Physical Properties Evaluation of Chicken Eggs on Soaking in Sour Starfruit Extract (Averrhoa bilimbi L.)
Research Article
Physical Properties Evaluation of Chicken Eggs on Soaking in Sour Starfruit Extract (Averrhoa bilimbi L.)
Djoko Kisworo*, Martina Safitri, BRD Wulandani, Bulkaini, Syamsuhaidi
Faculty of Animal Science University of Mataram, Majapahit Street, number 62, Gomong, Mataram, Lombok, 83125, Tel: (0370). 633007, West Nusa Tenggara, Indonesia.
Abstract | The decrease in egg quality is mainly due to bacteria, evaporation of the egg contents, indicated by egg weight loss, Haugh Unit (HU), air cavity, and an increase in pH. This research aims to determine the best level of sour starfruit (SSF) extract on the shelf life of chicken eggs. A Completely randomized design (CRD) was used in a 3 x 3 factorial pattern with five replications. The Factors were the level of SSF (0%, 10%, and 20%) and storage time (2, 4, and 6 weeks). Data were analyzed according to the analysis of variance followed by the Least Significant Difference test (LSD). The parameters measured were the egg’s weight, air cavity, pH, and Haugh Unit of chicken eggs that were stored at room temperature for six weeks. The results showed that immersion in SSF and storage decreased (P<0.5) the HU and egg’s weight. While the air cavity and pH increased significantly (P<0.05). The treatment of 0% SSF, 10% SSF and 20% SSF decreased HU values (66.56 ± 0.84 - 58.22 ± 2.83), (63.33 ± 7.43 - 64.94 ± 3.49), (72.07 ± 2.10 - 68.64 ± 3.32) during storage. Likewise, the rate of egg weight loss increased successively (2.20 ± 0.11 - 5.91 ± 0.18), (1.18 ± 0.15 - 5.63 ± 0.47), (1.10 ± 0.03 - 3.80 ± 0.41) during storage. While the air cavity increased during storage successively (21.12 ± 0.23 - 26.12 ± 0.36), (19.08 ± 0.05 - 25.66 ± 0.32), (19.74 ± 0.24 - 24.00 ± 0.60). During storage, the pH was relatively constant. The study concluded that a 20% SSF and two weeks of storage was the best treatment to maintain the quality of chicken eggs based on the egg weight, air cavity, pH, and HU, and can extend the shelf life up to six weeks. The interaction between the level of 20% SSF and the Storage Time (ST) factor for two weeks was the best treatment based on the egg weight and pH.
Keywords | Chicken egg, Physical Properties, Sour Star-fruit, Storage time.
Received | July 10, 2022; Accepted | August 15, 2022; Published | September 01, 2022
*Correspondence | Djoko Kisworo, Faculty of Animal Science University of Mataram, Majapahit Street, number 62, Gomong, Mataram, Lombok, 83125, Tel: (0370). 633007, West Nusa Tenggara, Indonesia; Email: djokokisworo@unram.ac.id
Citation | Kisworo D, Safitri M, Wulandani BRD, Bulkaini, Syamsuhaidi (2022). Physical properties evaluation of chicken eggs on soaking in sour starfruit extract (Averrhoa bilimbi L.). Adv. Anim. Vet. Sci. 10(9): 2051-2058.
DOI | http://dx.doi.org/10.17582/journal.aavs/2022/10.9.2051.2058
ISSN (Online) | 2307-8316

Copyright: 2022 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
INTRODUCTION
Raised chicken eggs are livestock products with nutrition content that the body need (Lestari, 2019). The yolk is the highest nutrition content of purebred chicken eggs. Moreover, the egg yolks contain essential amino acids and several minerals such as iron, phosphorus, calcium, and vitamin B complex (Mirnawati, 2018). The high nutrition content of broiler eggs not only has a very positive effect on the growth of children under five and increases the immune system of adults, but also hurts the length of shelf life of eggs (Ariyana et al., 2020). High nutrient content in eggs is a food ingredient that is very easily contaminated with microbes both directly and indirectly so that it can cause various kinds of damage and become one of the causes of the short shelf life of broiler eggs (Ariyana et al., 2020).
