Prevalence and Intensity of Ectoparasites in Catfish (Clarias sp) Cultivated with Biofloc System
Research Article
Prevalence and Intensity of Ectoparasites in Catfish (Clarias sp) Cultivated with Biofloc System
Zhulmaydin C. Fachrussyah1*, Indra G. Ahmad2, Iin S. Lantu3, Arafik Lamadi2, Wila R. Nento3
1Department of Aquatic Resources Management, Faculty of Marine Affairs and Fisheries Technology, Gorontalo State University, Kota Gorontalo, Indonesia; 2Department of Aquaculture, Faculty of Marine Affairs and Fisheries Technology, Gorontalo State University, Kota Gorontalo, Indonesia; 3Department of Fisheries Product Technology, Faculty of Marine Affairs and Fisheries Technology, Gorontalo State University, Kota Gorontalo, Indonesia.
Abstract | Catfish is one of the most common high-protein food sources. Biofloc is a collection of microorganisms that promote fish growth and development. The purpose of this study is to inventory ectoparasites in catfish (Clarias sp.) cultivated with Biofloc in Gorontalo. This study was carried out from November to December 2023 at Gorontalo State University’s Integrated Laboratory of Maritime Affairs and Fisheries Technology. A total of 40 catfish samples measuring 20-25 cm from cultivation sites using a biofloc system were selected using saturated sampling based on the sustainability of the catfish business (5 years), which included 20 businesses. Ectoparasite observations were conducted using a ZEISS Axioscope 5 Smart Laboratory Microscope. The descriptive method was used to identify and analyze the observational results. Gyrodactylus and Dactylogyrus parasites were discovered in mucus and gills. The Vorticella parasite was found on the gills, while Lernea was discovered on the skin. In this study, the prevalence and intensity of ectoparasites were 15% and 0.35 for Gyrodactylus sp; 10% and 0.26 for Dactylogyrus sp, while Lernea sp and Vorticella sp were 7.5% and 0.18, respectively. The most common infection was Gyrodactylus sp., which was classified as a “frequent infection.” The infection with Lernea sp. and Vorticella sp. had the lowest prevalence rate, classified as “infection sometimes.” All parasites found had a low attack intensity (<1). More research is needed to examine the environment factors where the catfish were reared, the parasites discovered, and the economic losses caused by parasitic infection.
Keywords | Ectoparasites identification, Microscopy, Prevalence, Intensity biofloc, Catfish
Received | July 03, 2024; Accepted | August 30, 2024; Published | September 03, 2024
*Correspondence | Zhulmaydin C. Fachrussyah, Department of Aquatic Resources Management, Faculty of Marine Affairs and Fisheries Technology, Gorontalo State University, Kota Gorontalo, Indonesia; Email: fachrussyah@ung.ac.id
Citation | Fachrussyah ZC, Ahmad IG, Lantu IS, Lamadi A, Nento WR (2024). Prevalence and intensity of ectoparasites in catfish (clarias sp) cultivated with biofloc system. Adv. Anim. Vet. Sci. 12(10): 2029-2033.
DOI | https://dx.doi.org/10.17582/journal.aavs/2024/12.10.2029.2033
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright: 2024 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
INTRODUCTION
The aquaculture industry is a fisheries sub-sector that plays an important role in national development because it contributes to national economic growth and food security (Oktopura et al., 2020). Due to overfishing, aquaculture now supplies 50% of global fish demand (FAO, 2018; Naylor et al., 2021). Fish is a popular food commodity in the community. Gorontalo’s freshwater fish production capacity has increased to 1 048.00, 1 087.00, and 1 267.00 (Tons) in the last three years (BPS Gorontalo, 2022), including catfish. Catfish, also known as the order Siluriformes, are a large and diverse group of freshwater fish with ray fins. Catfish is a popular fish due to its low price, high protein content, adaptability to environmental changes, rapid growth, and high market demand. It is widely grown by Indonesians due to high consumer demand, which requires fish farmers to produce on a regular basis to meet demand (Senthilkumaran and Kar, 2021; Rizki et al., 2016; Salsabila et al., 2021). Catfish can tolerate low dissolved oxygen (DO) levels in water and can be grown in fish cages, concrete tanks, or ponds (Vaishnav et al., 2017). Catfish is widely cultivated in many countries, including Myanmar, Thailand, Cambodia, Vietnam, and Indonesia. Some catfish species are reared as research animals due to their more adaptability for culture and artificial spawning (Ng and Kottelat, 2008).
