Preference and Seasonality of Allogrooming Posture and Body Site of Wild White-Headed Black Langurs (Trachypithecus leucocephalus) in Guangxi, China: Functional Implications
Preference and Seasonality of Allogrooming Posture and Body Site of Wild White-Headed Black Langurs (Trachypithecus leucocephalus) in Guangxi, China: Functional Implications
Qiu-Cheng Zhao1,2, Xin-Ling Gan1,2, Zhou-Quan Wei1,2, Zhong-Hao Huang1,2* and You-Bang Li1,2*
1Key Laboratory of Ecology of Rare and Endangered Species and Environmental Protection (Guangxi Normal University), Ministry of Education, China
2Guangxi Key Laboratory of Rare and Endangered Animal Ecology, Guangxi Normal University, China
ABSTRACT
Allogrooming behavior is ubiquitous among nonhuman primates and considered to be highly preferences regarding to allogrooming postures and body sites. In order to investigate the allogrooming preference and seasonality of the white-headed black langurs (Trachypithecus leucocephalus), we studied the allogrooming posture and body site of the animal via focal animal sampling and continuous recording in the Chongzuo White-Headed Langur National Nature Reserve from February 2016 to January 2017. Results showed that totally proportions of non-eye contact and eye contact allogrooming postures of the animals accounted for 47.86% and 52.14%, respectively. The most frequently used allogrooming posture in the dry season was sprawl (32.73%), and that of in the rainy season was sit side (33.56%). There were significant differences among allogrooming postures throughout the year (p < 0.001). Proportion of allogrooming in inaccessible area in the dry season was higher than in the rainy season. The grooming preference index was greater than 0 in the dry season and less than 0 in the rainy season. The proportion of difficult to reach area was opposite and there was a significant difference between dry season and rainy season (p = 0.04), and both grooming preference index was greater than 0. The grooming preference index of easy to reach area was less than 0 in dry season and rainy season. Animals were selective in allogrooming sites, the anogenital area had the largest grooming preference index in both dry and rainy seasons. The allogrooming of white-headed black langur appeared to be consistent with the social function hypothesis. In addition, allogrooming was in line with the hygiene function hypothesis during dry season, but not in rainy season. The reason may be associated with variation of food supply between the two seasons. It is necessary to further study before generalizing the function of allogrooming of the langur.
Article Information
Received 20 December 2022
Revised 20 February 2023
Accepted 10 March 2023
Available online 04 September 2023
(early access)
Published 15 January 2025
Authors’ Contribution
Q-cZ: Formal analysis (Equal), writing-original draft (Lead), writing-review and editing (Equal). X-lG: Writing-review and editing (Equal). Z-qW: Behavioral observations (Lead). Z-hH: Writing-review and editing (Equal). Y-bL: Conceptualization (Lead), formal analysis (Equal), funding acquisition (Lead), methodology (Lead), project administration (Equal), supervision (Lead), writing-review and editing (Equal).
Key words
Allogrooming, Seasonal variations, Grooming postures, Grooming areas, Trachypithecus leucocephalus
DOI: https://dx.doi.org/10.17582/journal.pjz/20221220141244
* Corresponding author: hzh773@126.com, lyb_2001@126.com
0030-9923/2025/0001-0365 $ 9.00/00
Copyright 2025 by the authors. Licensee Zoological Society of Pakistan.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
INTRODUCTION
Grooming behavior is ubiquitous among primates, accounting for 2% ~ 10% of the daily time budget (Tahir et al., 2017; Maurice et al., 2019). Grooming can be divided into autogrooming and allogrooming (Hutchins and Barash, 1976; Pérez and Baró, 1999). The former is an individual combing their own hair, sometimes picking up small particles from the hair and putting them in their mouths to chew or use their mouths to bite. The latter, as a supplement to autogrooming, is to comb the hair between individuals, and occasionally exposed skin to pick up small particles into the mouth to chew or bite directly with the mouth, thus it has a more complex social function (Vea et al., 1999). Most studies of grooming in primates (Wolovich et al., 2017; Jiang et al., 2019), and in several other mammals, such as bats (Carter and Leffer, 2015), deer (Heine et al., 2017), horses (Shimada and Suzuki, 2020), mice (Lawande et al., 2020), coati (Hirsch et al., 2012), cows (de Freslon et al., 2020) show that grooming serves important social functions. Two popular ecological hypotheses, the hygienic function hypothesis and the social function hypothesis, can better explain the important biological significance of primate grooming behavior.
The hygienic function hypothesis holds that social primates groom each other to remove salt and parasites from the body surface to control disease (Barton, 1985; Borries, 1992; Grueter et al., 2013). Therefore, regarding grooming body sites, allogrooming mostly occurs in areas of the body that are inaccessible to groomee and are susceptible to parasite infection (Borries et al., 1994; Grueter et al., 2013). For example, the allogrooming behavior of Yunnan snub-nosed monkeys (Rhinopithecus bieti) focuses on parts of the body that are difficult to reach by groomees (Zhang et al., 2014). This is also true in wild black capuchin monkeys (Sapajus nigritus), which preferentially groom inaccessible body sites (e.g., back and head) (Pfoh et al., 2021). Also, the anogenital area and the corpus callosum are more frequently groomed among individuals of the narrow-nosed monkeys (Pérez and Baró, 1999; Li et al., 2002; Allanic et al., 2021) and François’ langur (Trachypithecus francoisi) (Zhou et al., 2006), which possibly since the area is difficult to autogrooming, and also contains some information about reproductive status and social status (Moser et al., 1991). Wild Bonobos (Pan paniscus) mothers groom more frequently to their pups body site that are less accessible to themselves to prevent ectoparasite-related diseases (Allanic et al., 2020). In addition, the number of grooming partners in vervet monkeys (Chlorocebus aethiops) is associated with hookworm infection, and the number of grooming partners in vervet monkeys vary significantly by hookworm infection and sex (Wren et al., 2016).
