Physicochemical Properties of Duck Egg White Treated with Different Ultrasound Time
Research Article
Physicochemical Properties of Duck Egg White Treated with Different Ultrasound Time
Ho Shi Hui1, Eng-Keng Seow2, Nurul Huda1*
1Faculty of Food Science and Nutrition, Universiti Malaysia Sabah, Jalan UMS, 88400, Kota Kinabalu, Sabah, Malaysia; 2Department of Food Science and Technology, School of Industrial Technology, Faculty of Applied Sciences, Universiti Teknologi MARA, 40450 Shah Alam, Selangor, Malaysia.
Abstract | The effect of ultrasound treatment duration (0, 10, 20, 30, 40, 50 and 60 minutes) on physicochemical properties of duck egg white was studied. Duck egg white were treated with ultrasound generated by sonicator (frequency 40±0.2 kHz) for different times, and their pH, colour, foaming capacity and stability, emulsifying activity, tube inverting test, folding test, expressible moisture and texture profile analysis were compared. Ultrasound treatment had caused a significant (p<0.05) change in pH, foaming capacity, and the folding test score of egg white gel. However, texture profile analysis showed that the hardness, springiness, gumminess and chewiness of egg white gel had decreased after ultrasound treatment. Ultrasound-treated duck egg white for 40 minutes showed a significantly (p<0.05) higher foaming capacity and improved folding test score. Result showed that the increase of ultrasound treatment time may have conferred detrimental effects on duck egg white protein that influenced the physicochemical properties of egg white. Hence, ultrasound treatment for 40 minutes was suggested to produce duck egg white with better physicochemical properties.
Keywords | Duck egg, Egg white, Physicochemical properties, Ultrasound, Foaming
Received | September 15, 2021; Accepted | October 28, 2021; Published | January 05, 2022
*Correspondence | Nurul Huda, Faculty of Food Science and Nutrition, Universiti Malaysia Sabah, Jalan UMS, 88400, Kota Kinabalu, Sabah, Malaysia; Email: drnurulhuda@ums.edu.my
Citation | Hui HS, Seow EK, Huda N (2022). Physicochemical properties of duck egg white treated with different ultrasound time. J. Anim. Health Prod. 10(1): 43-50.
DOI | http://dx.doi.org/10.17582/journal.jahp/2022/10.1.43.50
ISSN | 2308-2801
INTRODUCTION
Eggs are one of the protein sources that often consumed by human other than milk, meat and fish. Egg consists of high protein content, lipid, essential vitamins (riboflavin and niacin) and minerals (Zaheer, 2015). In Asian countries such as Malaysia, China, Thailand and Taiwan, hen and duck eggs are the most common avian eggs consumed as compared to quail, geese and turkey (Quan & Benjakul, 2018a). Domestic duck production is divided into meat production and egg production. The Pekin ducks and Muscovy ducks are bred to produce meat whereas Khaki Campbell and Indian Runner are bred for egg production (Ismail et al., 2010; Aronal et al., 2012; Arthur, 2017). In Malaysia, the common breeds for egg production are the local Java ducks and Khaki Campbell (Nurkhoeriyati et al., 2011; Huang & Lin, 2011). Khaki Campbell are well known for their laying ability. They can lay about 250-240 eggs per year on average when they reach 5 to 7 months old (Bhattacherjee et al., 2018).
Eggs consist of three components, which are eggshell (9-12%), egg white (60%) and egg yolk (30-33%). Egg white consists of 88% of water and 11% of protein, and the remaining 1% are carbohydrate, ash and trace amounts of lipids (Abeyrathne et al., 2013). Egg white is widely used in food industries due to its functional properties such as gelling, foaming, emulsifying, binding adhesion, viscosity enhancement, water holding capacity and moisturizing (Arzeni et al., 2012; Quan & Benjakul, 2018a). The functional properties of egg white are important to bakery products, desserts, meat and fish products because egg white gives textural characteristics in term of hardness, stickiness, springiness and cohesiveness to food products (Zayas, 2012) and build up the food structure. Recent research also studied the use of monosaccharides (i.e. glucose, D-galactose and D-xylose) in molecular structural modification of duck egg white protein conjugates to improve emulsifying capacity (Ai et al., 2021), and effect of calcium hydroxide addition to the structural enhancement of duck egg white gel with or without heating treatment (Ai et al., 2020).
Application of ultrasound treatment in food processing that involves interaction of sound waves and food matrix with frequencies of 16-100 kHz may modify or improve some functional, chemical and physical properties of food ingredients (Stefanovic et al., 2017). The cavitation phenomenon occurs during ultrasound treatment causes protein denaturation, which modifies or improves the functional properties of egg white (Gharbi & Labbafi, 2018). The main purpose of the present study was to determine the effect of different times ultrasound treatment by using sonicator on physicochemical properties (pH, colour, foaming capacity and stability, emulsifying activity, tube inverting test, folding test, expressible moisture and texture profile analysis) of duck egg white.
