso-nitrogenous = 21) formulated according to the nutritional recommendations of NRC (1994). The composition and calculated analysis of basal diets are shown in Table (2). The chicks were raised at 33.0 ± 0.5 °C, and then the temperature was gradually decreased until 28.0 ± 1 °C was reached by day 15, and then left with the case of natural temperature.
Growth Performance and Carcass traits
Live body weight (LBW), feed intake (FI) g/chick were at 21 and 42 days recorded, body weight gain (BWG) g/chick, and feed conversion rate (FCR) g FI/g BWG were calculated for the overall period and corrected for mortality rate. At the end of the experiment (42 days), 80 birds (20 birds from each group) were sacrificed and then dissected to measure the relative weights of the dressing, abdominal fat, breast, and thigh to live body weight. Chicken meat from dietary treatments were collected, packed and frozen at -20ºC for subsequent analyses.
Digestibility experiment
On day 42, ten broilers per group were randomly assigned to metabolism cages with wire mesh bottom and excreta collection trays. Before the digestibility experiment started, the bird was adapted for 3 days then, excreta from each cage were collected 3 times daily (each 8-h intervals) during 4 -day collection period, a known quantity of the feed was given daily to each bird. Then remaining feed in the excreta trays was carefully removed and weighed, also feathers were removed, and stored in sealed bags at −20 °C after being dried till analyzed. Excreta samples were subsequently analyzed for dry matter (DM), crude protein (CP), ether extract (EE), and nitrogen-free extract (NFE) using routine procedures (AOAC, 1990).
Blood Samples and Biochemical Analysis
At day 42 days, blood samples (5 ml) from 20 birds from the group were collected during slaughtering (gathered into heparinized test tubes), and then rapidly centrifuged (3500 rpm for 15 min) to separate the plasma and stored at −20 °C until analysis. Plasma total protein (TP), albumin (AL), uric acid (UA), creatinine (CR), total cholesterol (TC), high-density lipoprotein (HDL), low-density lipoprotein (HDL), triglyceride (TG), glucose (GLU), alanine aminotransferase (ALT), and aspartate aminotransferase (AST) were measured calorimetrically by using a commer
Table 2: Feed ingredients and chemical analyses of experimental diets
| Ingredients (%) |
Starter(1-21 d) |
|
|
Grower(22-42 d) |
|
|||
|
CON |
Q5 |
Q10 |
Q15 |
CON |
Q5 |
Q10 |
Q15 |
|
| Yellow corn |
53.35 |
49.60 |
45.95 |
43.44 |
57.70 |
54.05 |
50.12 |
45.56 |
| Soybean meal (44%) |
33.14 |
32.14 |
31.00 |
29.50 |
28.65 |
28.26 |
27.34 |
26.8 |
| Corn gluten meal (62%) |
6.35 |
6.35 |
6.35 |
6.35 |
5.75 |
5.20 |
5.20 |
5.20 |
| Quinoa |
0.00 |
5.00 |
10.00 |
15.00 |
0.00 |
5.00 |
10.00 |
15.00 |
| Soybean oil |
3.00 |
2.70 |
2.39 |
1.40 |
3.95 |
3.50 |
3.28 |
3.30 |
| Calcium carbonate |
1.23 |
1.23 |
1.23 |
1.23 |
1.07 |
1.17 |
1.15 |
1.15 |
| Di-calcium phosphate |
1.93 |
1.93 |
2.03 |
2.03 |
1.98 |
1.85 |
1.88 |
1.90 |
| Broiler premix* |
0.35 |
0.35 |
0.35 |
0.35 |
0.35 |
0.35 |
0.35 |
0.35 |
| Salt |
0.45 |
0.45 |
0.45 |
0.45 |
0.40 |
0.40 |
0.40 |
0.40 |
| DL-methionine |
0.20 |
0.20 |
0.20 |
0.20 |
0.15 |
0.17 |
0.20 |
0.22 |
| L-lysine |
0.00 |
0.05 |
0.05 |
0.05 |
0.00 |
0.05 |
0.08 |
0.12 |
| Total |
100 |
100 |
100 |
100 |
100 |
100 |
100 |
100 |
| Chemical analysis |
|
|
|
|
|
|
|
|
|
ME (kcal kgG-1) |
3050 |
3050 |
3050 |
3050 |
3150 |
3150 |
3150 |
3150 |
| Crude protein |
23.00 |
23.00 |
23.00 |
23.00 |
21.00 |
21.00 |
21.00 |
21.00 |
| Calcium |
1.00 |
1.00 |
1.00 |
1.00 |
0.95 |
0.95 |
0.95 |
0.95 |
| Av. phosphorus |
0.48 |
0.48 |
0.48 |
0.48 |
0.45 |
0.45 |
0.45 |
0.45 |
*Premix: (1%) provided the following (per Kilogram of complete diets). 50 mg vitamin E, 4 mg vitamin K, 3 mg Vitamin B6, 6 mg Vitamin B12, 1400 IU vitamin A, 3000 IU Vitamin D3,60 mg Niacin, 20 mg Pantothenic acid, 0.20 mg folic acid, 48 mg Ca, 3.18 mg P, 150 mg Choline, 100 mg Mn, 50 mg Fe, 80 mg Zn, 10 mg Cu, 0.25 mg Co, and 1.5 mg Iodine.