The shelf life of broiler eggs is affected by several factors, namely: the physical quality of fresh eggs both internally and internally (Thohari, 2018), handling methods (Saud, 2014), shell thickness, and storage temperature (USDA, 2000). Storage of eggs carried out in the open air (room temperature) will cause the quality and freshness of eggs to decrease (Syaifulloh et al., 2021; Cornelia et al., 2014; Fibrianti et al., 2012). The structure of the egg shell significantly affects the evaporation of water content through the pores of the eggshell, changes in the chemical composition of the egg, and the dilution of the egg contents (Djaelani, 2016).
Evaluation of the physical properties of the eggs seen from the size of the air cavity in the egg (Geveke et al., 2016); pH of egg whites and yolks; egg index, egg white, and yolk index (Wibawanti et al., 2017); and the Haugh Unit (HU) (Menezes et al., 2012). The formation of air cavities in eggs is affected by the difference in room temperature with the parent’s body temperature after being removed from the mother (Jazil, 2012). Fresh eggs have a pH of around 7 (Soekarto, 2013) and tends to increase to 9.0 - 9.7 after being store.
Chicken eggs circulating in traditional markets such as in the Bandar traditional market have a pH of around 7.59; The Manisrenggo market has a pH of 7.68 and the Setono Batik market has a pH of 8.02 (Afiyah and Rahmawati, 2017). Local chicken eggs stored at a temperature of 15 oC – 18 oC have a HU value of 72.80 - 89.96 (Lengkey et al., 2012), while those stored at room temperature around 30 oC – 32 oC have a HU value of 64.43 - 70.17 with an egg index value of 0.76 - 0.78 which was smaller than the ideal egg index of 0.80 (Afiyah and Rahmawati, 2017). Eggs stored at room temperature and circulated in traditional markets have an egg’s white index of 0.09 - 0.10 while an egg’s yolk index of 0.26 - 0.38. Eggs that are normal and fit for consumption have an egg’s white index of 0.050 - 0.134 with the egg’s yolk index of 0.330 - 0.458 (BSN, 2008).
Strategies to extend the egg shelf life can be done by soaking in various solutions. For example, in lime solution (Djaelani, 2016), immersion in lime water, and boiling water (Koswara, 2009), soaking using herbal ingredients such as guava leaf solution (Ernawati et al., 2019), and mangosteen rind extract (Tindjabate et al., 2014). Another herbal solution that has high potential as an ingredient for egg preservation is sour starfruit (SSF) extract because SSF contains 5.07 % tannins (Utami et al., 2020). The tannins contained in the SSF can cover the pores of the egg’s shell causes CO2 gas to inhibited, and microorganisms cannot enter the egg. The reaction between the protein in the egg shell and tannins form an impermeable layer to gases (Lestari et al., 2013). Other phytochemical substances contained in SSF are flavonoids, oxalate, phenol, and pectin that are function as anti-microbial substances (Mukhlisoh, 2010). In optimizing the use of SSF in the egg preservation process, a study was conducted to evaluate the physical properties of broiler eggs by immersion in SSF solution with different storage times.
MATERIALS AND METHODS
Research Material
The materials used in the study consisted of: (1) Two hundred and twenty-five fresh chicken eggs with a weight range of 50-55 g obtained from UD farms Utama Adji East Lombok; (2) sufficient H2O; (3) sufficient CaCO3; and (4) SSF that is not yet ripe. The types of equipment used include digital scales, pH meters, calipers, depth micrometers, glass, egg trays, and thermohydrometers.
Research Methods
Sour Starfruit Extract Preparation: The SSF was washed using running water, then sliced thinly and dried in the sun for about 16 hours. The dried SSF was boiled for 15 minutes at 80 oC. The boiling results were cooled and squeezed and filtered to take SSF. The SSF juice added with lime solution aims to reduce the acidity of the fruit juice so that it does not cause damage to the egg shell. The extract was used to soak the broiler eggs for 24 hours. After completion of the soaking period, the eggs were removed and placed in the egg rack systematically according to the treatment and stored at room temperature. The room temperature was monitored by measuring the temperature and humidity of the room using a thermohydrometer. Evaluation of the physical properties of eggs was caried out after storage for two, four, and six weeks, flow chart for the manufacture of SSF extract is presented in Figure 1.