MATERIALS AND METHODS
Experimental Design
All trials were done using completely randomized design. Egg white was divided into 7 portions and placed in 500 mL conical flask. Each conical flask contain 500ml sample. The egg white samples were treated with ultrasound treatment by using sonicator bath (Branson Model 8510, America) for 10, 20, 30, 40, 50 and 60 minutes, respectively, at frequency 40±0.2 kHz and a constant temperature (25±1℃) was maintained during the treatment (Stefanovic et al., 2018). Frequency 40±0.2 kHz used due to the previous report that frequencies higher than 100 kHz are showed no improvement on the physicochemical properties of the sample protein. The untreated egg white sample served as a control.
Sample Preparation
Khaki Campbell duck eggs were purchased from Tuaran, Sabah, Malaysia. The eggs are around less than one week old after laying. The weight of duck eggs are between 61 to 70g. Eggshell were cleaned to remove dirt and allowed to dry. Egg white and egg yolk were separated manually into a 5L measuring jug (Wong et al., 2018). Egg white was stirred gently manually using glass rod to prepare homogeneous mixture without foam formation and was filled into conical flask for ultrasound treatment.
Egg White Gel Preparation
About 300 mL of each treated and untreated egg white were stirred evenly and filled into plastic tube (diameter 25 mm) with the end of tubes tightly tied. Samples were heated in water bath at 90 ℃ for 30 minutes. Gel samples were cooled in refrigerator at 4℃ for 24 hours (Quan & Benjakul, 2018b) prior to expressible moisture test, folding test and texture profile analysis.
pH Measurements
The pH of egg white treated for 10 to 60 minutes, and untreated egg white were measured by using pH meter (Eutech Instruments pH2700, America). The pH meter was calibrated with the known pH buffer standard solution. The measurement of each sample was repeated three times and reported as average and standard deviation.
Expressible Moisture
Egg white gels were cut into slices with thickness of 5 mm. The initial weight of sample was recorded and placed between two pieces of Whatman filter paper No. 41. A standard 5 kg weight was placed on top of the sample and held for 2 minutes. The final weight of sample was weighed and recorded. The expressible moisture of each sample was calculated as follows (Huda et al., 2013a; Nik Aisyah et al., 2017):
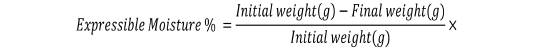
Folding Test
Egg white gels were cut into slices with thickness of 3 mm. Samples were folded slowly with thumb and index finger. The way the gels broke were observed and rated with the following scales: 1 = breaks by finger pressure, 2 = cracks immediately when folded in half, 3 = cracks gradually when folded in half, 4 = no cracks showing after folding in half, 5 = no cracks showing after folding twice (Huda et al., 2013b; Nik Aisyah et al., 2017).
Colour
The colour of egg white samples, and egg white gel samples were analysed by colourimeter (Hunterlab Colorflex Spectrophotometer, America). The colourimeter was calibrated with a black calibration plate followed by white calibration plate. Each sample was filled in a glass cup designed for colourimeter and covered with an opaque cup for analysis. The parameters analysed were L* (lightness), a* (redness) and b* (yellowness) (Quan & Benjakul, 2018a).
Tube Inverting Test
Each treated and untreated egg white solution sample with the ratio of egg white: distilled water (10:90; 20:80; 30:70; 40:60; 50:50) were prepared in test tube (Martins et al., 2019). Egg white solution was heated in water bath at 90 ℃ for 10 minutes until gel was formed. The samples were cooled at room temperature for a few minutes and test tube were inverted for 5 minutes to observe the formation of stable gel. Unstable gel will collapse (Niehoff et al., 2013) and was recorded as negative result.
Texture Profile Analysis
Egg white gels were cut into a cylindrical shape with the height of 20 mm. Gel hardness, springiness, cohesiveness, gumminess and chewiness were analysed by using texture analyzer (Brookfield CT3 Texture Analyzer, America). Analyzer was calibrated with load cell weight and probe height before analysis of samples. The parameters were set as: pre-test speed = 5 mm/s, test speed = 2 mm/s, post-test speed = 5 mm/s, strain = 50% and trigger force = 5 g (Wong et al., 2018).