Table 3: Effects of feeding quinoa seeds on growth performance and carcass traits (% body weight) in broilers at 42 days.
| Phase | Items | CON |
QS5 |
QS10 |
QS15 |
SEM | P-value |
| Starter (1 to 21 d) | BWG (g/d) | 32.8 | 33.6 | 34.7 | 34.2 | 0.703 | 0.62 |
| FI(g/d) | 46.1 | 45.8 | 46.6 | 45.1 | 0.515 | 0.19 | |
| FCR (g feed /g gain) | 1.40 | 1.37 | 1.34 | 1.32 | 0.032 | 0.21 | |
| Grower (22 to 42 d) | BWG (g/d) |
67.3b |
70.3ab |
72.5a |
73.8a |
0.777 | 0.04 |
| FI (g/d) | 115.9 | 118.4 | 119.2 | 120.6 | 1.031 | 0.06 | |
| FCR (g feed /g gain) |
1.72a |
1.70a |
1.64ab |
1.63b |
0.025 | 0.05 | |
| Overall (1 to 42 d) | BWG (g/d) |
50.2b |
51.9ab |
53.6a |
53.9a |
0.701 | 0.02 |
| FI (g/d) | 80.6 | 81.2 | 82.7 | 82.5 | 0.590 | 0.09 | |
| FCR (g feed /g gain) |
1.62a |
1.57ab |
1.54b |
1.53b |
0.012 | 0.03 | |
| Carcass traits (%) | Dressing |
70.7b |
71.2ab |
72.8a |
72.3a |
0.971 | 0.01 |
| Breast | 26.71 | 26.94 | 27.15 | 27.02 | 0.544 | 0.10 | |
| Thigh | 16.28 | 16.36 | 16.48 | 16.27 | 0.422 | 0.35 | |
| Abdominal Fat |
2.08a |
1.81b |
1.72b |
1.79b |
0.131 |
0.02 |
a-b means within the same row with different superscripts letters are different (P<0.05). CON: control diet, QS5: diet contains 5% of quinoa seeds, QS10: diet contains 10% of quinoa seeds, and QS15: diet contains 15% of quinoa seeds. SEM: Standard error of means, BWG: Body weight gain, FI: Feed intake, FCR: Feed Conversion Ratio.
Table 4: Effects of feeding quinoa seeds on nutrient digestibility in broilers at 42 days.
| Items | CON |
QS5 |
QS10 |
QS15 |
SEM | P-value |
| DM (%) | 71.3 | 71.8 | 72.2 | 71.6 | 0.631 | 0.19 |
| CP (%) |
66.4b |
67.3ab |
68.9a |
68.2a |
0.805 | 0.03 |
| EE (%) | 70.1 | 69.7 | 71.0 | 70.6 | 1.671 | 0.17 |
| NFE (%) | 82.8 | 84.2 | 83.7 | 84.3 | 0.457 |
0.09 |
a-b means within the same row with different superscripts letters are different (P<0.05). CON: control diet, QS5: diet contains 5% of quinoa seeds, QS10: diet contains 10% of quinoa seeds, and QS15: diet contains 15% of quinoa seeds. SEM: standard error of means. DM: dray matter, CP: crude protein, EE: ether extract, and NFE: Nitrogen-free extract.
cial kit (Diamond Diagnostics, Cairo, Egypt), using spectrophotometry analysis.
Fatty acid profile
The fatty acid profiles of broiler chicken meat were analyzed as described by AOAC (2012). The fatty acids are methylated with boron trifluoride in methanol, extracted with heptanes, and determined on a gas chromatograph with FID detector (PE Auto System XL) with an auto-sampler and Eȥchrom integration system. Carrier gas (He); ca. 25Psi- air 450ml/min- Hydrogen 45ml- split100 ml/min. Oven temperature 200 °C, injectors, and detector 250 °C. Lipid extraction and direct methylation were performed as described by Folsch et al. (1957).
pH value
pH values of raw chicken meat samples were determined as described by Hood (1980). Using a digital pH-meter (Jenway 3310 conductivity and pH meter) Values of pH were determined in triplicate for each treatment.
Water Holding Capacity (WHC)
The water holding capacity of raw meat was measured as described by Grau and Hamm (1953).
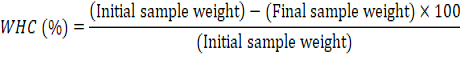
Cooking loss and Shear force value
Chicken meat samples of each treatment were cooked in a water bath at 85°C until the internal temperature reached 75 ±3°C. The cooking loss was determined as reported by Meek et al. (2000) as follows:
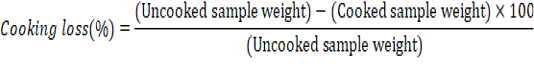
Cooked chicken meat samples of each treatment were sheared three times at different positions by using Intron Universal Testing Machine (Model 2519-105, USA). The average shear force was calculated from the three obtained results (Kg/f).
Color measurements
The color of raw chicken meat samples was measured by Chroma meter (Konica Minolta, model CR 410, Japan) calibrated with a white plate and light trap supplied by the manufacturer (CIE, 1976). The color was expressed as L* (lightness), a* (the redness), and b* (the yellowness). Color measurements were calculated as the average of three spectral readings at different locations for each treatment.
Statistical analysis
All data were analyzed by a one-way analysis procedure (ANOVA) using the General Linear Models (GLM) procedure of SAS software (SAS., 2004). Differences in the mean values between the groups were assessed via Duncan’s multiple range test (Duncan, 1955), the effects were considered significant if P < 0.05.
