Obtaining Osteological Material using Dermestes maculatus De Geer, 1774 (Coleoptera: Dermestidae) in Veterinary Anatomy
Obtaining Osteological Material using Dermestes maculatus De Geer, 1774 (Coleoptera: Dermestidae) in Veterinary Anatomy
Sedef Selviler Sizer1, Semih Kurt1*, Burcu Onuk1, Gokmen Zafer Pekmezci2 and Murat Kabak1
1Department of Anatomy, Faculty of Veterinary Medicine, Ondokuz MAYIS University, Samsun, Turkey
2Department of Preclinical Science, Faculty of Veterinary Medicine, Ondokuz Mayıs University, Samsun, Turkey
ABSTRACT
Dermestid beetles being low-cost, efficient, and environmentally friendly are commonly used to prepare osteological materials in museums. This study was carried out to produce osteological material from the heads of roe deer, cows, and cats using a Dermestes maculatus (De Geer, 1774) colony and reveal the colony’s meat consumption performance in terms of time. In addition, using hydrogen peroxide solution to kill remaining beetles in the cleaned osteological material and determine the whitening and degreasing efficiency of this solution was also aimed. In the study, approximately 10,000 Dermestes maculatus, along with the heads of six roe deer, three cows, and three cats were used. To observe the meat-cleaning performance, the initial and final weights of the heads were measured before they were placed in the beetle colony. All tissues, including eye and brain tissues, of the heads placed in the Dermestes colony were cleaned in one day in cats, two days in roe deer, and three days in cows. In addition, it was observed that the Dermestes colony was not a preference priority when consuming soft tissues such as eyes, brain and muscle. This current manuscript has revealed the advantages of the hydrogen peroxide application in whitening and degreasing bone materials and killing remaining beetles at the end of the process on skull materials cleaned by Dermestes beetles.
Article Information
Received 11 May 2022
Revised 25 September 2022
Accepted 19 October 2022
Available online 14 January 2023
(early access)
Published 29 March 2024
Authors’ Contribution
All authors contributed to the conception and design of the study. Material preparation, data collection, and analysis were performed by SSS, SK, BO, and MK. DNA barcoding was done by GZP. All authors read and approved the final version of the manuscript.
Key words
Dermestes maculatus, Environmental pollution, Osteological material, Hydrogen peroxide
DOI: https://dx.doi.org/10.17582/journal.pjz/20220511120524
* Corresponding author: semih.kurt@omu.edu.tr
0030-9923/2024/0003-1125 $ 9.00/0
Copyright 2024 by the authors. Licensee Zoological Society of Pakistan.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
INTRODUCTION
Visuality has an important place in anatomy education, which is one of the basic building blocks of medical education (Özdemir, 2003). Different methods, such as boiling, maceration, and burial, are used to obtain the real skeletal models used in traditional education methods (Fenton et al., 2003; Couse and Connor, 2015). Of these, a unique system is needed for the boiling method, and its cost is high (Tompsett, 1970; Rowley, 2015). Additional work is required to clean tissues other than bone after the procedure (Rowley, 2015). In the maceration method, water or chemicals are used, generating a bad odor due to the long duration of water maceration and intense bacterial growth (Kamath et al., 2016). Although chemical maceration takes less time than water maceration, the cost increases, depending on the type of chemical used and bone tissue damage which may occur (Simonsen et al., 2011; Onwuama et al., 2012). Another method, burial, requires considerable time, and there is a possibility that carnivores may damage the buried material (Sarma et al., 2017). Due to the disadvantages of boiling, maceration, and burial, there have been attempts to develop alternative methods to prepare skeletons. For this purpose, the Dermestes genera are used in forensic entomology, zoology, and anthropology and to prepare materials in museums (Kulshrestha and Satpathy, 2001; Mccarthy, 2015; Pahl, 2020). Since