Foaming Properties
Approximately 20 mL of each treated and untreated egg white sample were required to determine the foaming properties. About 20 mL of the sample was whipped at 1500 rpm for 3 min using blender (Waring Commercial Laboratory Blender 7010HS, America) and immediately transferred the solution into a 100 mL measuring cylinder. The volume of foam and solution was recorded (Wang et al., 2013). Foaming capacity (FC) and foaming stability (FS) of treated, and untreated egg white solution were calculated as follows:
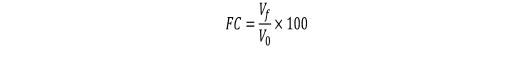
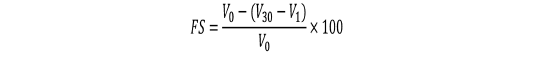
where V0 is the volume of egg white solution before whipping (m3); Vf is the volume of bubbles (m3); V1 is the volume of solution after whipping and V30 is the volume of bubbles after whipping for 30 min (m3) (Wong et al., 2018).
Emulsion Activity
About 10 mL of egg white and 10 mL of sunflower oil were filled into a 50 mL centrifuge tube. Solution of egg white with oil was vortexed for 1 minute by using vortex mixer (VELP Scientifica ZXClassic Vortex Mixer, Italy) and centrifuged at 3200 rpm for 5 minutes (Eppendorf Centrifuge 5702, German). Emulsion activity (EA) was calculated as follows (Lim et al., 2019):

Statistical Analysis
All tests were carried out in triplicate (n = 3) and the data were expressed as means ± standard deviation. Data was statistically analyzed with IBM SPSS Statistics 26. One-way analysis of variance (ANOVA) was performed to determine the significance level at p<0.05 (Arzeni et al., 2012).
RESULTS AND DISCUSSION
pH
The pH result of egg white treated with ultrasound from 0 to 60 minutes are shown in Table 1. When the duration of ultrasound treatment increased, pH value of egg white increased. There was a significant difference (p<0.05) between untreated egg white and ultrasound-treated egg white. Sert et al. (2011) reported that ultrasound treatment increases pH of egg white due to loss of carbon dioxide in egg white. Degradation of gel structure of egg white causes carbon dioxide migration and the pH of egg white increases. Egg white contains around 0.35% of carbon dioxide with pH 7.6 to 8.5. Release of carbon dioxide increases the pH of egg white to 9.7 (Banerjee et al., 2011). Ultrasound treatment causes degassing of carbon dioxide. When ultrasound wave is transmitted to the sample, cavitation phenomenon occurs causing formation and expansion of air bubbles in liquid sample, follows by flotation of air bubbles to the surface of sample and releasing gas (Eksin, 2015).
Foaming Properties And Emulsion Activity
Table 1 shows the foaming capacity, foaming stability and emulsion activity of egg white at different treatment time. There was a significant difference (p<0.05) among the samples in foaming capacity and foaming stability. Foaming capacity increased as ultrasound time treatment increased due to the exposure of egg white protein to ultrasound. Surface denaturation of egg white protein during the treatment increases protein surface hydrophobicity and flexibility. Protein molecules are adsorbed more efficiently onto the air-liquid interface (Zhang et al., 2011; Stefanovic et al., 2018). Jambrak et al. (2008) reported that ultrasound treatment on whey protein for 30 minutes showed an improvement on foaming capacity but a decrement afterward. The homogenization effect of ultrasound treatment caused a more even dispersion of protein with improved foaming properties. Foaming capacity decreased after certain treatment period due to the over denaturation of protein, that insufficient amounts are adsorbed onto air-liquid interface
Table 1: Physicochemical properties of egg white and egg white gel at different ultrasound time
| Time (min) | pH | Foaming capacity (%) | Foaming stability (%) | Emulsion activity (%) | Expressible moisture (%) | Folding Test Score |
| 0 |
8.97±0.31b |
61.33±4.37cd |
55.83±3.82a |
15.17±7.32 |
26.70±1.78a |
2.00±0.00b |
| 10 |
9.17±0.05ab |
59.67±8.02d |
56.67±7.64a |
15.33±4.73 |
27.13±2.54a |
2.00±0.00b |
| 20 |
9.43±0.07a |
74.67±5.60bc |
51.33±7.09ab |
17.33±8.74 |
26.37±2.88a |
2.00±0.00b |
| 30 |
9.37±0.11a |
83.33±4.27b |
40.17±2.57bc |
16.67±6.66 |
25.73±1.75a |
3.00±0.00a |
| 40 |
9.37±0.06a |
94.50±0.87a |
38.21±2.93c |
23.34±7.64 |
17.65±1.48b |
3.00±0.00a |
| 50 |
9.53±0.06a |
73.83±3.81bcd |
41.67±2.89bc |
25.00±8.66 |
22.78±0.55ab |
3.00±0.00a |
| 60 |
9.47±0.06a |
65.00±5.00cd |
49.83±2.75abc |
25.00±8.54 |
26.22±1.42a |
2.00±0.00b |
Data presented as mean ± standard deviation (n = 3). Different letters in the same column indicate significant differences (p <0.05).