Experimental Research Design
Physical properties evaluation of broiler eggs was carried out using a completely randomized design with a 3 x 3 factorial pattern (Steel and Torrie (2015). The first factor was the level of use of SSF extract (0%, 10%, and 20%), and the second factor was the storage time (ST) at room temperature (two weeks, four weeks, and six weeks) so that there were nine treatment combinations occurred. Each treatment combination consisted of five repetitions, and five eggs were used in each, so that 225 eggs was used. The treatments combination in the study is as follows:
SSF1-ST1 = Without SSF extract with two weeks ST,
SSF1-S21 = Without SSF extract with four weeks ST,
SSF1-ST3 = Without SSF extract with six weeks ST,
SSF2-ST1 = 10% of SSF extract with two weeks ST,
SSF2-ST2 = 10% of SSF extract with four weeks ST,
SSF2-ST3 = 10% of SSF extract with six weeks ST,
SSF3-ST1 = 20% of SSF extract with two weeks ST,
SSF3-ST2 = 20% of SSF extract with four weeks ST,
SSF3-ST3 = 20% of SSF extract with six weeks ST,
Measurement Of Research Variables
Egg Weight: The percentage of egg weight loss was calculate using the formula (Saud, 2014):
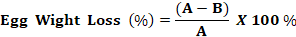
Where:
A: Egg weight before storage
B: Egg weight after storage
Egg Air Cavity Diameter: The measurement of the diameter of the broiler egg air cavity carried out as follows: (1) Fraction of the blunt part of the egg, namely the part that contains air cavities; (2) Measuring the vertical width of the air cavity; (3) Measuring the width of the air cavity; and (4) Determining the value of the egg air cavity using the formula (Djaelani, 2016):
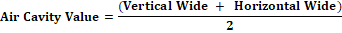
Mechanism of measuring the diameter of the air cavity of the eggs as shown in Figure 2.
Haugh Unit: The Haugh unit value was calculate using the formula:
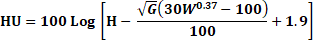
Where:
H = albumen height (mm)
G = conversion factor = 32.2 (only G is rooted)
W = egg weight (g)
Degree of acidity (pH) Chicken Eggs: The pH measurement of broiler eggs was carried out by shaking until they were evenly mixed, then measuring the pH of the broiler eggs using a pH meter.
Statistical Analysis
Data were analyzed according to the analysis of variance followed by the Least Significant Difference test (LSD). Furthermore, the treatment that gave a significant difference was following the Least Significant Difference (LSD) follow-up test using the SAS software, Steel and Torrie (2015).
RESULTS AND DISCUSSION
The decrease in egg quality is mainly due to evaporation of the egg contents, indicated by egg weight loss, HU, air cavity, and an increase in pH. The decrease in egg quality can be reduced using vegetable tanners such as tannins. Storage of broiler eggs by immersion in SSF extract at an average room temperature of 28.35 oC, with an average air humidity of 75 %, data on egg weight loss, pH, HU, and air cavity, is presented in Table 1.
Percentage Of Egg Weight Loss
The average weight loss of broiler eggs during storage at room temperature increased successively at 0% SSF, 10% SSF, and 20% SSF immersion (Figure 3). The immersion in SSF solution can inhibit egg weight loss during storage. Egg weight loss was slow at the beginning of storage. However, the relatively high temperature of the storage space causes the evaporation of water and CO2 gas to
Table 1: Data on egg weight loss, pH value, HU, and air cavity, as affected by sour starfruit and storage time.