this method creates much less medical waste, it is also deemed environmentally friendly (Botero-González and Agudelo, 2019; Pahl, 2020). Dermestes species (Coleoptera: Dermestidae) are known as scavengers (Rowley, 2015; Sanger-Ciarleglio et al., 2020; Hess, 2021). The most common species used for cleaning skeletons from these genera is D. maculatus (Krinsky, 2019). The use of Dermestes species in material preparation saves time, cost, and labor (Laurie and Hill, 1951; Sommer and Anderson, 1974). However, the main factor affecting the speed with which the skeleton is cleaned in this method is the need to have a strong knowledge of the environmental conditions and life cycles of beetles (İnce and Onar, 2004; Zanetti et al., 2016; Oliveira, 2018). It is very important to know these elements in detail to ensure a sustainable system. D. maculatus, a black-colored species, has white stripes on the abdomen. The surface of the larvae of this species, which is brown-colored, is covered with hairs (Sanger-Ciarleglio et al., 2020). For the sustainability of a colony kept in a closed environment, the ambient temperature should be 21–26°C, and the humidity should be 50% (Russell, 1947; Laurie and Hill, 1951; Sanger-Ciarleglio et al., 2020). The colony’s water requirements are met by spraying water on a sponge placed in the environment. Coarse chips are used to make a suitable colony base (İnce and Onar, 2004; Pahl, 2020). The life cycle of these beetles has four stages: Egg, larvae, pupae, and adult (Muñoz-Saba et al., 2020; Hess, 2021). They do not like to feed on feathers, skin, or internal organs (Muñoz-Saba et al., 2020; Sanger-Ciarleglio et al., 2020). Contamination with invasive insect species must be prevented in order to maintain colony health (Sanger-Ciarleglio et al., 2020; Hinshaw, 2021). To remove larvae of dermestid beetles from the material cleaned by the colony, processes such as immersion in hot water, ammonium hydroxide, or 50% alcohol solution, and holding in the deep freeze or sodium perborate, are used (Borell, 1938; İnce and Onar, 2004; Anderson, 2020; Pahl, 2020).
This study aimed to prepare osteological material from the heads of roe deer, cows, and cats using a D. maculatus colony and to reveal the meat consumption performance of the colony in terms of time. In addition, it was purposed to determine the effectiveness of hydrogen peroxide, used for the first time in killing the beetles likely to remain in osteological materials. It is thought that this study will contribute to the literature by providing additional information on the bone cleaning methods with Dermestidae.
MATERIALS AND METHODS
Dermestes maculatus culture and confirmation using DNA barcoding
One male and one female live adult D. maculatus (De Geer 1774) (Coleoptera: Dermestidae) were purchased from “dermestidae.tr” in Turkey, and these specimens were morphologically confirmed, based on a standard taxonomic key using a stereomicroscope (Olympus SZ2-STU2 Tokyo, Japan) and the photographs were taken with a digital camera (Olympus C-5060) (Gennard, 2007). After confirmation of the species, the protocols for rearing and maintenance of cultured D. maculatus laboratory colonies (egg to adult) according to Xiang et al. (2015), and Zanetti et al. (2016) were followed. Then, to molecularly verify the identity of the species, two representative specimens were randomly selected from among all the larvae. Genomic DNA was extracted from individual larvae using the DNA extraction kit (Thermo Scientific). The mitochondrial cytochrome C oxidase subunit I (mtCO1) gene was amplified using LCO1490/HCO2198 primers (Folmer et al., 1994). PCR conditions followed the protocol given by Mashaly et al. (2018). The mtCOI gene amplification products were sequenced with the same primers using an ABI PRISM 310 genetic analyzer (applied biosystems). The quality of the raw sequence data was assessed with Phred scores (Q ≥ 20) before editing and assembly were carried out with Geneious R12 (Kearse et al., 2012). Assembled sequences were compared to Dermestes species sequences using BLAST searches in GenBank (Altschul et al., 1997).