Table 2: Colour analysis result of egg white and egg white gel at different ultrasound time
| Time (min) | Egg white | Egg white gel | ||||
| L* | a* | b* | L* | a* | b* | |
| 0 |
26.11±0.46a |
-0.88±0.03a |
-2.70±0.54a |
89.35±0.07c |
-2.09±0.15b |
4.46±0.02b |
| 10 |
24.76±0.06ab |
-1.57±0.01bc |
-3.51±0.01ab |
89.12±0.04d |
-2.18±0.02c |
4.47±0.01a |
| 20 |
23.48±0.14bcd |
-1.13±0.01a |
-4.49±0.02bc |
89.84±0.03a |
-2.16±0.01c |
3.54±0.04d |
| 30 |
24.11±0.71abc |
-1.20±0.29ab |
-3.66±0.76ab |
88.82±0.01e |
-2.09±0.01b |
3.34±0.01e |
| 40 |
22.62±0.24cd |
-1.13±0.11a |
-4.46±0.07bc |
89.57±0.03b |
-1.88±0.01a |
3.63±0.01c |
| 50 |
19.95±1.65e |
-1.78±0.10c |
-5.39±0.80c |
89.32±0.08c |
-2.31±0.01d |
3.51±0.01d |
| 60 |
22.05±0.28d |
-1.64±0.18c |
-4.49±0.21bc |
88.47±0.13f |
-2.49±0.02e |
3.12±0.03f |
Data presented as mean ± standard deviation (n = 3). Different letters in the same column indicate significant differences (). L*: lightness, a*: redness, b*: yellowness
Table 3: Tube inverting test result for egg white at different ultrasound time
| Time (min) | Concentration of egg white to form gel (%) | ||||||||||
| 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 | 100 | ||
| 0 | - | - | - | + | + | + | + | + | + | + | |
| 10 | - | - | - | + | + | + | + | + | + |
+ |
|
| 20 | - | - | - | + | + | + | + | + | + | + | |
| 30 | - | - | - | + | + | + | + | + | + | + | |
| 40 | - | - | - | + | + | + | + | + | + | + | |
| 50 | - | - | - | + | + | + | + | + | + | + | |
| 60 | - | - | - | + | + | + | + | + | + |
+ |
|
Note: + present; - absent
Table 4: Textural properties of egg white gel at different ultrasound time
| Time (min) | Hardness (g) | Springiness (mm) | Cohesiveness | Gumminess (g) | Chewiness (g/mm) |
| 0 |
1853.17±184.85a |
6.20±0.47a |
0.74±0.01 | 1429.01±137.96 |
82.28±14.34a |
| 10 |
1703.83±22.67ab |
5.51±0.21b |
0.73±0.04 | 1347.50±77.46 |
72.67±2.51ab |
| 20 |
1685.66±43.00ab |
5.62±0.15ab |
0.73±0.04 | 1317.51±83.87 |
72.49±2.67ab |
| 30 |
1636.00±39.25ab |
5.54±0.18ab |
0.76±0.05 | 1309.67±44.50 |
71.17±1.56ab |
| 40 |
1534.67±59.82b |
5.36±0.19ab |
0.75±0.04 | 1227.50±77.79 |
66.68±2.93b |
| 50 |
1612.67±96.07b |
5.54±0.11b |
0.72±0.07 | 1239.92±121.91 |
65.33±8.72b |
| 60 |
1688.00±28.97ab |
5.55±0.15ab |
0.78±0.03 | 1386.67±62.57 |
75.40±2.00ab |
Data presented as mean ± standard deviation (n = 3). Different letters in the same column indicate significant differences (p <0.05).
for foaming (Lomakina & Mikova, 2006).
Ultrasound-treated duck egg white for 40 minutes had the highest foaming capacity (94.50%) but the lowest foaming stability (38.21%). Zhou et al. (2015) reported that foaming capacity has an opposite trend to foaming stability due to the prolong ultrasound treatment that caused a greater damage on foam stability of egg white protein. As higher foam volume is produced during whipping, more foam will collapse due to drainage and thinning of foam film after leaving the foam for a certain period (Chang, 2016). Yu et al. (2020) reported that ultrasound-irradiation combined pretreatment increased the solubility and reduced the pH and particle size of ovalbumin and lysozyme, thus enhancing the foamability of egg white. Based on the result of emulsion activity of egg white in Table 1, emulsion activity increased as the treatment time increased. Emulsion activity of egg white protein gradually increased with the time of ultrasound treatment (Stefanovic et al., 2017). Ai et al. (2021) also reported the ability of high-intensity ultrasound together with heat treatment to improve the interfacial properties of egg white peptides and to enhance the stability of prepared emulsions. The improvement of emulsifying properties that are related to conformational changes during treatment, results in an improved interfacial adsorption of protein molecules onto oil interface. Partial unfolding of protein after ultrasound treatment leads to the exposure of hydrophobic amino acid residue and enhances protein adsorption at oil-water interface for emulsifying (Zhou et al., 2014).