| Sour Starfruit (SSF) | Storage Time (ST) | ||
| 2 weeks | 4 weeks | 6 weeks | |
| Egg weight loss (%): | |||
| SSF1 | 2.20 ± 0.11 | 4.67 ± 0.67 | 5.91 ± 0.18 |
| SSF2 |
1.18 ± 0.15 |
3.88 ± 0.68 | 5.63 ± 0.47 |
| SSF3 | 1.10 ± 0.03 | 2.52 ± 0.24 | 3.80 ± 0.41 |
| pH: | |||
| SSF1 | 7.03 ± 0.03 | 7.07 ± 0.08 | 7.09 ± 0.01 |
| SSF2 | 6.92 ± 0.02 | 6.98 ± 0.02 | 6.96 ± 0.02 |
| SSF3 | 6.80 ± 0.08 |
6.92 ± 0.05 |
6.94 ± 0.02 |
| Haugh Unit: | |||
| SSF1 | 66.56 ± 0.84 | 53.86 ± 0.57 | 58.22 ± 2.83 |
| SSF2 | 63.33 ± 7.43 |
73.13 ± 3.54 |
64.94 ± 3.49 |
| SSF3 | 72.07 ± 2.10 | 67.29 ± 3.50 | 68.64 ± 3.32 |
| Air Cavity (mm): | |||
| SSF1 | 21.12 ± 0.23 | 23.83 ± 0.34 | 26.12 ± 0.36 |
| SSF2 | 19.08 ± 0.05 | 22.59 ± 0.83 | 25.66 ± 0.32 |
| SSF3 | 19.74 ± 0.24 | 21.77 ± 0.20 | 24.00 ± 0.60 |
occur morequickly. The longer the ST, the supply of liquids and gas will shrink or decrease (Joubrane et al., 2019). Applying 20% SSF extract storage at room temperature for six weeks was the best treatment. Tannin contained in SSF extract can cover the pores of the egg’s shell. Therefore, the evaporation of water, carbon dioxide gas, ammonia, and nitrogen were little. Mukhilsah et al. (2020) stated that increasing levels of melinjo leaf extract can maintain the quality of duck eggs, while storage time decreases the quality of duck eggs. The principle of using tanners is the occurrence of a reaction on the egg shell by tanning substances (tannins) to prevent the release of water and gas from the egg. Meanwhile, eggs as control threatment experienced more evaporation of water and carbon dioxide, ammonia, and nitrogen gases, resulting in a decrease in egg weight. Mukhilsah et al. (2021) said that egg weight continues to decrease during storage caused to evaporation of water and carbon dioxide gas (CO2).
Based on the description above and the LSD test, it was known that the use of a 20% level of SSF extract and an ST of 2 weeks and the interaction between a 20% level of SSF and an ST of 2 weeks (SSF3-ST2) was the best treatment to inhibit egg weight loss. In line with Novika et al. (2017) eggs stored in bay leaf extract for seven days at 4 ºC have weight loss of 0.35 g and 0.68 g at 20 ºC. The longer eggs stored, the bigger it gets weight loss. Eggs stored for ten days at 4 ºC have a weight loss of 0.43 g and increased storage fourteen days to 0.56 g.
Egg Air Cavity
The air cavity in the egg is formed momentarily after laying due to the temperature difference lower than the parent body temperature the contents of the eggs become colder and shrinks to separate the membrane’s inner and outer shells separate. This membrane usually occurs in the egg blunt. The longer the storage time, the greater the depth of the air cavity. It is caused by the loss of egg weight caused by the evaporation of water and the release of gases that occur during storage. During storage, the eggs will lose their fluid, and contents shrink so that it enlarges air cavities (Jazil, 2013).