Results
Performance and Carcass trait
Growth performance did not differ during the starter period (1-21 d) with the inclusion of quinoa seeds in broiler diets, as shown in Table (3). However, the body weight gain increased significantly, and the feed conversion ratio improved with the increase in the inclusion of quinoa seeds in the diet during the grower period (22-42 d) and overall, while the feed intake was not affected during the experiment. The inclusion of quinoa seeds in broiler diets led to a significant increase in carcass dressing and a decrease in abdominal fat compared to the control group (p<0.05), while the relative weights of the breast and thigh did not differ among the groups, as shown in Table (3).
Nutrient Digestibility
Total digestibility of dry matter (DM), crude protein (CP), ether extract (EE), and nitrogen-free extract (NFE) in broilers at day 42 are presented in Tables (4). The total digestibility of CP in the QS group was significantly higher than in the control group (P< 0.05). While, the total digestibility of DM, EE, and NFE were insignificantly affected by the addition of QS.
Table 5: Effects of feeding quinoa seeds on blood metabolites and lipid profile in broilers at 42 days.
|
|
Items |
CON |
QS5 |
QS10 |
QS15 |
SEM |
P-value |
|
Plasma metabolites |
AST (U/L) |
218.5 |
214.0 |
221.3 |
209.6 |
3.116 |
0.15 |
|
|
ALT (U/L) |
27.28 |
26.87 |
26.45 |
26.69 |
0.655 |
0.09 |
|
|
TP (g.dl-1) |
3.63 |
3.55 |
3.68 |
3.64 |
0.023 |
0.13 |
|
|
AL (g.dl-1) |
1.72 |
1.69 |
1.71 |
1.66 |
0.019 |
0.34 |
|
|
UA (mg.dl-1) |
6.12a |
6.07a |
5.25b |
5.19b |
0.038 |
0.02 |
|
|
CR (mg.dl-1) |
0.461a |
0.405b |
0.396b |
0.391b |
0.091 |
0.03 |
|
Lipid profile |
TC (mg.dl-1) |
174.7 |
170.2 |
168.5 |
171.9 |
3.545 |
0.14 |
|
|
LDL (mg.dl-1) |
124.1a |
108.7ab |
95.7b |
96.9b |
3.062 |
0.01 |
|
|
HDL (mg.dl-1) |
46.2b |
45.7b |
47.5ab |
48.5a |
0.894 |
0.04 |
|
|
TG (mg.dl-1) |
231.6a |
214.5b |
219.1b |
206.5c |
2.677 |
0.03 |
|
|
GLU (mg.dl-1) |
242.3 |
235.0 |
239.2 |
236.7 |
4.051 |
0.11 |
a-c means within the same row with different superscripts letters are different (P<0.05). CON: control diet, QS5: diet contains 5% of quinoa seeds, QS10: diet contains 10% of quinoa seeds, and QS15: diet contains 15% of quinoa seeds. SEM: standard error of means. ALT= Alanine aminotransferase, AST: Aspartate aminotransferase, TP: Total Protein, UA: Uric Acid, AL: Albumin, CR: Creatinine, TC: Total Cholesterol, TRI: Triglyceride, GLU: Glucose, HDL: high-density lipoprotein, LDL: low-density lipoprotein
Table 6: Effects of feeding quinoa seeds on fatty acid composition (% of total fatty acids) of broiler chicken meat
| Fatty acids | CON |
QS5 |
QS10 |
QS15 |
SEM | P-value |
|
Lauric acid C12:0 |
2.08b |
0.58d |
4.71a |
0.75c |
0.02 | 0.03 |
|
Myristic acid C14:0 |
1.00b |
0.58c |
2.26a |
0.47c |
0.04 | 0.01 |
|
Palmitic acid C16:0 |
22.03d |
27.31b |
29.65a |
23.59c |
0.25 | 0.04 |
|
Stearic acid C18:0 |
6.84c |
8.39b |
8.70a |
6.22d |
0.05 | 0.02 |
| ∑SFA |
33.46c |
37.38b |
46.78a |
31.46d |
0.25 | 0.01 |
|
C16:1ω7 |
3.84b |
5.24a |
2.63c |
5.33a |
0.12 | 0.05 |
|
Oleic acid C18:1ω9 |
40.50a |
40.52a |
35.51c |
40.35b |
0.04 | 0.02 |
|
Eicosaenoic acid C20:1ω11 |
0.31c |
0.49b |
0.60a |
0.31c |
0.01 | 0.01 |
| ∑MUFA |
47.99b |
48.99a |
43.83c |
48.29b |
0.16 | 0.04 |
|
Linoleic acid C18:2ω6 |
14.80b |
11.89c |
7.86d |
18.51a |
0.03 | 0.01 |
|
C20:2ω6 |
0.26a |
0.13b |
0.22a |
0.11b |
0.01 | 0.05 |
|
Linolenic acid C18:3ω3 |
0.88b |
0.44c |
0.28d |
1.14a |
0.01 | 0.02 |
|
Arachidonic acid C 20:4ω6 |
1.18a |
0.32c |
0.40b |
0.25d |
0.01 | 0.04 |
| ∑PUFA |
18.14b |
13.49c |
9.41d |
20.15a |
0.05 | 0.02 |
| ∑UFA |
66.13b |
62.49c |
53.24d |
68.44a |
0.16 | 0.02 |
| UFA/SFA |
1.97b |
1.66c |
1.14d |
2.17a |
0.01 | 0.03 |
| MUFA/ SFA |
1.43b |
1.30c |
0.93d |
1.53a |
0.01 | 0.04 |
| PUFA/ SFA |
0.54b |
0.36c |
0.20d |
0.64a |
0.04 | 0.05 |
|
∑ω6 |
16.95b |
12.58c |
9.12d |
19.01a |
0.04 | 0.05 |
|
∑ω3 |
1.19a |
1.06b |
0.62c |
1.14a |
0.01 | 0.03 |
| n-6:n-3 |
14.24b |
11.88c |
14.71b |
16.67a |
0.21 | 0.04 |
| Non identified | 0.31 | 0.1 | 0.0 | 0.1 | 0.15 |
0.09 |
a-d means within the same row with different superscripts letters are different (P<0.05). CON: control diet, QS5: diet contains 5% of quinoa seeds, QS10: diet contains 10% of quinoa seeds, and QS15: diet contains 15% of quinoa seeds. SEM: standard error of means. ∑SFA: sum of saturated fatty acids. ∑MUFA: sum of monounsaturated fatty acids. ∑PUFA: sum of polyunsaturated fatty acids. ∑UFA: sum of total unsaturated fatty acids (MUFA + PUFA). ∑ω6: sum of polyunsaturated fatty acids n-6. ∑ω3: sum of polyunsaturated fatty acids n-3. n-6:n-3: ∑ω6/∑ω3.