Efforts to increase catfish production can be done through the biofloc technique. Biofloc are microorganisms (bacteria, fungi, algae, worms, and protozoa) that form beneficial clumps or flocs in rearing media to support better fish growth and development (Direktorat Produksi dan Usaha Budidaya, 2017). The biofloc technique, which involves multiplying beneficial microorganisms, is used to support better fish growth and development. However, increasing catfish productivity through the biofloc system is influenced by internal and external factors such as environment, feed, and disease attacks. Parasites, such as Trichodina sp., Zoothamnium sp., Glossatella sp., Ichthyophthirius multifiliis, Gyrodactylus sp., and Dactylogyrus sp., can cause significant losses, slow growth, and even death in catfish cultivators in Gorontalo. Other issues include declining water quality due to pollution, low knowledge and skills in fish cultivation, and inefficient production factors.
The success of catfish cultivation can be influenced by the presence of parasites that attack the external and internal organs of the catfish’s body, disrupting their metabolism. This research aims to identify and measure the prevalence level of ectoparasite types in catfish kept in a biofloc system, as it can help control the presence of parasites and improve the overall growth and development of catfish.
MATERIALS AND METHODS
This research was conducted in November–December 2023. A total of 40 catfish samples used were taken from biofloc cultivation located in Gorontalo city, with a size of 20–25 cm. Saturated sampling based on the sustainability of the catfish business (5 years) with a total of 20 businesses was used in the study. Saturated sampling is a sampling technique where all members of the population are used as samples.Catfish farming in Gorontalo is very limited; business capacity sizes were 1000-2000 (5 businesses), 2000-5000 (3 businesses), >5000 (1 business), and <1000 (11 businesses). The fish’s body length was measured using a caliper. Ectoparasite examination was conducted at the Integrated Laboratory of the Faculty of Maritime Affairs and Fisheries Technology, Gorontalo State University. The target organs for examination were gills, mucus, and fins. Prevalence category of ectoparasites can be seen in Table 1.
|
No |
Prevalence (%) |
Category |
Description |
|
1 |
100 – 99 |
Always |
Very severe infection |
|
2 |
98 – 90 |
Almost always |
Severe infection |
|
3 |
89 – 70 |
Usually |
Moderate infection |
|
4 |
69 – 50 |
Very often |
Very frequent infection |
|
5 |
49 – 30 |
Generally |
Common infection |
|
6 |
29 – 10 |
Often |
Frequent infection |
|
7 |
9 – 1 |
Sometimes |
Infection sometimes |
|
8 |
<1 – 0,1 |
Seldom |
Rare infection |
|
9 |
<0,1 – 0,01 |
Very rarely |
Very rare infection |
|
10 |
<0,01 |
Almost never |
Never |
Source: (Syukran et al., 2017).
Ectoparasite examination of the fish was carried out using a ZEISS Axioscope 5 Smart Laboratory Microscope. The detection of parasites from the gills of the sampled fish was done by using the methods described by Ekanem et al. (2014). The operculum was cut open to expose the gills by using dissecting scissors; the exposed gills were detached on object glass and dripped with distilled water; and the gill filaments and the gill arches were examined under the microscope. The same thing was done with the fin inspection. The fins examined were the tail fin, dorsal fin, pectoral fin, and pelvic fin. Observation of mucus was carried out by scraping off the mucus on the surface of the catfish’s body. Then, placing it on a glass object and dripping it with distilled water, observe it under a microscope. Intensity Category of ectopasites attack can be seen in Table 2.
|
No |
Prevalence (%) |
Category |
|
1 |
<1 |
Very low |
|
2 |
1 – 5 |
Low |
|
3 |
6 – 55 |
Currently |
|
4 |
51 – 100 |
Critical |
|
5 |
>100 |
Awfully |
|
6 |
>1000 |
Super infection |
Source: (Syukran et al., 2017).