Table 1 shows that based on the LSD test, it is known that the use of a 20% level of SSF with an ST of 2 weeks and the interaction between a 10% level of SSF and an ST of 2 weeks is the best treatment to inhibit the enlargement of the air cavity diameter of the egg (egg preservation). It was in line with the decrease in egg weight. The highest air cavity diameter of broiler chicken eggs (26.12 mm) was at a level of 0% SSF for six weeks of storage at room temperature. The small diameter of the air cavity of the eggs stored at room temperature with the level of 10% and 20% SSF was caused by the pores of the eggs being covered by tannins and inhibiting the evaporation of water, carbon dioxide, ammonia, and nitrogen gases of the eggs. On the other hand, the large diameter of the air cavity of eggs that was not preserved with SSF (level 0%) was caused by a large amount of evaporation of water and carbon dioxide, ammonia, and nitrogen gases (Figure 4). Gary et al. (2009) stated that the large diameter of the cavity is caused by the inner membrane of the egg being detaches so that it attaches to the albumen due to evaporation of water in the egg. The same thing was also stated by Pescatore and Jacob (2011) that as the age of the egg increases, the egg experience fluid loss so that it enlarges the air cavity. Similarly, Yuwanta (2010) stated that the enlargement of air pockets in eggs is influenced by storage temperature, humidity, and changes in egg contents. It’s in line with the decrease in egg weight during storage.
EGG pH
The average pH of broiler eggs stored at room temperature for six weeks was the highest (7.06) in the uncured treatment with SSF (0% level) and the lowest (6.88) in the treatment level 20% SSF. The low pH of eggs stored at room temperature for six weeks preserved at a level of 20% SSF was caused by the tannins in the SSF juice closing the pores of the egg shell so that CO2 gas evaporation was low. On the other hand, the high pH of eggs stored at room temperature for six weeks that were not preserved with SSF juice at 0% level was caused by the evaporation of CO2 gas. Novika et al. (2017) stated that the loss of CO2 gas in eggs causes the concentration of bicarbonate ions to decrease and the buffer system to be damaged, increasing pH. Saud (2014) explained that albumen dilution was caused by the breakdown of ovomucin fibers that bind egg whites, increasing egg white pH.
Table 1 shows that based on the LSD test, the use of 20% SSF level with two weeks ST was the best treatment to inhibit the increase in pH. It can shorten the shelf life of eggs. It is in line with the decrease in egg weight and air cavity diameter of broiler eggs stored at room temperature for six weeks. Moreover, Syaifulloh et al. (2021); Cornelia et al. (2014); and Fibrianti et al. (2012) stated that storage of eggs carried out at room temperature will cause the quality and freshness of eggs to decrease. In general, preserving SSF can reduce increasing the pH during the storage period. Figure 5 shows that treating an egg with SSF can increase the pH gradually during the storage period compared to the egg without SSF.
Haugh Unit Eggs
The Haugh Unit value is a value that describes the state of the egg white to determine egg quality. Eggs of good quality usually have high HU values, Hajrawati et al., (2012). According to Kurtini et al. (2014), high HU eggs reflect the condition of fresh eggs. HU values more than 72 are categorized as AA quality eggs, HU values 60-72 as A quality eggs, HU values 31-60 as B quality eggs, and HU values less than 31 are categorized as C quality eggs (USDA 2007).
The average HU of broiler eggs stored at room temperature for six weeks was the highest (69.22) in the treatment preserved with SSF at a 20% level and the lowest (59.59) at the level of 0 % SSF. Analysis of variance showed that the level of SSF juice had a very significant effect (P<0.01) on the HU of eggs stored at room temperature for six weeks. ST and SSF had a significant interaction effect (P<0.05) certainly for eggs stored for six weeks (Figure 6). The average difference test between treatments using the LSD test showed that the HU of eggs stored at room temperature for six weeks in the treatment with SSF juice at levels of 0%, 10%, and 20% were very significantly different, respectively (P< 0.01).
Table 1 shows that the higher the concentration level, the longer the durability. It was Saud’s statement (2014) that
the higher the concentration level of a solution, the higher the quantity of SSF solute, and the longest the freshness of the eggs. It produced at 20% immersion, which was six weeks of storage. The soluble substances contained in the extract of SSF used as a marinade for consumption of chicken eggs are tannin and polyphenolic compounds, namely chavicol and capitol. The HU value represents the egg weight per egg in grams and albumen height in millimeters. Evaporation CO2, NH3, and H2S, as well as the microbial activity in eggs during storage, will decrease the HU value of eggs. In addition, storage longer than two weeks will reduce the HU value of eggs. Ismawati (2011) states that the period eggs in storage cause the eggs to experience liquid evaporation and the release of gases such as carbon dioxide from the egg’s contents, which results in the expansion of the egg’s white surface. In addition, the longer the egg storage period, the more microbes that enter the shell pores can damage the eggs.