Table 7: Effects of feeding quinoa seeds on physical properties of broiler chicken meat
| Items | CON |
QS5 |
QS10 |
QS15 |
SEM | P-value |
| pH |
5.71a |
5.64a |
5.27b |
5.31b |
0.05 | 0.01 |
| Cooking loss (%) |
28.71a |
26.43a |
28.57a |
26.58a |
1.35 | 0.02 |
| WHC (%) |
25.43b |
31.24a |
23.36b |
24.02b |
1.35 | 0.03 |
|
Shear force(kg/f) |
2.87a |
2.20b |
2.90a |
2.14b |
0.15 |
0.01 |
a-b means within the same row with different superscripts letters are different (P<0.05). CON: control diet, QS5: diet contains 5% of quinoa seeds, QS10: diet contains 10% of quinoa seeds, and QS15: diet contains 15% of quinoa seeds. SEM: standard error of means. WHC: water holding capacity.
Table 8: Effects of feeding quinoa seeds on color measurement of broiler chicken meat
|
Items |
CON |
QS5 |
QS10 |
QS15 |
SEM |
P-value |
|
L* |
54.99a |
53.48b |
49.50d |
52.57c |
0.25 |
0.03 |
|
a* |
9.99c |
12.09b |
13.39a |
11.98b |
0.23 |
0.01 |
|
b* |
12.24b |
9.80c |
14.33a |
11.81b |
0.43 |
0.01 |
a-d means within the same row with different superscripts letters are different (P<0.05). CON: control diet, QS5: diet contains 5% of quinoa seeds, QS10: diet contains 10% of quinoa seeds, and QS15: diet contains 15% of quinoa seeds. SEM: standard error of means. L*: (lightness), a*: (redness), b* (yellowness).
Blood metabolites
Data presented in Table (5) showed that the QS group exhibited a significant decrease in TG and LDL compared with the control group. However, the concentration of total protein, albumin, glucose, cholesterol, and HDL didn’t show any significant changes. Results of liver and kidney functions were presented in Table (5) revealed that QS decreased (P< 0.05) creatinine and uric acid level compared with the control group, while there was no significant effect of QS on AST and ALT.
Fatty acid profile
It was observed that meat of broiler chicken fed on diets containing a higher level of quinoa seeds (QS15) had a lower content of saturated fatty acids than those fed on control and lower levels of quinoa seeds (QS5, and QS10), as shown in Table (3). Conversely, meat from feeding groups of control and lower level of quinoa seeds (CON and QS5) showed significantly higher content of oleic acid (C18:1ω9) than that containing higher levels of quinoa seeds (QS10, and QS15). Regarding linoleic acid (C18:2ω6) the highest value was found in the meat of QS15 followed by meat of CON (control group) and QS5, while the lowest value was found in the meat of QS10. Similar trends were found in linolenic acid. The polyunsaturated fatty acids (PUFA) content was significantly higher in broiler chicken of QS15. No significant differences were found in ω3 between the control (CON) and QS15 groups. On the other hand, broiler fed on different levels of quinoa seeds had a significantly higher n-6: n-3 ratio compared with broiler fed on the control group.
Physical properties
Results of pH values indicated that no significant differences were found in pH values between the meat of CON and QS5 feeding groups, as shown in Table (7). However, no significance was found in pH values between the meat of QS10 and QS15 feeding groups. Generally, meat from feeding groups of control and lower level of quinoa seeds (CON and QS5) showed higher pH values than that feeding on higher levels of quinoa seeds (QS10, and QS15). Data of cooking loss of chicken meat samples revealed that feeding on different levels of quinoa seeds did not significantly affect the cooking loss of meat. No significant differences were found in WHC of broiler meat fed on the control group (CON) and different levels of quinoa seeds groups (QS10 and QS15) except for meat of QS5. Results of shear force values of meat exhibited that meat of CON and QS10 had the highest shear force value (low tender) while; meat of QS5 and QS15 had the lowest value (more tender).
Color measurements
The results of L* values revealed that the meat of the control feeding group (CON) had the highest L* value, followed by the meat of QS15 and QS5, as shown in Table (8). The lowest L* value was found in the meat of QS10. Conversely, the meat of CON showed the lowest a* value, while the highest value was found in the meat of QS10. On the other hand, data of b* values showed that no significant differences were found in the meat of CON and QS15, whereas the highest value was found in the meat of QS10 and the lowest found in the meat of the QS5 group.