Expressible Moisture
Table 1 shows the expressible moisture content of egg white gels from egg white treated with ultrasound. Expressible moisture of ultrasound-treated egg white gel for 40 minutes is the lowest (17.65%) as compared to other gels. Low expressible moisture content indicates that more water are retained in gel network due to the high water holding capacity (WHC) of gel (Hamzah et al., 2015). In the research of Huda et al. (2013b), the amount of entrapped water in protein gel decreased as the expressible moisture increased. Gels with a higher expressible moisture content was probably due to the denaturation of protein during treatment that causes formation of weak gel network with lower gel strength. Gel matrices fail to imbibe water and more water are being released (Panpipata & Chaijan, 2017).
Folding Test
Folding test is a simple method to determine the gel quality and a high test score indicates a good quality gel (Huda et al., 2013a). A significant difference (p<0.05) was found between untreated and ultrasound-treated egg white based on the result in Table 1. Gels from egg white treated for 0, 10 and 20 minutes cracked immediately when folded in half (score 2). When a little folding pressure was applied, the gels cracked immediately even though they were not completely folded in half. The increase of folding test in sample treated for 30, 40 ad 50 minutes (score 3) related with the increase number of crosslink sites. Jing et al. (2020) reported that number of cross-linking sites in egg white protein was increased significantly after ultrasound-assisted treatments 40kHz. These gels also had lower hardness and higher springiness as shown in Table 4. Gel samples with a higher hardness caused a higher fracturability (Mochizuki, 2001). Folding test is a subjective test and cannot represent the overall result of texture profile because this test is less sensitive to distinguish the functional properties of gel (Nik Aisyah et al., 2017).
Colour
The parameters of colour measured are lightness (L*), redness (a*) and yellowness (b*). The results of ultrasound-treated egg white and their gels are shown in Table 2. A significant difference (p<0.05) was found between parameters of untreated and ultrasound-treated egg white and their gels. Untreated egg white showed the highest values whereas egg white treated for 50 minutes had the lowest values. According to Zhou et al. (2015), the colour parameters of egg white and gels changed after ultrasound treatment because of the increment in exposure of protein surface hydrophobicity and reduction of sulfhydryl group content, that increases exposed area of riboflavin molecule embedded inside the macromolecular proteins. Riboflavin is a component that gives colour of egg white. Reduction of riboflavin has resulted in the decreasing of egg white colour.
Tube Inverting Test
According to Table 3, ultrasound treatment did not cause negative effect to duck egg white. There was no gel formation at concentration less than 40% of untreated or ultrasound-treated egg white and they present in a liquid state. However, when the egg white concentration was more than 40%, gel formation can be observed. Gelling properties of egg white are influenced by protein concentration. Protein interaction occurs within molecules instead of between molecules, and gel network fails to form at low protein concentration. High concentration of protein enhances intermolecular interaction, and a firmer gel will be formed when more water bind tightly to protein molecules (Zayas, 2012; Kuan et al., 2017). For gel formation at high protein concentration, denatured protein will form crosslinked soluble linear aggregates and three-dimensional gel structure. However, at low concentration, gel network cannot be developed but a viscous transparent sol is formed (Mine & Yang, 2010).
Texture Profile Analysis
Table 4 shows that textural properties of egg white at different treatment time were significantly different (p<0.05) among some of the egg white gels. Based on the result, when ultrasound treatment time increased, gel hardness, springiness, gumminess and chewiness decreased. Gel from egg white that was ultrasound-treated for 40 minutes had the lowest gel hardness (1534.67g) whereas untreated egg white gel had the highest hardness (1853.17g). Gel hardness is influenced by covalent crosslinking of egg white protein. Gel with higher degree of hardness was developed when numerous covalent bonds were formed in aggregation of protein molecules (Chen et al., 2015). Ultrasound treatment has caused partial denaturation of protein which decreased the number of crosslinking between protein molecules and weakened hydrogen bonds that resulted in a lower gel hardness (Zhang et al., 2011).
Springiness measures the amount of gel structure broken down by the first compression (Lau et al., 2000). Egg white gel that had undergone 40 minutes of treatment showed a significantly (p<0.05) lower gel springiness (5.36 mm) than other gels. Untreated egg white has the highest gel springiness (6.20 mm) as compared to ultrasound-treated egg white. As the treatment time increased, gel springiness decreased. Chen et al. (2015) reported that gel springiness is affected by pH of egg white. Increasing egg white pH causes a decrease in gel springiness due to the extensive formation by electrostatic repulsive forces of internal bonding of gel network structure.