Another hypothesis of grooming is the social function hypothesis. It argues that grooming is crucial for promoting social cohesion (Kanngiesser et al., 2011). Since grooming can reduce the tension and thus maintain the intimacy, the social hierarchy relationship between individuals, as well as the stability of the community (Terry, 1970; Dunbar, 1991). In white crowned mangabeys (Cercocebus torquatus lunulatus), low-rank females groom to reduce the frequency of attack (Vea et al., 1999). Similarly, Japanese macaque (Macaca fuscata) females tend to invest in grooming of high-rank females (Kurihara, 2016). Female mandrills (Mandrillus sphinx) will give priority to grooming individuals of high status in order to gain advantage in social competition (Schino and Lasio, 2018). In wild gelada (Theropithecus gelada) females, infant handling affects grooming exchanges to strengthen society (Caselli et al., 2021). Primate grooming is often thought of as a kind of currency that can be exchanged for other services or goods in biological market theory, such as alliance support (Borgeaud and Bshary, 2015), infant care (Jiang et al., 2019; Pereira et al., 2019), tolerance for food sources (Wubs et al., 2018), positive food sharing (Wolovich et al., 2017), or mating opportunities (Rathinakumar et al., 2017).
In gregarious primates, grooming behavior varies with ages (Tombak et al., 2019), genders (Lhota et al., 2019), dominance hierarchy (Wu et al., 2018), kinship (Wu et al., 2018; Allanic et al., 2020), seasonal food availability (Jasso del Toro et al., 2020), grooming postures (Zhao et al., 2019) and other factors. Grooming of different body sites can mean different costs and benefits (Schino and De Angelis, 2020). Many primates have seasonality in grooming time. For instance, the grooming time of captive hamadryas baboons (Papio hamadryas) is reduced in the cold winter (Chen, 2016). Similarly, grooming time in Sichuan snub-nosed golden monkey (Rhinopithecus roxellana) in autumn and winter is shorter because it had less food to eat and was reduced to more indigestible bark, which led to longer rest periods and shorter grooming periods (Li, 2004).
The white-headed black langur (Trachypithecus leucocephalus) is a rare and endangered species endemic to the limestone forests in southwest Guangxi, China (Huang et al., 2008). Lime forests are dominated by evident seasonal variation of food supply and rainfall; therefore, activity of limestone-associated langurs vary accordingly (Zhang et al., 2020). To date, increasingly studies, including diet (Li et al., 2016; Lu et al., 2021), habitat use (Liu et al., 2022), ranging behavior (Huang et al., 2017), activity pattern and time budget (Zhang et al., 2020) of white-headed black langurs have been conducted. However, limited information concerning allogrooming seasonality and preference of the free-ranging group is available. The white-headed black langur had a distinct social structure and obvious mutual grooming behavior, which was conducive to our observation. Thus, we conducted field observations on the allogrooming postures and body sites to explore the following questions: (1) What are the allogrooming characteristics of the white-headed black langur? (2) Are allogrooming preference and function of the white-headed black langurs vary with seasonality?
MATERIALS AND METHODS
Study site
The research site is located in the Jiuchongshan in the Chongzuo White-Headed Langur National Nature Reserve, Guangxi, China. The reserve is dominated by limestone landscapes, which can be divided into three parts from top to bottom: including hilltop, the cliff and the gentle slope (Li and Elizabeth Rogers, 2006). The elevation of the peak ranges 200~300 m (Tan, 2014). The climate belongs to the north tropical humid monsoon climate, with an annual rainfall of about 1200 mm, which can be clearly divided into a rainy season (April to September) and a dry season (October to March of the following year) (Huang et al., 2010). The average temperature is around 22.0 °C, and a humidity around of 78% (Tan, 2014).
Study subjects
The observation group G10 ranged several hills ca. 0.4 square kilometer. The group had 10 individuals at the beginning of the study. According to Huang (2002) criterion, the group included six adult females, one adult male, two sub-adult females, and one juvenile female. During the observation the adult male replacement happened twice, and hence group composition changed throughout the observation (Table I). In this area, photographers often observe and photo the langurs, and local farmers work on the flat land around the hills. The langurs are tolerant to observers within 50 m.
Table I. Group composition of the white-headed black langur in this study.
|
Stage |
Adult male |
Adult female |
Sub-adult male |
Sub-adult female |
Juvenile male |
Juvenile female |
|
1 |
1 |
6 |
2 |
1 |
||
|
2 |
1 |
6 |
2 |
2 |
3 |
1 |
|
3 |
1 |
6 |
1 |
1 |
Key: Stage 1: Initial study period. Stage 2: In March 2016, male replacement occurred, two sub-adult males and three juvenile males were added to the group. Stage 3: The second male replacement occurred in the observation group on November 21, 2016. After that the observation group consisted of one male and 8 females.