Discussion
Performance and Carcass trait
The present study was designed to study the possibility of using quinoa seeds (which are known to have high nutrient value) as a feed material for poultry by adding different levels of it and studying its effect on performance, carcass characteristics, physiological responses, and meat quality in broilers. It was hypothesized that adding QS in broiler feed enhancing the bird’s health and improving nutrient utilization would result in improving productive performance. Although the feed intake was not affected between the experimental groups, there was a noticeable improvement in the BWG and FCR, especially in birds fed on higher levels of quinoa seeds (QS10 and QS15). The improvement in the growth performance of birds fed on diets containing QS may be due to its higher biological value and possesses a balance of proteins and lipids, also rich protein content (15.7% CP). As quinoa does not contain only a high amount of protein, but it also contains all the essential amino acids in high quantities and balance (Filho, et al., 2017), meaning that the bioavailability of amino acids is high (high-quality proteins), especially the methionine, lysine, and tryptophan (Comai, 2007), which the chicks need. It is rich in micronutrients, such as vitamins (riboflavin, pyridoxine, and folic acid) and minerals (Alvarez-Jubete et al., 2010). Similar results were obtained by Marzoni et al. (2014) who reported that dietary inclusion of plants or herbal extracts was reported to have a positive effect on growth by improvement of nutrients digestion and absorption. Shaimaa et al. (2020) also reported that adding quinoa led to the protection of the hepatic tissue and the enhancement of digestive function, immunological responses, and antioxidant status, which led to an enhanced productive performance.
The improvement in carcass dressing in broilers fed quinoa may be due to the higher nutritional value of quinoa, which is reflected in an increase in the digestibility of nutrients and improved growth performance. Our results are in agreement with Vilcacundo et al. (2017) who found that improve performance in broilers, means increasing carcass dressing percentage. Statistical analysis showed less fat abdominal in chickens fed QS than that fed control diet. This represents an economic benefit in the poultry industry, where abdominal fat is considered a waste. Lippens, (2003); Jennen, (2004) indicated that abdominal and subcutaneous fat (excess fat), which is the main source of waste in the slaughterhouse, is one of the problems facing the poultry industry because it reduces carcass yield. On the other hand, the presence of fatty acids in the tissues or muscles contributes to the quality, succulence, and flavor development of meat and is essential for the nutritional value of meat (Wood, 2008). From here, it can be said that adding quinoa to broiler diets is economically feasible because of its ability to improve the distribution of fat within the carcass.
Nutrient Digestibility
Statistical analysis of the data showed significantly higher CP digestibility of the diet supplemented with QS fed to broilers compared with the control group. Higher crude protein digestibility might be due to chemical composition, which revealed that QS is a top-quality protein and contain several bioactive compounds (natural antioxidants) that improve nutrient digestion. Wang et al. (2008) found improvement in broilers’ performance due to the antioxidant positive effect on health and nutrient digestibility when fed broiler on dietary inclusion of natural antioxidants, such as QS.
Blood metabolites
Blood biochemical indices are essential indicators of broilers’ physiological status, especially with environmental changes or feed additions. The obtained results indicated that adding QS reduced the plasma concentration of LDL and TG and gave a healthy result for the consumer. There was also an insignificant decrease in the total cholesterol level of chickens fed quinoa. These results demonstrate that the inclusion of QS in broilers feed may have a possible role in altering the lipid profile in the blood. Also, the inclusion of QS in broilers diet may reduce the accumulation of cholesterol and LDL, and the reason may be due to the distinctive fat content of quinoa, which is considered beneficial to the bird and the consumer (Çiftçi, et al. 2018).
Moreover, the beneficial changes in the blood lipids of chickens fed QS can be characterized by its distinct content of fatty acids, which can play a role in lipid metabolism and enhance enzyme activity that promotes the biliary cholesterol secretion, lowering fractional absorption of diet cholesterol (Abd El-Hack et al., 2020). Another effect of adding QS to the diet on blood lipids can be explained by its ability to deconjugate and dehydroxylate bile acids which results in inhibiting lipids absorption, fatty acid synthesis, and increasing fatty acids fecal excretion, which results in lowering blood lipid profiles (Abd El- Moneim and Sabic, 2019). In addition, the present study revealed that increasing the level of QS in the diets resulted in a higher level of plasma HDL. It is known that the high level of HDL in the blood has a beneficial effect on the bird’s body and conditioning the transport of cholesterol from the peripheral tissue to the liver. Our data did not a significant clear effect of adding different levels of QS on plasma total protein and albumin. While, a significant difference (P<0.05) was noticed in the levels of creatinine and uric acid. The main protein metabolism product in birds is uric acid. It is known that the lower the level of uric acid in the blood indicates good protein metabolism, which is affected by dietary protein quality, total food intake, and others. This confirms the high quality of quinoa protein, as shown by several studies (Nowak et al., 2016; Lin et al., 2019). Liver enzymes as ALT and AST have been studied as an indicator of liver tissue integrity and function, as the liver is involved in many metabolic processes (Minafra et al., 2009). Any changes in the physiological state of the bird are a result of environmental or nutritional changes that may affect the liver tissue state, whether beneficial or harmful, resulting in changes in ALT and AST levels. Our results also indicated that ALT and AST levels were not affected, which indicated that the liver tissue was not affected by any damage with the addition of quinoa seeds. Results of the current study indicated that the inclusion of quinoa seeds in the diet led to an improvement in the physiological condition of the broiler, which has a positive impact on growth performance.