Cohesiveness measures the strength of endurance of gel at second deformation or difficulty in breaking internal structure of gel (Lau et al., 2000; Kaewmanee et al., 2011). No significant difference (p>0.05) was observed in gel cohesiveness among egg white at different treatment time. Based on the result, ultrasound treatment increased the gel cohesiveness. Egg white that had undergone 60 minutes of treatment had the highest value of gel cohesiveness whereas egg white with 50 of minutes treatment had the lowest cohesiveness. High cohesiveness value indicates the strength of gel network structure, whereas low cohesiveness indicates the risk of gel to collapse (Chen et al., 2015).
Gumminess is an attribute of semisolid food that has low degree of hardness and high degree of cohesiveness. Chewiness measures the energy to masticate food (Chandra & Shamasundur, 2015). No significant difference (p>0.05) was found in gel cohesiveness among egg white at different treatment time. Gel gumminess and hardness are proportional. Gumminess is an important attribute for semisolid food (Rahman & Al-Mahrouqi, 2009). Gel chewiness from egg white treated for 40 and 50 minutes were significantly different (p<0.05) to untreated egg white. Ultrasound treatment had reduced the gel gumminess and chewiness of egg white.
CONCLUSION
The qualities of duck egg white are influenced by the duration of ultrasound treatment. According to the results obtained in this research, pH values, foaming capacity, and emulsion activity of duck egg white increased as time taken for ultrasound treatment increased to 60 minutes. However, the texture profile of duck egg white gel decreased after longer ultrasound treatment. Hence, it was suggested to subject the egg white samples to ultrasound treatment for 40 minutes in order to produce duck egg white with better functional properties in term of foaming capacity, emulsion activity, expressible moisture and folding test.
ACKNOWLEDGEMENTS
The authors acknowledge with gratitude the support provided by Universiti Malaysia Sabah.
conflict of interest
The authors declare no conflict of interest.
authors contribution
Ho Shi Hui: Involved in the data collection, data analysis, and drafted the manuscript. Eng-Keng Seow: Involved in the writing review and editing the manuscript. Nurul Huda: Involved in the conceptualization of idea, supervision, funding, writing review and editing the manuscript.
REFERENCES
Abeyrathne EDNS, Lee HY, Ahn DU (2013). Egg white proteins and their potential use in food processing or as nutraceutical and pharmaceutical agents – a review. Poult. Sci. 92(12): 3292-3299. https://doi.org/10.3382/ps.2013-03391
Ai M, Xiao N, Jiang A (2021). Molecular structural modification of duck egg white protein conjugates with monosaccharides for improving emulsifying capacity. Food Hydrocoll. 111:106271. https://doi.org/10.1016/j.foodhyd.2020.106271
Ai M, Zhang Z, Fan H, Cao Y, Jiang A (2021). High-intensity ultrasound together with heat treatment improves the oil-in-water emulsion stability of egg white protein peptides. Food Hydrocoll. 111: 106256 https://doi.org/10.1016/j.foodhyd.2020.106256
Ai M, Zhou Q, Xiao N, Guo S, Cao Y, Fan H, Ling Z, Zhou L, Li S, Long J, Jiang A (2020). Enhancement of gel characteristics of NaOH-induced duck egg white gel by adding Ca(OH)2 with/ without heating. Food Hydrocoll. 105654. https://doi.org/10.1016/j.foodhyd.2020.105654
Aronal AP, Huda N, Ahmad R (2012). Amino acid and fatty acid profiles of Peking and Muscovy duck meat. Int. J. Poult. Sci. 11(3):229-236. https://doi.org/10.3923/ijps.2012.229.236
Arthur J (2017). Chapter 3: Duck Eggs. In Hester, P.Y. (eds). Egg Innovations and Strategies for Improvements. London: Elsevier Inc. pp 23-32. https://doi.org/10.1016/B978-0-12-800879-9.00003-2
Arzeni C, Perez OE, Pilosof AMR (2012). Functionality of egg white proteins as affected by high-intensity ultrasound. Food Hydrocoll. 29(2): 308-316. https://doi.org/10.1016/j.foodhyd.2012.03.009
Banerjee P, Keener KM, Lukito VD (2011). Influence of carbon dioxide on the activity of chicken egg white lysozyme. Poult. Sci. 90(4): 889-895. https://doi.org/10.3382/ps.2010-00854