Results were identified and recorded, i.e., the type of parasite and fish organs where the parasite was found. The prevalence and intensity of ectoparasites were calculated using
the formula Kabata (1985), then analyzed descriptively.
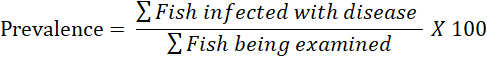
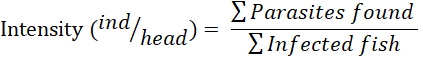
RESULTS AND DISCUSSION
The results of the research found four types of ectoparasites that attack catfish cultivated using the biofloc system, namely Gyrodactylus sp., Dactylogyrus sp., Lernea sp., and Vorticella sp., whose prevalence and intensity levels can be seen in Table 3. Ectoparasites found in the study showed the same research results as those by Afolabi et al. (2020) on African catfish and Mavuti et al. (2017) on catfish in Kenya, i.e., Gyrodactylus sp. and Dactylogyrus sp.; Salsabila et al. (2021) on Catfish (Clarias sp.) in AquaculturePonds and Bioflocs System in Aceh Besar, Indonesia, i.e., Dactylogyrus sp.; Vorticella sp.; Yusni and Rambe (2019) on Fry Tilapia (Oreochromis niloticus) in Aquaculture Pond, i.e., Dactylogyrus sp.
Table 3: Prevalence and Intensity of Ectoparasites infected Catfish in Biofloc Cultivation Ponds.
|
Type of parasites |
Predilection |
Number of infected fish |
Prevalence (%) |
Intensity |
|
Gyrodactylus sp |
Gills |
6 |
15 |
0,35 |
|
Dactylogyrus sp |
Gills |
4 |
10 |
0,26 |
|
Lernea sp. |
Skin |
3 |
7,5 |
0,18 |
|
Vorticella sp. |
Gills |
3 |
7.5 |
0,18 |
Ectoparasites like Gyrodactylus sp., Dactylogyrus sp., and Vorticella sp. (Figure 1) are commonly found in the gill organs. This is consistent with the explanation (Suhardi et al., 2014) that both types of parasites belong to the monogenea class, which only attacks the outside of the fish’s body and is commonly found in freshwater fish. Gyrodactylus are viviparous skin and gill parasites found in many freshwater and marine fish (Přikrylová et al., 2012).
Lernea sp. is found on catfish skins. According to the Indonesian Institute of Sciences (2019), Lernea-type parasites can be found in the gills, eyes, and skin (fins and scales). The area infected by this parasite is damaged and will serve as the starting point for secondary infections of other diseases. Lernaea cyprinacea, or anchor worm, is a parasitic cyclopoid copepod that parasitizes culture systems in freshwater aquaculture ponds and hatcheries. Lernea sp. infestations have been found in catfish, minor carps, major carps, and perches. Lernaea infestation included fins, skin, the oral cavity, and gills. Female parasites attach to the fish by burrowing deep into its tissue. The attachment site may experience intense hemorrhage and focal inflammation. A small number of parasite infections are not fatal, but they are extremely irritating to the fish. Lernaea’s intense inflammation can cause secondary bacterial and fungal infections. Secondary infections can worsen and kill fish. A larger number of parasites on the gill can interfere with respiration, resulting in death. Chronic Lernaea infestation causes poor growth and makes fish more susceptible to secondary infection by fungus and bacteria, which eventually kills them (Hossain et al., 2013; Sayyadzadeh et al., 2015).