Fatty acid profile
Recently, the consumer has become more interested in the healthy aspects of food, especially the association of many diseases with the type of fatty acids in meat (Russo, 2009). Some studies have also shown that nutrition plays an effective role in the formation of fatty acid profiles in poultry meat (Viveros et al., 2009). Therefore, it was necessary to shed light on the effect of feeding chicken a diet containing quinoa seeds on the fat content of meat. In this study, feeding broiler chicken on different levels of QS had a significant effect on the fatty acid profile of broiler chicken meat. Where the meat of broilers fed a diet containing a higher level of quinoa seeds (QS15) had the lowest content of saturated fatty acids. Higher content of linoleic acid (C18:2ω6) was found in the meat of QS15 than in the control group. Likewise, the meat of the feeding group QS15 had the highest content of linolenic acid (C18:3ω3). Similar results were obtained by Schilling et al. (2010). The fatty acids profile of chicken meat from dietary groups is reflecting the composition of fatty acids of quinoa seeds as found by Altuna et al. (2018). Additionally, the chemical analysis of fatty acids indicated a balance in them because they contain: unsaturated fatty acids oleic (19.7%-29.5%), linoleic (49.0%-56.4%), and alpha-linolenic (8.7%-11.7%) acids, while palmitic acid (10 - 11.5%) is the major saturated fatty acid found in quinoa, which affects blood lipids (Repo-Carrasco, et al., 2003). In view of the current study, it could be concluded that including quinoa seeds in broilers diets resulted in modifying the fatty acid profile and improving the nutritional value of chicken meat.
Physical properties
Data of pH values indicated that the addition of quinoa seeds had no significant effects on pH values of CON and QS5 or between QS10 and QS15 feeding groups. These results are coming in accordance with that obtained by Marino et al. (2018) they found that no significant differences were found in pH values of meat fed a diet containing quinoa seeds and /or quinoa with linseed. On the same basis, George et al. (2017) found that pH values were not affected by the addition of sorghum in broiler diets. Shim et al. (2018) found that no significant difference was found in pH values of broiler meat fed on different levels of dried grains. The addition of quinoa seeds had no significant effects on the cooking loss of broiler chicken meat. These results are in line with that obtained by Parpinelli et al. (2020) who indicated that cooking loss % of breast meat was not affected by the level of dried grains in the broiler diet. Similarly, was found by Shim et al. (2018). The incorporation of different levels of quinoa seeds into the broiler diet had no significant effect on the WHC of chicken meat. These results are consistent with Parpinelli et al. (2020) they indicated that supplemented broiler diet with different levels of dried grains had no significant effect on the WHC of meat.
Data of shear force values indicated that supplemented broiler diet with different levels of quinoa seeds did not significantly affect the tenderness of chicken meat. These results are close to that obtained by Marino et al. (2018) they found that the shear force value of meat from the control group was higher than that of feeding on quinoa seed and /or linseed. Concurrently, the differences in shear force values of meat from the quinoa and linseed feeding group were not significant. On the other hand, Shim et al. (2018) found that the addition of dried grains in the broiler diet resulted in a significant increase in shear force values of breast meat. Our results are in line with that obtained by Parpinelli et al. (2020) they found that using different levels of dried grains in broiler diets had no significant effect on the shear force values of meat.
Color measurements
Data of color measurements indicated that the color characteristics of broiler chicken meat were significantly affected by feeding on different levels of quinoa seeds. Generally, the meat of quinoa feeding groups had higher a* values (more red) than the meat of the control group. This may be attributed to the higher content of phenolic and flavonoid compounds of quinoa seeds, which play as a source of natural antioxidant agents resulting in improvement of meat color (Bastidas et al., 2016). Also, Easssawy et al. (2016) reported that the addition of quinoa seed extract in broiler diets can be delayed the lipid oxidation of chicken meat. On the same basis, the results of our study are close to that obtained by Marino et al. (2018) they found that a* values of meat fed on quinoa seeds and /or linseed were higher than the control meat. On the other hand, Shim et al. (2018) found that using dried grains in a broiler diet resulted in a significant difference in meat color.
Conclusion
Results of the current study revealed that the inclusion of quinoa seeds in broilers diet resulted in enhancing productive performance, improving blood metabolites, and nutrient digestion. Further, quinoa seeds in broilers diet improved the fatty acid profile and the nutritional value of chicken meat without any negative effect on meat quality. These results uphold the potential of adding quinoa seed to broiler feed to reduce costs and enhance productive performance and meat quality. Moreover, we need more research into the possibility of adding larger quantities of quinoa seed to broilers feed.
Acknowledgments
Quinoa seeds were supplied by the following research project: “Climate Smart Agriculture Entrepreneurship Development of Quinoa Value in Egypt” funded by (HSBC) bank in Dubai and the International Center for Bio saline Agriculture (ICBA).
Compliance with ethical standards Ethical approval
All the procedures used in this trial were approved by the Local Experimental Animals Care and Welfare Committee in Desert Research Center.
Conflict of interest
The authors declare no competing interests.
authors contribution
A.M.E., E.F.Z., A.A.S. Formal analysis: A.M.E., E.F.Z., Investigation: A.M.E., E.F.Z., A.A.S. Methodology: A.M.E., E.F.Z. Writing—original draft preparation: A.M.E., E.F.Z. Writing—review, and editing: A.M.E., E.F.Z. Validation and supervision: A.M.E., E.F.Z. All authors read and approved the final manuscript.