Bhattacherjee A, Acharya CP, Rana N, Mallik BK, Mohanty PK (2018). Haematological and morphometrical analysis of blood cells of Khaki Campbell duck (Anas platyrhynchos) in different age groups with respect to sexual dimorphism. Comp. Clin. Path. 27(6): 1465-1472. https://doi.org/10.1007/s00580-018-2758-6
Chandra MV, Shamasundar BA (2015). Texture profile analysis and functional properties of gelatin from the skin of three species of fresh water fish. Int. J. Food Proteins 18(3): 572-584. https://doi.org/10.1080/10942912.2013.845787
Chang Q (2016). Colloid and Interface Chemistry for Water Quality Control. London: Academic Press. https://doi.org/10.1016/B978-0-12-809315-3.00002-5
Chen Z, Li J, Tu Y, Zhao Y, Luo X, Wang J, Wang M (2015). Changes in gel characteristics of egg white under strong alkali treatment. Food Hydrocoll. 45: 1-8. https://doi.org/10.1016/j.foodhyd.2014.10.026
Eksin DG (2015). Ultrasonic degassing of liquids. In Juarez, J.A.G. and Graff, K.F. (eds). Power Ultrasonics: Applications of High-Intensity Ultrasound. United Kingdom: Woodhead Publishing. pp 611-631. https://doi.org/10.1016/B978-1-78242-028-6.00020-X
Gharbi N, Labbafi M (2018). Effect of processing on aggregation mechanism of egg white proteins. Food Chem. 252: 126-133. https://doi.org/10.1016/j.foodchem.2018.01.088
Hamzah N, Sarbon NM, Amin AM (2015). Physical properties of cobia (Rachycentron canadum) surimi: Effect of washing cycle at different salt concentrations. J. Food Sci. Technol. 52(8): 4773-4784. https://doi.org/10.1007/s13197-014-1622-1
Huang JF, Lin CC (2011). Chapter 21: Production, composition and quality of duck eggs. In Nys, Y., Bain, M. and Immerseel, F.V. (eds). Improving the safety and quality of eggs and egg products: Volume 1 Egg chemistry, production and consumption. First Edition. United Kingdom, Cambridge: Woodhead Publishing Limited. pp 487-504. https://doi.org/10.1533/9780857093912.4.487
Huda N, Seow EK, Normawati MN, Nik Aisyah NM, Fazilah A, Easa AM (2013b). Preliminary Study on Physicochemical Properties of Duck Feet Collagen. Int. J. Poult. Sci. 12(10):615-621. https://doi.org/10.3923/ijps.2013.615.621
Ismail I, Huda N, Ariffin F, Ismail N (2010). Effects of washing on the functional properties of duck meat. Int. J. Poult. Sci. 9 (6): 556-561. https://doi.org/10.3923/ijps.2010.556.561
Jambrak AR, Mason TJ, Lelas V, Herceg Z, Herceg IL (2008). Effect of ultrasound treatment on solubility and foaming properties of whey protein suspensions. J. Food Eng. 86(2): 281-287. https://doi.org/10.1016/j.jfoodeng.2007.10.004
Jing H, Sun J, Mu Y, Obadi M, McClements DJ, Xu B (2020). Sonochemical effects on the structure and antioxidant activity of egg white protein-tea polyphenol conjugates. Food Func. 11(8):7084 – 7094. https://doi.org/10.1039/D0FO01636E
Kaewmanee T, Benjakul S, Visessanguan W (2011). Effects of salting processes and time on the chemical composition, textural properties and microstructure of cooked duck eggs. J. Food Sci. 76(2): 139-147. https://doi.org/10.1111/j.1750-3841.2010.01975.x
Kuan, YH, Nafchi AM, Huda N, Ariffin F, Karim AA (2017) Comparison of physicochemical and functional properties of duck feet and bovine gelatins. J. Sci. Food Agric. 97(5):1663-1671. https://doi.org/10.1002/jsfa.7970
Lau MH, Tang J, Paulson AT (2000). Texture profile and turbidity of gellan/gelatin mixed gels. Food Res. Int. 33(8): 665-671. https://doi.org/10.1016/S0963-9969(00)00111-3
Lim JW, Abbas FM, Huda N (2019). Physicochemical properties of egg yolk powder from eggs of different types of bird. Int. J. Adv. Sci. Eng. Inf. Technol. 9(1): 373-378. https://doi.org/10.18517/ijaseit.9.1.3046
Lomakina K, Mikova K (2006). A study of the factors affecting the foaming properties of egg white – a review. Czech J. Food Sci. 24(3): 110-118. https://doi.org/10.17221/3305-CJFS
Martins AJ, Silva P, Maciel P, Pastrana LM, Cunha RL, Cerqueira MA, Vicente AA (2019). Hybrid gels: Influence of oleogel/ hydrogel ratio on rheological and textural properties. Food Res. Int. 116: 1298-1305. https://doi.org/10.1016/j.foodres.2018.10.019
Mine Y, Yang M (2010). Chapter 29: Functional properties of egg components in food systems. In Legarreta, I.G. and Hui, Y.H. (eds). Handbook of Poultry Science and Technology. Volume 1: Primary Processing. New Jersey: John Wiley and Sons. pp 579-630. https://doi.org/10.1002/9780470504451.ch29