Vorticella sp. is found in catfish gills. According to Khotimah et al. (2018), Vorticella sp. is an ectoparasite that attacks the gills of fish and crustaceans. This ectoparasite attack disrupts the respiratory system of fish and prevents crustaceans from molting.
The results show that the highest prevalence and intensity was the ectoparasite type Gyrodactylus sp. compared to other types of ectoparasites, namely 15% and 0.35, then Dactylogyrus sp. 10% and 0.26, Lernea sp., and Vorticella sp., respectively 7.5% and 0.18. With a prevalence value below 30%, catfish cultivated using the biofloc system are categorized as frequently and occasionally infected. Meanwhile, for intensity values below 1, they are categorized as very low. With relatively low prevalence and intensity of ectoparasite attacks, the possibility of death from catfish is also low. This can be caused by the biofloc system cultivation; there is a role of good bacteria that influence the immune system and also the environmental conditions of the cultivation medium (water quality). As explained by Suhardi et al. (2014), low parasite attacks can be caused by good water quality, which causes fish to be stressed so that parasites develop quickly. The ectoparasite prevalence study yielded lower results than Afolabi et al. (2020) study on African catfish, which had the lowest prevalence of 20% and the highest of 75%. The highest prevalence was found in fish from rivers with natural habitats. The prevalence of Dactylogyrus sp. in the study was lower than that of (Salsabila et al., 2021), but Vorticella sp. was more common. Dactylogyrus sp. from the study and previous studies by Salsabila et al., 2021) infected the most. This is because Dactylogyrus sp. is a parasite that frequently infects both freshwater fish and ornamental fish. Dactylogyrus sp. frequently attacks koi, tilapia, and catfish, which live in fish gills. Gills of fish affected by Dactylogyrus sp look pale. Changing behavior was found in fish infected with Dactylogyrus sp. (Mohammadi et al., 2012). Low prevalence from cultured habitat such as biofloc system may imply that there had been an improvement in management practices of cultured fish (Ayanda 2009). Meanwhile, higher prevalence from natural habitat may be due to many factors such as pollution of water, feeding habit, and availability of intermediate hosts which harbor the infective larval stage of parasites (Kawe et al., 2016).
CONCLUSIONS AND RECOMMENDATIONS
The study found four types of ectoparasites in catfish cultivated using biofloc systems: Gyrodactylus sp, Dactylogyrus sp, Lernea sp, and Vorticella sp. Gyrodactylus sp had the highest prevalence rate which categorized as “often” or “Frequent infection”, while Lernea and Vorticella sp had the lowest which categorized as “sometimes” or “Infection sometimes”. The intensity attack of all parasites was very low. Further research is needed to understand environment factors, economic losses caused by parasitic infection in catfish.
ACKNOWLEDGEMENTS
The authors thank the Integrated Laboratory Assistant of the Faculty of Maritime Affairs and Fisheries, especially the Microbiology and Feed Laboratory division, who assisted in collecting and observing samples for this study.
NOVELTY STATEMENT
Ectoparasites in catfish have been studied, but research on ectoparasites in catfish cultivated with biofloc is limited, so it should be considered when selecting a cultivation method to avoid disease attacks caused by ectoparasites.
AUTHOR’S CONTRIBUTIONS
Zhulmaydin C. Fachrussyah and Indra G. Ahmad: Idea and design.
Iin S. Lantu, Arafik Lamadi and Wila R. Nento: Material sample collection and lab analysis.
Zhulmaydin C. Fachrussyah, Indra G. Ahmad and Iin S. Lantu: Write the manuscript.
Zhulmaydin C. Fachrussyah: Revision.
Conflict of Interest
The authors confirm that there is no conflict of interest in the manuscript.