References
Abd El-Hack ME, Alagawany M, Abdel-Moneim A-ME, Mohammed NG, Khafaga AF, Bin-Jumah M, Othman SI, Allam AA, Elnesr SS (2020). Cinnamon (Cinnamomum zeylanicum) Oil as a Potential Alternative to Antibiotics in Poultry, Antibiotics. 9: 210-221. Https://doi.org/ 10.3390/antibiotics9050210
Abd El-Moneim AE, Sabic EM (2019). Beneficial effect of feeding olive pulp and Aspergillus awamori on productive performance, egg quality, serum/yolk cholesterol and oxidative status in laying Japanese quails. J. Anim. Feed Sci. 28: 52-61.
Https://doi.org/ 10.22358/jafs/105537/2019
Ahmed SA, El-Rahman A, Ghada I, Behairy A, Beheiry RR, Hendam BM, Alsubaie FM, Khalil SR (2020). Influence of Feeding Quinoa (Chenopodium quinoa) Seeds and Prickly Pear Fruit (Opuntia ficus indica) Peel on the Immune Response and Resistance to Aeromonas sobria Infection in Nile Tilapia (Oreochromis niloticus). Animals. 10(12): 2266. Https://doi.org/ 10.3390/ani10122266
Altuna JL, Silva M, Alvarez M, Quinteros MF, Morales D, Carrillo W (2018). Ecuadorian quinoa (Chenopodium quinoa Willd) fatty acids profile. Asian J. Pharm. Clin. Res. 11:209. Https://doi.org/10.22159/ajpcr.2018.v11i11.24889
Alvarez-Jubete L, Wijngaard H, Arendt EK, Gallagher E (2010). Polyphenol composition and in vitro antioxidant activity of amaranth, quinoa buckwheat, and wheat as affected by sprouting and baking. Food Chem. 119(2):770-8. Https://doi.org/ 10.1016/j.foodchem.2009.07.032
AOAC (2012). Official method of analysis. 19th ed. Gaithersburg Maryland: Association of Official Analytical Chemists; 2012.
Bastidas EG, Roura R, Rizzolo DA, Massanés T, Gomis R (2016). Quinoa (Chenopodium quinoa Willd), from nutritional value to potential health benefits: An integrative review. J. Nutrit. Food Sci. 6(3): 497-507. Https://doi.org/ 10.4172/2155-9600.1000497
Choct M (2009). Managing gut health through nutrition. British Poult. Sci. 50(1):9-15. Https://doi.org/ 10.1080/00071660802538632
CIE (Commission International de l’E´ clairage) (1976). Official recommendations on uniform colour spaces. Colour difference equations and metric colour terms, Suppl. No. 2. CIE Publication No. 15 Colourimetry. Paris. Https://doi.org/10.1002/j.1520-6378.1977.tb00102.x
Çiftçi M, Şimşek ÜG, Dalkılıç B, Erişir Z, Mutlu Sİ, Azman MA, Özçelik M, Yılmaz Ö (2018). Effects of essential oil mixture supplementation to basal diet on fattening performance, blood parameters, and antioxidant status of tissues in Japanese quails exposed to low ambient temperature. Pak. Agric. Scient. Forum. Https://doi.org/20.500.12462/5757
Comai S, Bertazzo A, Bailoni L, Zancato M, Costa CV, Allegri G (2007). The content of proteic and nonproteic (free and protein-bound) tryptophan in quinoa and cereal flours. Food Chem. 100(4):1350-5. Https://doi.org/ 10.1016/j.foodchem.2005.10.072
Dini I, Tenore GC, Dini A (2005). Nutritional and antinutritional composition of Kancolla seeds: an interesting and underexploited and food plant. Food Chem. 92(1):125-32. Https://doi.org/10.1016/j.foodchem.2004.07.008
Duncan DB (1955). Multiple ranges and multiple F tests. Biometrics. 11: 1- 42. Https://doi.org/ 10.2307/3001478
Easssawy MM, Abdel-Moneim MA, El-Chaghaby GA (2016). The use of quinoa seeds extracts as a natural antioxidant in broilers’ diets and its effect on chickens’ performance and meat quality. J. Anim. Poult. Prod. 7(5):173-80. Https://doi.org/10.21608/jappmu.2016.48696
Fadahunsi IF (2009). The effect of soaking, boiling, and fermentation with Rhizopus oligosporus on the water soluble vitamin content of bambara groundnut. Pakistan J. Nutrit. 8(6):835-40. Https://doi.org/pjnonline/fin1081.pdf
FAO (2013). Food Outlook – Biannual report on global food markets. 140 pp. Google Scholar
Filho AM, Pirozi MR, Borges JT, Pinheiro Sant’Ana HM, Chaves JB, Coimbra JS. Quinoa: nutritional, functional, and antinutritional aspects. Critical reviews in food science and nutrition. 2017 May 24;57(8):1618-30. Https://doi.org/ 10.1080/10408398.2014.1001811
George A, Habeanu M, Olteanu M, Turku PR, Dragomir K (2017). Effects of dietary sorghum and triticale on performance, carcass traits and meat pH in broiler chickens. Food Feed Res. 44(2):181-7. Https://doi.org/10.5937/FFR1702181G
Hood DE (1980). Factors affecting the rate of metmyoglobin accumulation in pre-packaged beef. Meat Sci. 4(4):247-65.
Jennen DG (2004). Chicken fatness: From QTL to candidate gene.