Mochizuki Y (2001). Texture profile analysis. Current Protocols in Food Analytical Chemistry, 00(1): H.2.3.1-H2.3.7. https://doi.org/10.1002/0471142913.fah0203s00
Niehoff A, Mantion A, McAloney R, Huber A, Falkenhagen J, Goh CM, Thunemann AF, Winnik MA, Menzel H (2013). Elucidation of structure of poly(γ-benzyl-L-glutamate) nanofibers and gel networks in a helicogenic solvent. Colloid Polym. Sci. 291(6): 1353-1363. https://doi.org/10.1007/s00396-012-2866-9
Nurkhoeriyati T, Huda N, Ahmad R (2011). Gelation properties of spent-duck meat surimi-like material produced using acid-alkaline solubilization methods. J. Food Sci. 76 (1): S48-S55. https://doi.org/10.1111/j.1750-3841.2010.01963.x
Panpipat W, Chaijan M (2017). Functional properties of pH-shifted protein isolates from bigeye snapper (Priacanthus tayenus) head by-product. Int. J. Food Prop. 20(3): 596-610. https://doi.org/10.1080/10942912.2016.1171778
Quan TH, Benjakul S (2018a). Quality, protease inhibitor and gelling properties of duck egg albumen as affected by storage conditions. J. Food Sci. Technol. 55(2): 513-522. https://doi.org/10.1007/s13197-017-2960-6
Quan TH, Benjakul S (2018b). Gelling properties of duck albumen powder as affected by desugarization and drying conditions. J. Texture Stud. 49(5): 520-527. https://doi.org/10.1111/jtxs.12339
Rahman MS, Al-Mahrouqi AI (2009). Instrumental texture profile analysis of gelatin gel extracted from grouper skin and commercial (bovine and porcine) gelatin gels. Int. J. Food Sci. Nutr. 60(S7): 229-242. https://doi.org/10.1080/09637480902984414
Sert D, Aygun A, Demir MK (2011). Effects of ultrasonic treatment and storage temperature on egg quality. Poult. Sci. 90(2): 869-875. https://doi.org/10.3382/ps.2010-00799
Stefanovic AB, Jonanovic JR, Dojcinovic MB, Levic SM, Nedovic VA, Bugarski BM, Knezevic-Jugovic ZD (2017). Effect of controlled high-intensity ultrasound on improving functionality and structural changes of egg white proteins. Food Bioprocess Technol. 10(7): 1224-1239. https://doi.org/10.1007/s11947-017-1884-5
Stefanovic AB, Jonanovic JR, Balanc BD, Sekuljica NZ, Tanaskovic SMJ, Dojcinovic MB, Knezevic-Jugovic ZD (2018). Influence of ultrasound probe treatment time and protease type on functional and physicochemical characteristics of egg white protein hydrolysates. Poult. Sci. 97(6): 2218-2229. https://doi.org/10.3382/ps/pey055
Yu Y, Zhang H, Zhu J, Liu J, Zhang T (2020). Effect of ultrasound-irradiation combined pretreatment on the foamability of liquid egg white. J Food Sci. 85(12):4312 – 4318. https://doi.org/10.1111/1750-3841.15530
Wang Y, Zhang M, Adhikari B, Mujumdar AS, Zhou B (2013). The application of ultrasound pretreatment and pulse-spouted bed microwave freeze drying to produce desalted duck egg white powders. Dry. Technol. 31(15): 1826-1836. https://doi.org/10.1080/07373937.2013.829851
Wong KS, Huda N, Dewi M, Hashim H (2018). Physicochemical properties of egg white powder from eggs of different types of bird. Int. J. Adv. Sci. Eng. Inf. Technol. 8(2): 384-389. https://doi.org/10.18517/ijaseit.8.2.4087
Zaheer K (2015). An updated review on chicken eggs: production, consumption, management aspects and nutritional benefits to human health. Food Nutr. Sci. 6(13): 1208-1220. https://doi.org/10.4236/fns.2015.613127
Zayas JF (2012). Functionality of Proteins in Food. New York: Springer.
Zhang H, Claver IP, Zhu KX, Zhou H (2011). The effect of ultrasound on the functional properties of wheat gluten. Molecules 16(5): 4231-4240. https://doi.org/10.3390/molecules16054231
Zhou B, Zhang M, Fang Z, Liu Y (2014). A combination of freeze drying and microwave vacuum drying of duck egg white protein powders. Dry. Technol. 32(15): 1840-1847. https://doi.org/10.1080/07373937.2014.952380
Zhou B, Zhang M, Fang Z, Liu Y (2015). Effects of ultrasound and microwave pretreatments on the ultrafiltration desalination of salted duck egg white protein. Food Bioprod. Process. 96: 306-313. https://doi.org/10.1016/j.fbp.2015.09.004
To share on other social networks, click on any share button. What are these?