Behavioral observations
We used focal animal sampling and continuous recording to study the animals (Altmann, 1974). The sampling period was 15 min, we observed and recorded the duration of allogrooming behavior during the first 5 mins, followed by a 10 min interval, and then sampled again. An allogrooming start if the allogrooming time exceeded 30s, and end if it stopped for 30 s (Altmann, 1974).
Field observation was conducted from February 2016 to January 2017. The observation time was 59 days in total, ranging from four days in September 2016 to five days in the remaining months. The observation time in summer was 7:00-19:00, and 7:30-18:30 in other seasons. A monocular (Nikon, 20-40x, Japan) or a binocular (Zeiss, TERRA ED 10X42, German) were used to observe the animals from 50-200 m. Grooming sampling data record included time spent on body sites and grooming postures. The grooming postures consisted of six items, sitting in the same direction, sprawl, quadrupedal stand, back lie, sit face to face, and sit side, which were further assigned to two categories, with eye contact and without eye contact (Zhang et al., 2014) (Table II).
In order to estimate the relative proportion of the body area of the langurs, following Ghiglieri (1984) and Boccia (1983), we divided the animal body site into 3 areas: Inaccessible area (IA), easy to reach area (ERA) and difficult to reach area (DRA) (Table III). Thus, we measured the body regions of a female white-headed black langur specimen in the Guangxi Normal University Biodiversity Herbarium and a male one in the white-headed black langur Exhibition Hall of the Chongzuo white-headed black langur Nature Reserve. The proportions of the relative body area were used to represent that of the animal in the field.
Table II. Definition of grooming posture of white-headed black langur.
|
Grooming posture |
Definition |
|
Eye contact |
|
|
Sit face to face |
Groomer and groomee face each other abdomen |
|
Back lie |
The torso of the receiver is standing upright on a relatively horizontal support, and the body mass is mainly on the back |
|
Sit side |
Between the initiator and the receiver, the abdomen orientation of one individual is perpendicular to the abdomen orientation of the other individual, and the abdomen of one individual may be close to the body side of the other individual |
|
Without eye contact |
|
|
Quadrupedal stand |
The limbs of the receiver stand on a horizontal or sub-horizontal support; the elbow joints and knees are relatively extended, and the torso is close to level |
|
Sitting in the same direction |
The abdomen between groomer and groomee is facing the same, and the body mass is borne by the ischium and feet. The torso is vertical and may be curved |
|
Sprawl |
The torso of the groomee is standing upright on a relatively horizontal support, and the body mass is mainly in the abdomen |
Table III. Body sites partition of white-headed black langur (from Ghiglieri, 1984).
|
Body sites |
Body region |
|
Easy to reach area (ERA) |
|
|
Hand |
From wrist to fingertip, excluding wrist |
|
Forearm |
From wrist to elbow, excluding elbow |
|
Tail tip |
From the middle of the tail to the tip |
|
Shank |
From groin and buttocks to knees excluding knees |
|
Thigh |
From knee to foot stomp, including knee, excluding foot rash |
|
Foot |
From the foot to the toe, including the stomp |
|
Inaccessible area (IA) |
|
|
Face |
The front part of the head, including the eyes |
|
Head |
The part that covers the brain, including the ears and eyebrows |
|
Neck |
The annular part that connects the head to the torso |
|
Upper back |
The upper part of the back of the body |
|
Lower back |
The lower part of the back of the body |
|
Difficult to reach area (DRA) |
|
|
Arm |
From shoulders to elbows, including underarms |
|
Abdomen |
The frontal part of the body from the chest to the anogenital area |
|
Chest |
The front part of the thoracic cavity from the bottom of the neck to the ventral surface |
|
Tail head |
From the base of the tail to the middle of the tail |
|
Flank |
From the chest and abdomen to the side part of the back |
|
Anogenital area |
The part including the hip, umbilical body, sex skin and anal |
Data analysis
Following Post (1981), in each month, we calculated the mean diurnal grooming time from a sampled individual, and then we averaged the values across all individuals sampled. The total grooming time was calculated by summing up the across individual mean of each month. In the same way, the diurnal proportion of time spent grooming body sites (hand, head and arm, etc.) and grooming postures were calculated from a sampled animal in each month, and a mean across all individuals was then calculated. The across means of time spent on body sites were further classified into the cross-individual means of rainy months and dry months were averaged to calculate the rainy season and dry season proportion of time spent grooming, respectively. Following Tweheyo et al. (2004), we assessed grooming preference using a grooming preference index (GPI) calculated by the following formula:
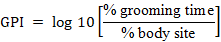
Where % grooming time is the proportion of grooming time in one body site relative to total grooming time; and % body site is the proportion in size of one part to the total body site area. When the value of GPI was 0 there was no preference of the body site; GPI > 0 indicated preference and GPI < 0 indicated avoidance the body site. The larger the GPI, the more preferred this part was.
We used the generalized linear mixed models (GLMM) to analyze the seasonal variation of grooming postures and body sites. The Kruskal-wallis test was used to analyze the annual variation of grooming postures. All data analysis was conducted in R 4.3.0 and SPSS 22.0. All tests were two-tailed, with significance levels of 0.05.