REFERENCES
Afolabi OJ, Olususi FC, Odeyemi OO (2020). Comparative study of African catfish parasites from cultured and natural habitats. Bull. National Res. Centre, 44: 1-9. https://doi.org/10.1186/s42269-020-00419-4
Amelia Novita Pebriani DAA, Kartika GRA (2023). Pola Kematian dan Intensitas Serangan Parasit pada Ikan Lele yang Dibudidayakan dengan Dua Sistem Yang Berbeda. J. Agroqua, 21(2): 327-333. https://doi.org/10.32663/ja.v21i2.3855
Ayanda OI (2009). Comparative parasitic helminth infection between cultured and wild species of Clarias gariepinus in Ilorin North Central Nigeria. Sci. Res. Essay, 4(1):18–21.
BPS Gorontalo (2022). Badan pusat Statistik Gorontalo https://gorontalo.bps.go.id/indicator/56/131/1/produksi-perikanan-tangkap.html
Direktorat Produksi dan Usaha Budidaya (2017). Buku Saku Budidaya Ikan Lele Sistem Bioflok. Kementerian Kelautan dan Perikanan
Ekanem AP, Eyo VO, Udoh JP, Okon JA (2014). Endoparasites of food-fish landing from the Calabar River Cross River State Nigeria. J. Sci. Res. Rep., 3(6):810–817. https://doi.org/10.9734/JSRR/2014/6918
FAO (2018). FAO The State of World Fisheries and Aquaculture-Meeting the Sustainable Development Goals FAO Rome Italy (2018)
Fransira I (2023). Identifikasi Ektoparasit Pada Insang Ikan Lele (Clarias sp.) dari Kolam Budidaya di Bakunase. J. Bahari Papadak, 4(2): 175-179.
Hasyimia US, Dewi NK, Pribadi TA (2016). Identifikasi ektoparasit pada ikan lele sangkuriang (Clarias gariepinus) yang dibudidayakan di Balai Benih Ikan (BBI) Boja Kendal. Life Sci., 5(2): 118-124.
Hossain MMM, Rahman MZ, Islam MA, Alam ME, Rahman H (2013). Lernaea (Anchor Worm) investigations in Fish. Int. J. Anim. Fish. Sci., 12-19
Kabata Z (1985). Parsites and Disease of Fish Cultured in The Tropics. Taylor and Francis London UK.
Kawe SM, God’spower RO, Balarabe MR, Akaniru RI (2016). Prevalence of gastrointestinal helminth parasites of Clarias gariepinus in Abuja Nigeria. Sokoto J. Vet. Sci., 14(2): 26–33. https://doi.org/10.4314/sokjvs.v14i2.4
Khotimah A, Rokhmani Riwidharso E (2018). Prevalensi dan Kelimpahan Vorticella sp. pada Kepiting Bakau (Scylla serrata) yang didaratkan di Tempat Pelelangan Ikan Sleko Kabupaten Cilacap Jawa Tengah. Prosiding Semin. Nasional Masyarakat Biodiv. Indones., 4(1): 87-91.
Lembaga Ilmu Pengetahuan Indonesia (2011). Berita Biologi Jurnal Ilmu-Ilmu Hayati. Pusat Penelitian Biol.,
Mavuti SK, Waruiru RM, Mbuthia PG, Maina JG, Mbaria JM, Otieno RO (2017). Prevalence of ecto-and endoparasitic infections of farmed tilapia and catfish in Nyeri County Kenya. Livestock Res. Rural Dev., 29(6): 55-59.
Mohammadi F, Mousavi SM, Rezaie A (2012). Histopathological study of parasitic infestation of skin and gill on Oscar (Astronotus ocellatus) and discus (Symphysodon discus). Int. J. Bioflux Soc., 5(2): 88-93.
Naylor RL, Hardy RW, Buschmann AH, Bush SR, Cao L, Klinger DH, Little DC, Lubchenco J, Shumway SE, Troell MA (2021). 20-year retrospective review of global aquaculture. Nature, 591(7851): 551-563.
