Prevalence of Toxoplasma gondii in Women Population of Rafha City, Saudi Arabia
Prevalence of Toxoplasma gondii in Women Population of Rafha City, Saudi Arabia
Akbar Ali1,*, Khalil Mohamed2 and Fawzia Toulah3
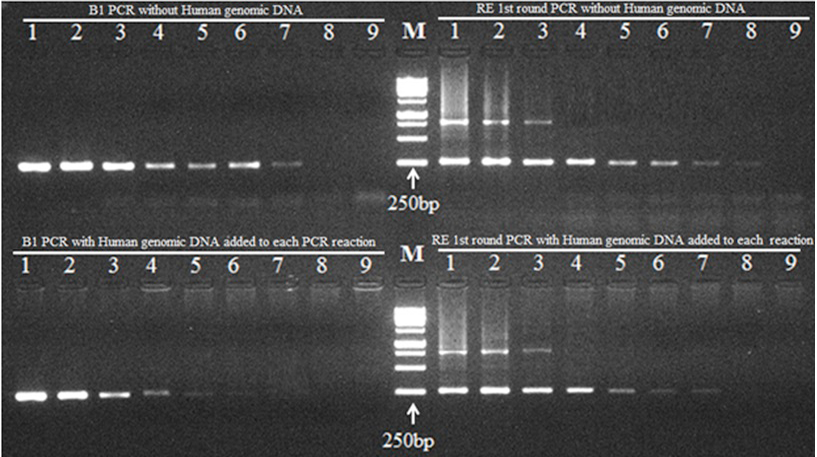
PCR optimization of B1 and RE (first round) reactions. Serial dilutions of the ABI Toxoplasma gondii (RH strain) quantitated DNA were used for PCR in both B1 and RE reactions. Lane 1, PCR reaction using 16000 copies of T. gondii genomic DNA (provided by supplier); Lane 2, 1600 copies T. gondii genomic DNA; Lane 3, 160 copies T. gondii genomic DNA; Lane 4, 16 copies T. gondii genomic DNA; Lane 5, 8 copies T. gondii genomic DNA; Lane 6, 4 copies T. gondii genomic DNA; Lane 7, 2 copies T. gondii genomic DNA; Lane 8, 1 copy T. gondii genomic DNA; Lane 9, negative control. The PCR products of RE first round with and without human genomic DNA were used as template for RE second round PCR. M, DNA Marker (SolisBiodyne 1Kb DNA ladder, cat# DM010-R500).
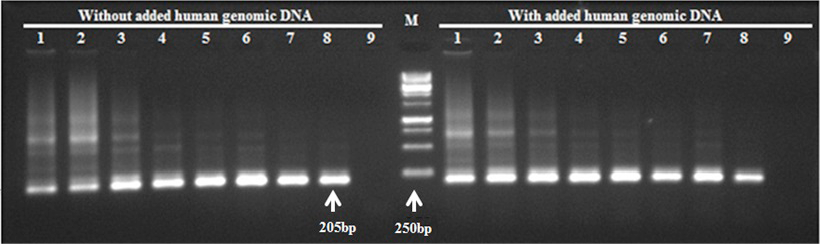
The RE second round PCR products; Lanes 1 to 8 on left represent PCR amplification using first round PCR amplified without human genomic DNA; Lane 9, negative control; Lanes 1-8 on the right represent PCR amplification using first round PCR amplified with added human genomic DNA; Lane 9, negative control; M, DNA marker (SolisBiodyne 1Kb DNA ladder, cat# DM010-R500).
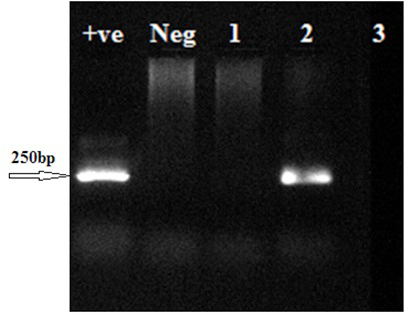
The representative agarose gel electrophoresis of RE second round PCR products using patient’s samples; Lanes 1 to 3, patient samples.
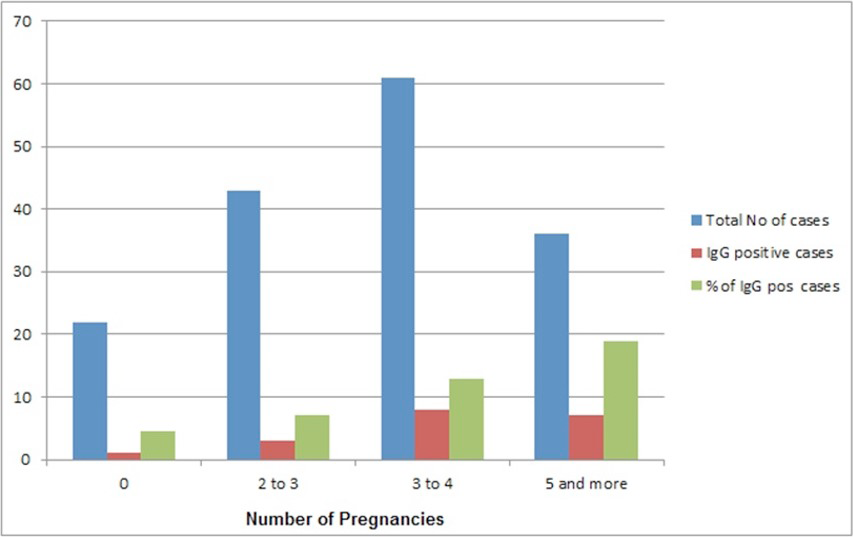
The percentage of IgG positive cases with increasing number of pregnancies.