RESULTS
Grooming postures
The total grooming time in the dry season accounted for 56.88% ± 4.09% of the annual total grooming time. There were significant differences among monthly grooming postures throughout the year (χ2 = 49.59, df = 5, p < 0.001). With the exception of sit side, the grooming posture in dry season was lower than that in rainy season (Fig. 1).
The grooming posture with eye contact and without eye contact accounted for 52.14% and 47.86% of the total grooming time, respectively. The proportion of time without eye contact was 52.01% in dry season and 43.71% in rainy season, and there was no significant seasonal variation throughout the year (Z = -0.05, n = 18, p = 0.96).
In the dry season, the dominant posture was sprawl (32.73% ± 11.90%), while in the rainy season, the dominant posture was sit side (33.56% ± 25.52%). Grooming postures did not significantly varied between the dry season and the rainy season (sit face to face: χ2 = 3.43, df = 1, p = 0.06, sitting in the same direction: χ2 = 0.48, df = 1, p = 0.49, sprawl: χ2 = 0.80, df = 1, p = 0.37, quadrupedal stand: χ2 =0.59, df = 1, p = 0.44, sit side: χ2 =2.66, df = 1, p = 0.10), but for the back lie posture (χ2 = 4.18, df = 1, p = 0.04).
Grooming body sites
The variation of grooming time between dry and rainy season was body sites-specific (Table IV). Among them, the proportion of grooming time in DRA in dry season (39.83% ± 4.69%) was significantly lower than that in rainy season (48.12% ± 5.31%) (χ2 = 4.05, df = 1, p = 0.04), but there was no seasonal significant difference between the other two body areas (ERA: χ2 = 0.07, df = 1, p = 0.79; IA: χ2 = 0.98, df = 1, p = 0.32).
Table IV. Time and body surface area ratio of different grooming areas of white-headed black langur. GPI is grooming preference index. The values are Mean±SD.
|
Body areas/ Body sites |
Body surface area ratio (%) |
Grooming time in |
GPI in |
||
|
Dry season (%) |
Rainy season (%) |
Dry season |
Rainy season |
||
|
Inaccessible area (IA) |
|||||
|
Face |
0.49 ± 0.01 |
1.71 ± 1.54 |
0.12 ± 0.20 |
0.54 |
-0.61 |
|
Head |
4.39 ± 0.06 |
2.53 ± 1.41 |
4.01 ± 4.98 |
-0.24 |
-0.04 |
|
Neck |
1.93 ± 0.36 |
3.36 ± 1.16 |
2.01 ± 1.77 |
0.24 |
0.02 |
|
Upper back |
7.34 ± 0.06 |
10.93 ± 5.94 |
6.45 ± 2.40 |
0.17 |
-0.06 |
|
Lower back |
8.03 ± 0.29 |
9.45 ± 3.82 |
7.24 ± 2.02 |
0.07 |
-0.04 |
|
Total |
22.17 ± 0.77 |
27.98 ± 10.02 |
19.82 ± 8.59 |
0.10 |
-0.05 |
|
Easy to reach area (ERA) |
|||||
|
Foot |
4.51± 0.74 |
2.02 ± 2.03 |
2.13 ± 2.26 |
-0.35 |
-0.33 |
|
Shank |
9.34 ± 0.13 |
7.34 ± 3.18 |
8.26 ± 2.20 |
-0.10 |
-0.05 |
|
Thigh |
14.21 ± 0.54 |
8.15 ± 4.01 |
6.30 ± 2.55 |
-0.24 |
-0.35 |
|
Tail tip |
5.71 ± 0.16 |
1.62 ± 1.38 |
5.14 ± 1.94 |
-0.55 |
-0.05 |
|
Forearm |
7.90 ± 0.92 |
8.79 ± 3.57 |
9.42 ± 3.22 |
0.05 |
0.08 |
|
Hand |
3.55 ± 1.41 |
2.14 ± 1.25 |
0.80 ± 0.31 |
-0.22 |
-0.65 |
|
Total |
45.21 ± 0.72 |
32.18 ± 6.11 |
32.06 ± 5.47 |
-0.15 |
-0.15 |
|
Difficult to reach area (DRA) |
|||||
|
Arm |
6.07 ± 1.05 |
10.50 ± 0.94 |
9.72 ± 3.32 |
0.24 |
0.20 |
|
Abdomen |
4.53 ± 0.27 |
1.74 ± 1.26 |
0.77 ± 0.69 |
-0.42 |
-0.77 |
|
Chest |
4.88 ± 0.27 |
2.20 ± 1.53 |
1.97 ± 1.33 |
-0.35 |
-0.39 |
|
Tail head |
7.49 ± 0.81 |
6.30 ± 4.47 |
11.55 ± 3.57 |
-0.08 |
0.19 |
|
Flank |
8.02 ± 0.22 |
6.79 ± 1.02 |
9.10 ± 1.72 |
-0.07 |
0.05 |
|
Anogenital area |
1.63 ± 0.52 |
12.31 ± 3.00 |
15.00 ± 2.51 |
0.88 |
0.96 |
|
Total |
32.62 ± 1.50 |
39.83 ± 4.69 |
48.12 ± 5.31 |
0.09 |
0.17 |
The anogenital area had the highest grooming time in both the dry season (12.31% ± 3.00%) and rainy season (15.00% ± 2.51%) (Table IV). Only 5 parts out of the 17 parts had significant difference between the rainy season and the dry season (tail head: χ2 = 4.78, df = 1, p < 0.05; tail tip: χ2 = 9.41, df = 1, p < 0.01; hands: χ2 = 9.00, df = 1, p < 0.01; thighs: χ2 = 10.45, df = 1, p < 0.01; flank: χ2 = 6.180, df = 1, p < 0.05).