The treatment of SSF 10% level with an ST of 2 weeks was not affected significant (P>0.05) compared to the treatment level of 0%, an ST of 2 weeks, the level of 20%, an ST of 4 weeks and a treatment level of 10%, ST of six weeks respectively. However, there was significant effect (P<0.05) compared to treatment levels of 20% with 2 and 6 weeks of ST, 10% SSF with four weeks of ST, and 0% with 4 and 6 weeks of ST. The treatment of giving SSF at a 20% level with an ST of 2 weeks, there was no significant difference (P>0.05) compared to the 10% level treatment, four weeks of ST, and the treatment level of 20%, four weeks and six weeks of ST, while significantly different. (P<0.05) compared to treatment at 0% level with two weeks, four weeks, and six weeks of ST, and with 10% level treatment, two weeks and six weeks of ST.
The treatment of giving SSF level of 0% with ST of 4 weeks was not significantly different (P>0.05) with treatment level of 0% SSF storage time of 6 weeks, while treatment level of 0% SSF for two weeks of ST, 10% level of two weeks, four weeks and six weeks of ST, and at 20% level two weeks, four weeks and six weeks of ST. The chicken egg HU value obtained in the study at two weeks of ST was 67.32±3.45; four weeks was 64.76±2.54, and six weeks was 63.93±3.21. The value was still in good condition compared to BSN (2008), where the HU obtained is in the range of quality II and includes eggs with quality B. According to BSN (2008), the freshness of eggs divided into Quality I has a HU = of 72; Quality II has a HU value of 62-72, and Quality III has a HU value = of 60. The HU value of quail eggs will decrease with the length of storage time. Jazil et al. (2012) stated that the longer the storage period, the value of the HU was low due to the evaporation of water and gases such as CO2 causes the thick egg white to become thinner.
CONCLUSIONS AND RECOMMENDATIONS
The research concluded that the use of 20% SSF was the best treatment to maintain the quality of chicken eggs and can extend the shelf life of eggs up to six weeks based on the percentage reduction in weight, air cavity diameter, pH, and HU of the eggs. Two weeks of egg storage was the best treatment to maintain the quality of broiler eggs and can extend the shelf life of eggs up to six weeks based on the percentage reduction in weight, air cavity diameter, pH, and HU of broiler eggs. The tannins contained in SSF (Averrhoa bilimbi L.) can perform as a natural preservative for eggs, and proven that the level of 20% SSF extract with a storage period of 2 weeks is a treatment. The best way to maintain the quality of broiler eggs is indicated by the weight loss, air cavity diameter, pH, and Haugh unit of broiler eggs.
ACKNOWLEDGMENT
The authors wish to thank the Rector of The University of Mataram for providing research grants. The authors also thank to the laboratory technicians of the Animal Products Processing Technology Laboratory for helping in conducting the research.
CONFLICT OF INTEREST
The authors have declared no conflict of interest.
NOVELTY STATEMENT
The research was conducted for the first time and found that the tannins contained in SSF (Averrhoa bilimbi L.) can perform as a natural preservative for eggs, and proven that at the level of 20% SSF extract with a storage period of 2 weeks is a treatment. The best way to maintain the quality of broiler eggs is indicated by the weight loss, air cavity diameter, pH, and Haugh unit of broiler eggs.
AUTHOR’S CONTRIBUTION
Djoko Kisworo, Martina Safitri, Bulkaini, Rani Dewi Wulandani and Syamsuhaidi were fully responsible for conducting the research and writing the paper. Djoko Kisworo, Martina Safitri, and Bulkaini were in charge of processing research data, while Rani Dewi Wulandani and Syamsuhaidi were in charge of editing the paper. Collectively revise the substance of the paper so that it is worthy of publication.