Material supply
In the study, approximately 10.000 D. maculatus larvae (Coleoptera: Dermestidae) produced by the reproduction of two insects, one female and the other male, which we obtained from the company “Dermestidae.tr” were used. The total number of these larvae was calculated with the following formula.

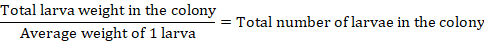
After these two insects were received from Dermestidae.tr company and firstly put in dermestidarium. Insects were reproduced with a 45-days breeding period. In about six months period, it was fed with fresh pieces of meat, which was increased daily depending on the number of insects, and the appropriate number of colonies was formed. Finally, the heads of contagious disease-free adult six roe deer, three cats, and three cows that could not be saved in the faculty clinic and were sent to our department for student practice were placed in the dermestidarium to be cleaned.
Dermestidarium
In a colony care system, where humidity and temperature were controlled through sensors, the environmental temperature was between 21oC and 24oC with 40–60% humidity. Poplar sawdust was used for the colony base, and water crystals were used for the water requirement. Approximately 10 g of water crystals were kept in 100 ml of water for 15 minutes and given to the colony every 5 days. The heads whose skin had been separated by dissection were weighed with a precision balance (Shaver SF-400, Turkey) before being given to the colony and after cleaning in the colony. The heads were photographed at every stage until they were cleaned and turned into osteological material.
Cleaning, degreasing, and whitening methods
After consumption, beetles on the skull surface were removed with a fine brush and air compressor. It was kept in a separate compartment for a week, as beetles could remain in the skull. At the end of a week, skulls were immersed in 5% hydrogen peroxide to whiten, degrease, and kill any living beetles (adults and eggs).
RESULTS
Adult and larvae of D. maculatus were morphologically identified according to Gennard (2007) (Fig. 1). The sequencing of mtCO1 of D. maculatus larvae produced 542 base pairs. There were no intraspecific nucleotide differences within two larval representatives. The mtCO1 of D. maculatus was submitted to GenBank under the accession number (OM533686). According to a BLASTn search, sequences of D. maculatus in the present study showed 99.8–100% identity with D. maculatus (MZ656890, MW278864‒MW278869, HM398864, HM398865, HM909035, MW200375) from Ecuador, Finland, Germany, and South Korea.
Table I. Daily meat consumption of D. maculatus colony.
|
Species |
Weight of the head before being given to the colony (g) |
Weight of the cleaned head (g) |
Daily consumption amount (g/day) |
|
Roe deer |
651.5±54.28 |
101.16±38.99 |
275.16±19.46 |
|
Cat |
313.66±8.08 |
29.66±1.52 |
289±3.60 |
|
Cow |
3102.3±15.69 |
2110.6±66.6 |
305±5 |
The heads were weighed before being given to the colony and after cleaning, and the daily meat consumption of the colony was calculated.
It was observed that the colony started to consume meat from the first day. The heads were weighed before being given to the colony and after cleaning, and the daily consumption of the colony was calculated (Table I). Accordingly, no difference was observed in the consumption performance of the colony according to the species. It was determined that cat heads were cleaned in one day (Fig. 2), roe deer heads in two days (Fig. 3), and cow heads in three days (Fig. 4). These differences in time were due to the size of the heads used. It was determined that the colony consumed the muscle tissue, brain (Fig. 5), and eye (Fig. 6) in the heads. The sutures between the cranium bones were undamaged and clearly revealed (Figs. 2, 3, 4). Interfrontal, frontal, and internasal sutures, often separated in boiling, were clearly observed in cow, roe deer, and cat heads. In addition, no damage was observed in the bone leaves forming endoturbunale I, endoturbunale II, and ectoturbinale, and these bone leaves were clearly revealed in all species (Figs. 2, 3, 4). When the skulls cleaned by the colony were immersed in 5% hydrogen peroxide solution, it was observed that the viability of the beetles ended. Just in case, the cleaned skulls were kept in a closed environment for one month, and no larvae were found during this period.