The GPI of IA was greater than 0 in dry season, but less than 0 in the rainy season; DRA was greater than 0 in both dry season and rainy season; and ERA was less than 0 in both dry season and rainy season (Table IV). Hence, according to the GPI, it could be seen that IA in dry season and DRA are more favored by monkeys, while ERA does not receive attention matching its area. In both dry and rainy seasons, the anogenital area has the greatest GPI of any part of the body, so monkeys preferentially groomed this area. The comparison chart of annual surface area and grooming time was shown in Figure 2.
DISCUSSION
The grooming posture adopted will affect the individual’s eye contact during grooming (Zhang et al., 2014). Some studies have pointed out that animals avoid eye contact when grooming each other to reduce tension and potential aggression (Boccia, 1983; 1989; Borries, 1992; Allanic et al., 2020). In Sichuan snub-nosed monkeys, when high-ranking individuals stare or threaten low-ranking ones, the latter often lower its head or crouch to avoid eye contact (Ren et al., 2000). However, other studies suggest that eye contact between individuals makes it easier to identify each other, promotes emotional /attentional engagement, and facilitates body language communication (Zhang et al., 2014; Zanoli et al., 2021). It is likely that eye contact makes it easier to identify other individuals and facilitates body language communication though eye contact postures increase the chances of conflict (Zhang et al., 2014). This slightly risky investment ensures that the group will reap the benefits of its social life (Boccia, 1989). In this study, the langurs used more without eye contact postures for grooming in the dry season, while in the rainy season they used more eye contact postures, but there was no significant difference. In general, there were more grooming posture with eye contact than without eye contact of the total grooming time. Thus, the grooming behavior of the langurs fit the social functional hypothesis.
On the basis of hygiene function hypothesis, the grooming time spent in different body sites are inconsistent, that is, the GPI of DRA and IA are greater than 0, while that of ERA is less than 0 (Pérez and Baró, 1999). In this research, GPI of DRA were greater than 0 but less than 0 of ERA in both dry and rainy seasons, suggesting that allogrooming of the animals is consistent with the hygiene function hypothesis, as is the case with François langur (Zhou et al., 2006). Some studies suggest that the anogenital area (belonging to DRA) is highly selective during allogrooming, for example, François’ langurs are more inclined to comb the anogenital area since this area is difficult to reach by groomee (Zhou et al., 2006). Similarly, this has been seen in the narrow-nosed monkeys (Dunbar, 1991; Pérez and Baró, 1999; Li et al., 2002). Social status and reproductive status can be checked through the anogenital area in primates, which may be the reason for the high selection of the anogenital area in addition to difficulty in autogrooming (Moser et al., 1991). In our research, the white-headed black langur was also highly selective in the grooming sites in both dry and rainy season. This is consistent with other studies.
However, the GPIs of IA were greater than 0 in the dry season, and less than 0 in the rainy season (Table IV), suggesting that allogrooming function may vary with seasons. Firstly, rainfall has a significant impact on the behavioral and ecological characteristics of primates (Li et al., 2018). The seasonal inconsistency of function of grooming in white-headed black langur may be related to this ecological factor. During the dry season, food availability decreases and animals spend less time searching for food to conserve energy and therefore have more time for grooming (Zhou et al., 2012). On the contrary, during the rainy season, when food is abundant, animals spend more time searching for high quality food to maximize net energy income (Dunbar, 1992), thus reducing grooming time.
Another factor influencing grooming is the number and gender of individuals that join in grooming, for example, the females form stable clusters with maternal kin-related female partners both during the mating and non-mating season, whilst, males were not included in the females’ clusters during the mating season (Xia et al., 2019). The third factors influencing preference of body site are age and kinship pecific, e.g., mother groom more their offspring more to prevent disease linked to ectoparasites, and matures individuals spend more time grooming the inaccessible back than immature individuals (Allanic et al., 2020). But to date, information concerning grooming variation between gender, among age groups and kinship are unavailable. Therefore, in the future, before we need more intensive study before generalizing the function of grooming of the langur.
CONCLUSION
In this study, we found that the animal was highly selective to the grooming site, preferring the difficult to reach area and the anogenital area. There was variation between the two seasons regarding the hygiene function hypothesis, but social function hypothesis has no different, which make it necessary to further study before generalizing the function of all grooming of the langur.
ACKNOWLEDGMENT
We are very grateful to the Guangxi Forestry Bureau and Chongzuo White-Headed Langur National Nature Reserve for permitting us to conduct research.
Funding
This study was supported by the National Nature Science Foundation of China (No. 31960104, 31960106).
Ethical statement
Data was collected in a noninvasive manner from free-ranging animals in the field. All research methods adhered to Chinese legal requirements, complied with protocols approved by the State Forestry Administration of China.Our field observations were approved by the state and local governments.
Statement of conflict of interest
The authors have declared no conflict of interest.