Lin M, Han P, Li Y, Wang W, Lai D, Zhou L (2019). Quinoa secondary metabolites and their biological activities or functions. Molecules. 24(13):2512. Https://doi.org/ 10.3390/molecules24132512
Marino R, Caroprese M, Annicchiarico G, Ciampi F, Ciliberti MG, Della Malva A, Santillo A, Sevi A, Albenzio M (2018). Effect of Diet Supplementation with Quinoa Seed and/or Linseed on Immune Response, Productivity and Meat Quality in Merinos Derived Lambs. Animals. 8(11):204. Https://doi.org/ 10.3390/ani8110204
Marzoni M, Chiarini R, Castillo A, Romboli I, De Marco M, Schiavone A (2014). Effects of dietary natural antioxidant supplementation on broiler chicken and Muscovy duck meat quality. Anim. Sci Papers Rep. 32 (4): 359– 68. Https://doi.org/ 279118250
Mazahib AM, Nuha MO, Salawa IS, Babiker EE (2013). Some nutritional attributes of bambara groundnut as influenced by domestic processing. Int. Food Res. J. 20(3):1165. Https://doi.org/ 202013.18
Meek KI, Claus JR, Duncan SE, Marriott NG, Solomon MB, Kathman SJ, Marini ME (2000). Quality and sensory characteristics of selected post-rigor, early deboned broiler breast meat tenderized using hydrodynamic shock waves. Poult. Sci. 79(1):126-36. Https://doi.org/10.1093/ps/79.1.126
Mujica A, Jacobsen S, Izquierdo J, Marathee J (2001). Quinua (Chenopodium quinoa Willd.): ancestral cultivo andino, alimento del presente y del futuro. Santiago: FAO. 2001:9-29.
Nowak V, Du J, Charrondière UR (2016). Assessment of the nutritional composition of quinoa (Chenopodium quinoa Willd.). Food Chem. 193:47-54. Https://doi.org/10.1016/j.foodchem.2015.02.111
Parpinelli W, Cella PS, Eyng C, Broch J, Savaris VD, Santos EC, Avila AS, Nunes RV. Impact of dried brewers’ grains supplementation on performance, metabolism, and meat quality of broiler chickens. South African Journal of Animal Science. 2020;50(2):186-95. Https://doi.org/ 10.4314/sajas.v50i2.2
Russo GL (2009). Dietary n− 6 and n− 3 polyunsaturated fatty acids: from biochemistry to clinical implications in cardiovascular prevention. Biochem. Pharmacol. 77(6):937-46. Https://doi.org/10.1016/j.bcp.2008.10.020
Ruales J, Nair BM (1993). Saponins, phytic acid, tannins, and protease inhibitors in quinoa (Chenopodium quinoa Willd.). J. Sci. Food Agric. 54:211-9. Https://doi.org/10.1016/0308-8146(93)90048-K
SAS (2004). User’s Guide: Statics. Release 9.1. SAS Institute Inc., Cary, NC.2004.
Schilling MW, Battula V, Loar II RE, Jackson V, Kin S, Corzo A (2010). Dietary inclusion level effects of distillers dried grains with solubles on broiler meat quality. Poult. Sci. 89(4):752-60. Https://doi.org/10.3382/ps.2009-00385
Shim Y, Kim J, Hosseindoust A, Choi Y, Kim M, Oh S, Ham H, Kumar A, Kim K, Jang A, Chae B (2018). Investigating meat quality of broiler chickens fed on heat processed diets containing corn distillers dried grains with solubles. Korean J. Food Sci. Anim. Res. 38(3):629. Https://doi.org/10.5851/kosfa.2018.38.3.629
Udensi EA, Arisa NU, Maduka M (2008). Effects of processing methods on the levels of some antinutritional factors in Mucuna flagellipes. Nigerian Food J. 26(2). Https://doi.org/10.4314/nifoj.v26i2.47437
Viveros A, Ortiz LT, Rodríguez ML, Rebolé A, Alzueta C, Arija I, Centeno C, Brenes A (2009). Interaction of dietary high-oleic-acid sunflower hulls and different fat sources in broiler chickens. Poult. Sci. 88(1):141-51. Https://doi.org/ 10.3382/ps.2008-00226
Vilcacundo R, Hernández-Ledesma B (2017). Nutritional and biological value of quinoa (Chenopodium quinoa Willd.). Curr. Opin. Food Sci. 14:1-6. Https://doi.org/10.1016/j.cofs.2016.11.007
Wang L, Piao XL, Kim SW, Piao XS, Shen YB, Lee HS (2008). Effects of Forsythia suspensa extract on growth performance, nutrient digestibility, and antioxidant activities in broiler chickens under high ambient temperature. Poult. Sci. 87(7):1287-94. Https://doi.org/10.3382/ps.2008-00023
Wood JD, Enser M, Fisher AV, Nute GR, Sheard PR, Richardson RI, Hughes SI, Whittington FM (2008). Fat deposition, fatty acid composition, and meat quality: A review. Meat Sci. 78(4):343-58. Https://doi.org/10.1016/j.meatsci.2007.07.019
Wood SG, Lawson LD, Fairbanks DJ, Robison LR, Andersen WR (1993). Seed lipid content and fatty acid composition of three quinoa cultivars. J. Food Composit. Analysis. 6(1):41-4. Https://doi.org/10.1006/jfca.1993.1005
Yao Y, Shi Z, Ren G (2014). Antioxidant and immunoregulatory activity of polysaccharides from quinoa (Chenopodium quinoa Willd.). Int. J. Molecul. Sci. 15(10):19307-18. Https://doi.org/10.3390/ijms151019307
To share on other social networks, click on any share button. What are these?