REFERENCES
Ariyana M. D., Sri Widyastuti, Nazaruddin, Baiq Rien Handayani, Moegiratul Amaro (2020). Aplikasi Anti Mikroba Alami Ekstrak Sargassum crassifolium Sebagai Agen Disinfeksi Untuk meningkatkan Mutu Mikrobiologis Telur Ayam Kampung. J. Penelitian. 3(1): 602-608.
Badan Standarisasi Nasional (BSN). (2008). SNI 01-3926-2008. Telur Ayam Konsumsi. Dewan Standarisasi Nasional. Jakarta.
Cornelia A., I. K. Suada, M. D. Rudyanto (2014). Perbedaan Daya Simpan Telur Ayam Ras yang Dicelupkan dan Tanpa Dicelupkan Larutan Kulit Manggis. Indonesia Med. Vet. 3(2): 112-119.
Djaelani M.A. (2016). Kualitas Telur Ayam Ras (Gallus L.) Setelah Penyimpanan yang dilakukan Pencelupan pada Air Mendidih dan Air Kapur Sebelum Penyimpanan. Buletin Anatomi dan Fisiologi., 24(1): 122-127. p-ISSN 2527 6751 https://doi.org/10.14710/baf.1.1.2016.24-29.
Ernawati T, Linda Ch. M. Karisoh, Rahmawaty Hadju, Surtijono E. (2019). Siswosubroto Pengaruh Konsentrasi Larutan Daun Jambu Biji (Psidium Guajava) Dan Lama Perendaman Terhadap Kualitas Telur Ayam Ras. J. Zoot., 39: 241 - 248. pISSN 0852 – 2626 eISSN 2615 – 869. https://doi.org/10.35792/zot.39.2.2019.24844
Gary D, DVM. Butcher, R. Miles (2009). Ilmu Unggas, Jasa Ekstensi Koperasi, Lembaga Ilmu Pangan dan Pertanian. Universitas Florida. Gainesville.
Geveke D.J., Gurtler J.B., Jones D.R., Bigley A.B. (2016). Inactivationof Salmonella in Shell Eggs by Hot Water Immersion and Its Effect on Quality. J. Food Sci. 81(3): 709-14. https://doi.org/10.1111/1750-3841.13233
Ismiwati (2011). Bobot, Komposisi Fisik dan Kualitas Interior Telur Puyuh (Coturnix coturnix japonica) yang diberi suplemen omega tiga. Skripsi. Institut Pertanian Bogor. Bogor
Jazil N, Hintono A, dan Mulyani S. (2013). Penurunan kualitas telur ayam ras dengan intensitas warna coklat kerabang berbeda selama penyimpanan. J. Aplikasi Teknol. Pangan., 2(1).
Jazil N., A. Hintono, dan S. Mulyani (2012). Penurunan Kualitas Telur Ayam Ras dengan Intensitas Warna coklat kerabang berbeda selama penyimpanan. J. Aplikasi Teknol. Pangan. 1(2): 43-47.
Joubrane K., Mnayer D., Hamieh T., Barbour G., Talhouk R., Awad E. (2019). Evaluation of Quality Parameters of White and Brown Eggs in Lebanon. American J. Analytic. Chem., 10: 488-503. https://doi.org/10.4236/ ajac.2019.1010035.
Koswara S. (2009). Teknologi Pengolahan Telur (Teori dan Praktek). eBook Pangan.com.
Kurtini T., Nova K., dan Septinova D. (2011). Produksi Ternak Unggas. Bandar Lampung: Universitas Lampung.
Kurtini T., K. Nova., dan D. Septinova (2014). Produksi Ternak Unggas. Universitas Lampung, Bandar Lampung.
Lengkey HA, Tuti W, Sjafril D. (2012). The effect of storage time in different temperature on native chicken egg haugh unit and yolk index. Anim. Sci., Series D, LV: 173-175.