DISCUSSION
It is known that different methods are used in the preparation of osteological material, such as boiling, maceration, and burial (Fenton et al., 2003; Atabo et al., 2019). Each method has advantages and disadvantages (Tompsett, 1970; Mairs et al., 2004; Allouch, 2014; Ajayi et al., 2016; Kamath et al., 2016; Sarma et al., 2017). Apart from these methods, the use of Dermestes in creating osteological material has become widespread in recent years (Leeper, 2015; Botero-González and Agudelo, 2019). In our study, osteological material was created using D. maculatus. It has been determined that this method is superior to other methods (maceration, burial, boiling) as it minimizes the amount of odor generated and time and labor required. One of the most important features of this method was that it minimized the amount of medical waste.
It is stated that D. maculatus tends to escape from the colony by flying due to the increase in temperature and humidity in the environment and that it should be kept in a closed system under optimum conditions to prevent this from occurring (İnce and Onar, 2004; Zanetti et al., 2016; Hinshaw, 2021). In our study, possible escape was prevented by using a specially produced care system (dermestidarium) adapted to the living conditions of the colony. In this system, when constant humidity (40–60%) and temperature (21–24oC) are provided, the absence of any tendency to escape in the colony is compatible with the literature (İnce and Onar, 2004). It is reported that the colony life cycle consists of four phases (İnce and Onar, 2004). It is stated that the highest amount of meat consumption is provided by the larvae (Russell, 1947; Russell et al., 2013). In our study, likewise, it was observed that the larvae consumed the most meat. It has been reported that beetles do not prefer to consume tissues such as internal organs, eyes, tongue, brain, skin and feathers, so these tissues should be removed from the cadaver and given to the colony (Leeper, 2015; Muñoz-Saba et al., 2020). However, in the study, it was observed that D. maculatus was not a preference priority when consuming soft tissues such as eyes, brain and muscle.
It has been reported in studies that methods such as alcohol baths, cleaning with ammonia, immersion in hot water, immersion in ammonium hydroxide, sodium perborate and holding in the deep freeze are used to remove the adult or its larvae in materials cleaned by the colony (Borell, 1938; İnce and Onar, 2004; Anderson, 2020; Muñoz-Saba et al., 2020; Pahl, 2020). In our study, 5% hydrogen peroxide was used for the first time in degreasing, whitening the material cleaned by the colony, and killing the remaining beetles. Three steps performed with a single chemical saves time and cost, suggesting that it can be an alternative to other methods. In addition, the necessary conditions for keeping the colony number constant and the factors affecting the cleaning time of the cadaver were determined in this study. The stability of the colony number depended on the amount of food and water in the system, and the cleaned time of the cadaver depended on the density of larvae in the colony, the humidity of the cadaver, the surface width, and the amount of meat.
ACKNOWLEDGMENTS
We thank the firm https://www.dermestidaetr.com/ for allowing us to use its closed maintenance system in this study.
Funding
There is no specific grant funding source.
Ethical statement
Since no live animals were used in the study, there is no need for an ethics committee.
Statement of conflict of interest
The authors have declared no conflict of interest.
REFERENCES
Ajayi, A., Edjomariegwe, O., and Iselaiye, O., 2016. A review of bone preparation techniques for anatomical studies. Malaya J. Biosci., 3: 76-80.
Allouch, G., 2014. Scientific technique for skeletons preservation and preparation of anatomical models to promote veterinary anatomy. J. Vet. Anat., 7: 133-139. https://doi.org/10.21608/jva.2014.44817
Altschul, S.F., Madden, T.L., Schäffer, A.A., Zhang, J., Zhang, Z., Miller, W. and Lipman, D.J., 1997. Gapped blast and psi-blast: A new generation of protein database search programs. Nucl. Acids Res., 25: 3389-3402. https://doi.org/10.1093/nar/25.17.3389
Anderson, R., 2020. Methods of collecting and preserving vertebrate animals. Read Books Ltd.