REFERENCES
Allanic, M., Hayashi, M., Furuichi, T. and Matsuzawa, T., 2020. Social influences on grooming site preferences in wild bonobos (Pan paniscus) at Wamba, DRC. Primates, 61: 213-223. https://doi.org/10.1007/s10329-019-00788-z
Allanic, M., Hayashi, M., Furuichi, T. and Matsuzawa, T., 2021. Body site and body orientation preferences during social grooming: A comparison between wild and captive chimpanzees and bonobos. Folia Primatol., 92: 1-12. https://doi.org/10.1159/000512901
Altmann, J., 1974. Observational study of behavior: Sampling methods. Behaviour, 49: 227–266. https://doi.org/10.1163/156853974X00534
Barton, R., 1985. Grooming site preferences and their functional implications. Int. J. Primatol., 6: 519-532. https://doi.org/10.1007/BF02735574
Boccia, M.L., 1983. A functional analysis of social grooming patterns through direct comparison with self-grooming in rhesus monkeys. Int. J. Primatol., 4: 399-418. https://doi.org/10.1007/BF02735602
Boccia, M.L., 1989. Comparison of the physical characteristics of grooming in two species of macaques (Macaca nemestrina and M. radiata). J. comp. Psychol., 103: 177-183. https://doi.org/10.1037/0735-7036.103.2.177
Borgeaud, C. and Bshary, R., 2015. Wild vervet monkeys trade tolerance and specific coalitionary support for grooming in experimentally induced conflicts. Curr. Biol., 25: 3011-3016. https://doi.org/10.1016/j.cub.2015.10.016
Borries, C., 1992. Grooming site preferences in female langurs (Presbytis entellus). Int. J. Primatol., 13: 19-32. https://doi.org/10.1007/BF02547725
Borries, C., Sommer, V. and Srivastava, A., 1994. Weaving a tight social net: Allogrooming in free-ranging female langurs (Presbytis entellus). Int. J. Primatol., 15: 421-443. https://doi.org/10.1007/BF02696102
Carter, G. and Leffer, L., 2015. Social grooming in bats are vampire bats exceptional? PLoS One, 10: 1-11. https://doi.org/10.1371/journal.pone.0138430
Caselli, M., Zanoli, A., Palagi, E. and Norscia, I., 2021. Infant handling increases grooming towards mothers in wild geladas (Theropithecus gelada). Behav. Process., 192: 104501. https://doi.org/10.1016/j.beproc.2021.104501
Chen, J., 2016. Diurnal activity pattern and seasonal variations of captive Papio hamadryas. Chin. J. appl. Ecol., 27: 3719-3726. https://doi.org/10.13287/j.1001-9332.201611.006
de Freslon, I., Peralta, J.M., Strappini, A.C., and Monti, G., 2020. Understanding allogrooming through a dynamic social network approach: An example in a group of dairy cows. Front. Vet. Sci., 7: 535. https://doi.org/10.3389/fvets.2020.00535
Dunbar, R.I.M., 1991. Functional significance of social grooming in primates. Folia Primatol., 57: 121-131. https://doi.org/10.1159/000156574
Dunbar, R.I.M., 1992. Time: A hidden constraint on the behavioural ecology of baboons. Behav. Ecol. Sociobiol., 31: 35-49. https://doi.org/10.1007/BF00167814
Ghiglieri, M.P., 1984. The Chimpanzees of kibale forest: A field study of ecology and social structure. Columbia University Press, New York, Berkeley.
Grueter, C.C., Bissonnette, A., Isler, K. and van Schaik, C.P., 2013. Grooming and group cohesion in primates: implications for the evolution of language. Evol. Hum. Behav., 34: 61-68. https://doi.org/10.1016/j.evolhumbehav.2012.09.004
Heine, K.B., DeVries, P.J. and Penz, C.M., 2017. Parasitism and grooming behavior of a natural white-tailed deer population in Alabama. Ethol. Ecol. Evol., 29: 292-303. https://doi.org/10.1080/03949370.2016.1179683
Hirsch, B.T., Stanton, M.A. and Maldonado, J.E., 2012. Kinship shapes affiliative social networks but not aggression in ring-tailed coatis. PLoS One, 7: 1-9. https://doi.org/10.1371/journal.pone.0037301
Huang, C., 2002. The white-headed leaf monkey of China. Guangxi Normal University Press, Guilin, China.
Huang, C., Li, Y., Zhou, Q., Feng, Y., Chen, Z., Yu, H. and Wu, Z., 2008. Karst habitat fragmentation and the conservation of the white-headed langur (Trachypithecus leucocephalus) in China. Primate Conserv., 23: 133-139. https://doi.org/10.1896/052.023.0116
Huang, Z., Yuan, P., Huang, H., Tang, X., Xu, W., Huang, C. and Zhou, Q., 2017. Effect of habitat fragmentation on ranging behavior of white-headed langurs in limestone forests in Southwest China. Primates, 58: 423-434. https://doi.org/10.1007/s10329-017-0600-4
Huang, Z.H., Huang, C.M., Zhou, Q.H., Wei, H. and Meng, Y.J., 2010. Diet and the seasonal changes of the François’ langur (Trachypithecus francoisi). Acta Ecol. Sin., 30: 5501-5508.