Lestari S., R. Malaka dan S. Garantjang. (2013). Pengawetan Telur Dengan Perendaman Ekstrak Daun Melinjo (Gnetum Gnemon Linn). J. Sains dan Teknol. 13(2).
Lestari (2019). Pengawetan Telur dengan Perendaman Ekstrak Daun Melinjo (Gnetum gnemon liin). Tesis. Pasca Sarjana Universitas Hasanuddin. Makassar.
Menezes de C P., E. R. de lima. J.P de mederios, W.N K. de oliveira, J. Evencio Neto (2012). Egg quality of laying hens in different conditions of storage, ages and Housing Densities. Revista Brasileira de Zootecnia, Brasil. https://doi.org/10.1590/S1516-35982012000900014
Mukhlisah A.N., E. Abustam, F Maruddin (2020). The effect from different level of Melinjo (Gnetum gnemon Linn) leaf extract and storage duration on the quality of duck eggs. IOP Conf. Series: Earth Environ. Sci. 492 (2020). https://doi.org/10.1088/1755-1315/492/1/012052.
Mukhlisah A.N., F. Maruddin, R. Faridah, A. Hafid, M. Irfan (2021). Quality test weight of duck eggs using level treatment of melinjo leaves extract (Gnetum Gnemon Linn) and storage time. The 3rd International Conference of Anim. Sci. Technol., 788. https://doi.org/10.1088/1755-1315/788/1/012119
Novika Z., M. Djaelani, S. Mardiati (2017). Kualitas Telur Itik setelah Perendaman dengan Ekstrak Daun Salam (Syzygium polyantha) dan disimpan pada Suhu 4ºC. Buletin Anatomi dan Fisiologi. 2(2). https://doi.org/10.14710/baf.2.2.2017.120-127
Saud A. (2014). Studi Penggunaan Kulit Pisang kapok (Musa paradisiacal normalis) Sebagai Bahan Pengawet Telur Ayam Ras. Tesis. UNG. Gorontalo
Soekart, S.T. (2013). Teknoogi Penanganan dan Pengolahan Telur. ALFABETA. Bandung
Steel RGD, Torrie JH (2015). Prinsip dan prosedur statistika. Penterjemah Bambang Sumantri. Gramedia Pustaka, Jakarta.
Syaifulloh M., Moelia EM., Wahyu DL. (2021). Pengaruh Perbedaan Suhu Dan Lama Penyimpanan Terhadap Kualitas Fisik Telur Ayam Ras, J. Ilmu Peternakan., 14(1): 52-62. https://doi.org/10.35457/aves.v12i1.1132.
Thohari I. (2018). Teknologi Pengawetan dan Pengolahan Telur. UB Press. Malang.
Tindjabate R.S., I.K. Suada dan, M.D. Rudyanto (2014). Pengawetan Telur Ayam Ras dengan Pencelupan Dalam Ekstrak Air Kulit Manggis pada Suhu Ruang. J. Indonesia Med. Vet. 3(4) : 310-316.
USDA (United States Departement of Agriculture) (2000). Gerading Manual Agricultural Handbook number 75, Washington DC.
USDA (United States Department of Agriculture) (2007). Agricultural Handbook No.75: Egg Grading Manual. Washington D.C: United States Department of Agriculture.
Utami S.W., Silifiatus S., dan Fatimatuz Z. (2020). The Effect of Wuluh Star Fruit (Averhoa Bilimbi L.) Leaves Concentration and Storage Duration to Physical Quality of Quail Eggs. J. Ilmiah Inovasi. 20 (3): 14-18. ISSN 1411-5549. https://doi.org/10.25047/jii.v20i3.2319
Wibawanti J. M.W., Ma M., Qiu N., Hintono, Pramono Y.B. (2017). The Influence of Liquid Smoke on The Chemical Characteristics of Salted Egg. J. Teknol. hasil ternak. 12(2): 76-82. https://doi.org/10.21776/ub.jitek.2017.012.02.3
Yuwanta Tri (2010). Telur dan Kualitas Telur. Gadjah Mada University Press. Yogyakarta.