Ng HH, Kottelat M (2008). The identity of Clarias batrachus (Linnaeus 1758) with the designation of a neotype (Teleostei: Clariidae). Zool. J. Linn. Soc., (4): 725-732. https://doi.org/10.1111/j.1096-3642.2008.00391.x
Oktopura AAD, Fauzi A, Sugema K, Mulyati H (2020). Aquaculture performance in Indonesia: economics and social perspectives. In IOP Conference Series: Earth and Enviro. Sci., 493(1): 012003. IOP Publishing. https://doi.org/10.1088/1755-1315/493/1/012003
Přikrylová I, Blažek R, Vanhove MP, (2012). An overview of the Gyrodactylus (Monogenea: Gyrodactylidae) species parasitizing African catfishes and their morphological and molecular diversity. Parasitol. Res., 110: 1185-1200. https://doi.org/10.1007/s00436-011-2612-0
Rizki A, Fahrimal P, Daud Y, Karmil R, Hambal ,F Zuhrawati M (2016). Identifikasi Parasit pada Ikan Lele Dumbo )Clarias gariepinus) di Desa Lambro Deyah Kecamatan Kuta Baro Kabupaten Aceh Besar. J. Medika Vet., 10(2): 157-158. https://doi.org/10.21157/j.med.vet..v10i2.4390
Salsabilla A, Putra DF, Octavina C, Maulana R (2021). Prevalence and Intensity of Ectoparasites on Cultivated Catfish (Clarias sp.) in Aquaculture Ponds and Bioflocs System in Aceh Besar Indonesia. In IOP Conference Series: Earth and Environ. Sci., 869: 012073. IOP Publishing. https://doi.org/10.1088/1755-1315/869/1/012073
Sayyadzadeh G, Esmaeili HR, Vatandoust S (2015). Infection of south sisorid catfish Glyptothorax silviae (Actinoptrygii: Sisoridae) with Anchor Worm Lernaea sp. (Lernaeidae) in Saimareh River Ilam province. J. Anim. Environ., 6(4): 241-244.
Senthilkumaran B, Kar S (2021). Advances in reproductive endocrinology and neuroendocrine research using catfish models. Cells, 10(11): 2807. https://doi.org/10.3390/cells10112807
Suhardi Raharjo E, Sunarto I (2014). Tingkat Serangan Ektoparasit pada Ikan Patin (Pangasius hypohtalmus) Yang Dibudidayakan dalam Karamba di Sungai Kapuas Kota Pontianak. J. Ruaya, 1(1): 42-52. https://doi.org/10.29406/rya.v1i1.228
Syukran M, El Rahimi SA, Wijaya S (2017). Intensitas dan Prevalensi Ektoparasit pada Ikan Cupan Hias (Betta splendens) di Perairan Kabupaten Aceh Besar dan Kota Banda Aceh. J. Ilmiah Mahasiswa Kelautan dan Perikanan Unsyiah, 2(1): 221-228.
Tuwitri R, Irwanto R, Kurniawa A (2020). Identifikasi Parasit pada Ikan Lele (Clarias sp) Di Kolam Budidaya Ikan Kabupaten Bangka. J. Teknologi Perikanan dan Kelautan, 11(2): 189-198. https://doi.org/10.24319/jtpk.11.189-198
Vaishnav M, Sharma SK, Sharma BK, Ojha ML (2017). Growth performance of Pangasius sp. cultured at different stocking density in floating net cages in Mahi Bajaj Sagar Dam of Banswara (Rajasthan). J. Entomol. Zool. Stud., 5(5):649-652.
Yusni E, Rambe N (2019). Identification of ectoparasites in fry Tilapia (Oreochromis niloticus) in aquaculture pond. In IOP Conference Series: Earth and Environ. Sci., 260: 012110. IOP Publishing. https://doi.org/10.1088/1755-1315/260/1/012110
To share on other social networks, click on any share button. What are these?