Hutchins, M. and Barash, D.P., 1976. Grooming in primates: Implications for its utilitarian function. Primates, 17: 145-150. https://doi.org/10.1007/BF02382848
Jasso del Toro, C., Mondragón-Ceballos, R. and Gutiérrez-García, G., 2020. Potential food availability influences social interactions of young individuals in a Neotropical primate (Alouatta palliata). Folia Primatol., 91: 31-47. https://doi.org/10.1159/000501408
Jiang, Q., Xia, D., Wang, X., Zhang, D., Sun, B. and Li, J., 2019. Interchange between grooming and infant handling in female Tibetan macaques (Macaca thibetana). Zool. Res., 40: 139-145. https://doi.org/10.24272/j.issn.2095-8137.2018.049
Kanngiesser, P., Sueur, C., Riedl, K., Grossmann, J. and Call, J., 2011. Grooming network cohesion and the role of individuals in a captive chimpanzee group. Am. J. Primatol., 73: 758-767. https://doi.org/10.1002/ajp.20914
Kurihara, Y., 2016. Low-ranking female Japanese macaques make efforts for social grooming. Curr. Zool., 62: 99-108. https://doi.org/10.1093/cz/zow006
Lawande, N.V., Ujjainwala, A.L. and Christian, C.A., 2020. A single test to study social behavior and repetitive self-grooming in mice. Bio-protocol, 10: e3499. https://doi.org/10.21769/BioProtoc.3499
Lhota, S., Roubová, V., Gregorová, V. and Konečná, M., 2019. Complex patterns of grooming and sexual activity in Barbary macaques (Macaca sylvanus). Am. J. Primatol., 81: e23040. https://doi.org/10.1002/ajp.23040
Li, B., Zhang, P., Watanabe, K. and Tan, C.L., 2002. Does allogrooming serve a hygienic function in the Sichuan snub-nosed monkey (Rhinopithecus roxellana)? Acta Zool. Sin., 48: 707-715.
Li, D., Yuan, P., Krzton, A., Huang, C. and Zhou, Q., 2016. Dietary adaptation of white-headed langurs in a fragmented limestone habitat. Mammalia, 80: 153-162. https://doi.org/10.1515/mammalia-2014-0152
Li, H., 2004. Time budgets of golden monkey (Rhinopithecus roxellana) in autumn and winter on Qinling Mountains. J. Shaanxi Normal Univ. (Natl. Sci. Ed.), 32: 86-89. https://doi.org/10.15983/j.cnki.jsnu.2004.02.027
Li, Y., Chen, Z., Huang, Z. and Zhou, Q., 2018. Activity time budget of Assamese macaque (Macaca assamensis) during rainy season in Nonggang Nature Reserve, Guangxi, China. J. Guangxi Normal Univ. (Natl. Sci. Ed.), 36: 80-86. https://doi.org/10.16088/j.issn.1001-6600.2018.03.012
Li, Z. and Elizabeth Rogers, M., 2006. Food items consumed by white-headed langurs in Fusui, China. Int. J. Primatol., 27: 1551-1567. https://doi.org/10.1007/s10764-006-9090-8
Liu, F., Li, Y., Zhang, K., Liang, J., Nong, D. and Huang, Z., 2022. Habitat use of the white-headed langurs in limestone forest of Southwest Guangxi, China: Seasonality and group size effects. Ecol. Evol., 12: e9068. https://doi.org/10.1002/ece3.9068
Lu, S., Chen, T., Huang, Z., Li, Y. and Lu, C., 2021. Interannual variation in food choice of white-headed langur inhabiting limestone forests in Fusui, southwest Guangxi, China. Ecol. Evol., 11: 9349-9360. https://doi.org/10.1002/ece3.7726
Maurice, M.E., Fuashi, N.A., Lengha, T.K. and Agiamte-Mbom, V.B., 2019. Activity budget of captive drill monkeys Mandrillus leucophaeus (Cuvier) in Limbe Wildlife Center, Southwest Region, Cameroon. Int. J. Biodivers. Conserv., 11: 69-77. https://doi.org/10.5897/IJBC2017.1096
Moser, R., Cords, M. and Kummer, H., 1991. Social influences on grooming site preferences among captive long-tailed macaques. Int. J. Primatol., 12: 217–230. https://doi.org/10.1007/BF02547585
Pereira, A.S., Rebelo, I.D., Casanova, C., Lee, P.C. and Louca, V., 2019. The dynamics of grooming interactions: Maintenance of partner choice and the consequences of demographic variation for female mandrills. PeerJ, 7: e6332. https://doi.org/10.7717/peerj.6332
Pérez, A.P. and Baró, J.J.V., 1999. Does allogrooming serve a hygienic function in Cercocebus torquatus lunulatus? Am. J. Primatol., 49: 223-242. https://doi.org/10.1002/(SICI)1098-2345(199911)49:3<223::AID-AJP2>3.0.CO;2-9
Pfoh, R., Tiddi, B., Bitetti, M.S.D. and Agostini, I., 2021. Grooming site preferences in black capuchin monkeys: Hygienic vs. social functions revisited. Am. J. Primatol., 83: e23336. https://doi.org/10.1002/ajp.23336
Post, D.G., 1981. Activity patterns of yellow baboons (Papio cynocephalus) in the Amboseli National Park, Kenya. Anim. Behav., 29. https://doi.org/10.1016/S0003-3472(81)80095-4
Rathinakumar, A., Cantor, M., Senthilkumar, K., Vimal, P., Kaliraj, P. and Marimuthu, G., 2017. Social grooming among Indian short-nosed fruit bats. Behaviour, 154: 37-63. https://doi.org/10.1163/1568539X-00003410
Ren, R., Yan, K., Su, Y., Li, J. and Zhou, Y., 2000. A field study of the society of Rhinopithecus roxellana. Peking University Press, Beijing.
Schino, G. and Lasio, F., 2018. Competition for grooming partners and interference in affiliation among female mandrills. Ethology, 124: 600-608. https://doi.org/10.1111/eth.12763
Shimada, M. and Suzuki, N., 2020. The contribution of mutual grooming to affiliative relationships in a feral Misaki horse herd. Animals, 10: 1564. https://doi.org/10.3390/ani10091564
Tahir, N.A., Ismail, A. and Rahman, F., 2017. Daily activity budget of silver leaf monkeys (Trachypithcus cristatus) in Kuala Selangor Nature Park (KSNP), Selangor, Peninsular Malaysia. Malay. Nat. J., 69: 337-343.
Tan, W., 2014. Nature reserves in Guangxi. China Environmental Press, Beijing.
Terry, R.L., 1970. Primate grooming as a tension reduction mechanism. J. Psychol., 76: 129-136. https://doi.org/10.1080/00223980.1970.9916830
Tombak, K.J., Wikberg, E.C., Rubenstein, D.I. and Chapman, C.A., 2019. Reciprocity and rotating social advantage among females in egalitarian primate societies. Anim. Behav., 157: 189-200. https://doi.org/10.1016/j.anbehav.2019.09.010
Tweheyo, M., Lye, K.A. and Weladji, R.B., 2004. Chimpanzee diet and habitat selection in the Budongo Forest Reserve, Uganda. For. Ecol. Manage., 188: 267-278. https://doi.org/10.1016/j.foreco.2003.07.028
Vea, J.J., Perez, A.P., Baldellou, M. and Alea, V., 1999. Cost-benefit analysis of allogrooming behaviour in Cercocebus torquatuslunulatus. Behaviour, 136: 243-256. https://doi.org/10.1163/156853999501306
Wolovich, C.K., Tapanes, E. and Evans, S., 2017. Allogrooming in male-female pairs of captive owl monkeys (Aotus nancymaae). Folia Primatol., 88: 483-496. https://doi.org/10.1159/000485134
Wren, B.T., Remis, M.J., Camp, J.W. and Gillespie, T.R., 2016. Number of grooming partners is associated with hookworm infection in wild vervet monkeys (Chlorocebus aethiops). Folia Primatol., 87: 168-179. https://doi.org/10.1159/000448709
Wu, C., Liao, Z., Sueur, C., Sha, J.C.M., Zhang, J. and Zhang, P., 2018. The influence of kinship and dominance hierarchy on grooming partner choice in free-ranging Macaca mulatta brevicaudus. Primates, 59: 377-384. https://doi.org/10.1007/s10329-018-0662-y
Wubs, M., Bshary, R. and Lehmann, L., 2018. A reinforcement learning model for grooming up the hierarchy in primates. Anim. Behav., 138: 165-185. https://doi.org/10.1016/j.anbehav.2018.02.014
Xia, D.P., Kyes, R.C., Wang, X., Sun, B.H., Sun, L.X. and Li, J.H., 2019. Grooming networks reveal intra- and intersexual social relationships in Macaca thibetana., Primates, 60: 223-232. https://doi.org/10.1007/s10329-018-00707-8
Zanoli, A., Gamba, M., Lemasson, A., Palagi, E. and Norscia, I., 2021. Looking into each other’s eyes makes it better: eye-to-eye contact enhances sexual interactions in wild geladas. Anim. Behav., 177: 269-276. https://doi.org/10.1016/j.anbehav.2021.05.011
Zhang, D., Li, D., Hu, J., Ren, B., Yuan, X., He, X., Li, Y. and Li, M., 2014. Allogrooming among female Yunnan snub-nosed monkeys (Rhinopithecus bieti). Acta Theriol. Sin., 34: 38-45.
Zhang, K., Zhou, Q., Xu, H. and Huang, Z., 2020. Effect of group size on time budgets and ranging behavior of white-headed langurs in limestone forest, southwest China. Folia Primatol., 91: 188-201. https://doi.org/10.1159/000502812
Zhao, D.P., Li, B.S. and Li, B.G., 2019. Postural effect on manual laterality during grooming in northern white-cheeked gibbons (Nomascus leucogenys). Zool. Res., 40: 449-455. https://doi.org/10.24272/j.issn.2095-8137.2019.059
Zhou, Q., Huang, C. and Li, Y., 2006. Allogrooming of captive François’ langur (Trachypithecus francoisi). Acta Theriol. Sin., 26: 221-225. https://doi.org/10.16829/j.slxb.2006.03.002
Zhou, Q., Huang, H., Tang, X. and Huang, C., 2012. Daily activity patterns and time budgets of an all-male group in the white-headed langur. J. Guangxi Normal Univ. (Natl. Sci. ed.,) 3: 282-287. https://doi.org/10.16088/j.issn.1001-6600.2012.03.045
To share on other social networks, click on any share button. What are these?