Atabo, S., Hena, S., Jaji, A. and Bodinga, A., 2019. Bovine skeleton preparation using hot water technique for anatomical studies. Asian J. Res. Anim. Vet. Sci., 4: 1-7.
Borell, A.E., 1938. Cleaning small collections of skulls and skeletons with dermestid beetles. J. Mammal., 19: 102-103. https://doi.org/10.1093/jmammal/19.1.102-a
Botero-González, D. and Agudelo, M., 2019. Comparison of macerations with dermestid larvae, potassium hydroxide and sodium hypochlorite in wistar rat crania. Anatomy, 13: 149-154.
Couse, T. and Connor, M., 2015. A comparison of maceration techniques for use in forensic skeletal preparations. J. Forensic Invest., 3: 1-6. https://doi.org/10.13188/2330-0396.1000021
Fenton, T.W., Birkby, W.H. and Cornelison, J., 2003. A fast and safe non-bleaching method for forensic skeletal preparation. J. Forensic Sci., 48: 274-276. https://doi.org/10.1520/JFS2002034
Folmer, O., Black, M., Hoeh, W., Lutz, R., and Vrijenhoek, R., 1994. DNA primers for amplification of mitochondrial cytochrome C oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol., 3: 294–299.
Gennard, D.E., 2007. Forensic entomology. John Wiley and Sons Ltd., England: John Wiley and Sons Ltd., England.
Hess, B., 2021. University of Michigan Herbarium (mich) and University of Michigan Museum of Zoology (ummz) Annual Report.
Hinshaw, S.H., 2021. Dermestarium. Avaible: https://webapps.lsa.umich.edu/ummz/mammals/dermestarium/default.asp.
İnce, N. and Onar, V., 2004. Dermestid böcekleri: Biyolojisi ve koloni oluşturulması. Istanbul Univ. Vet. Fak. Derg., 30: 133-140.
Kamath, V., Bhat, S., Asif, M., and Avadhani, R., 2016. Anatomy museums of southern india and medical education: An original research. Indian J. clin. Anat. Physiol., 3: 45-49. https://doi.org/10.5958/2394-2126.2016.00013.X
Kearse, M., Moir, R., Wilson, A., Stones-Havas, S., Cheung, M., Sturrock, S., Buxton, S., Cooper, A., Markowitz, S., and Duran, C., 2012. Geneious basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics, 28: 1647-1649. https://doi.org/10.1093/bioinformatics/bts199
Krinsky, W.L., 2019. Chapter 9 beetles (coleoptera). In: Medical and veterinary entomology (third edition) (eds. G.R. Mullen and L.A. Durden). Academic Press, pp. 129-143. https://doi.org/10.1016/B978-0-12-814043-7.00009-1
Kulshrestha, P. and Satpathy, D., 2001. Use of beetles in forensic entomology. Forensic Sci. Int., 120: 15-17. https://doi.org/10.1016/S0379-0738(01)00410-8
Laurie, E. and Hill, J., 1951. Use of dermestid beetles for cleaning mammalian skeletons. Mus. J., 51: 206-207.
Leeper, B.J., 2015. Evaluation of current methods of soft tissue removal from bone. University of Pittsburgh, PhD thesis.
Mairs, S., Swift, B., and Rutty, G.N., 2004. Detergent: An alternative approach to traditional bone cleaning methods for forensic practice. Am. J. Forensic Med. Pathol., 25: 276-284. https://doi.org/10.1097/01.paf.0000147320.70639.41
Mashaly, A.M., Al-Ajmi, R.A., and Al-Johani, H.A., 2018. Molecular identification of the carrion beetles (Coleoptera) in selected regions of saudi arabia. J. med. Ent., 55: 1423-1430. https://doi.org/10.1093/jme/tjy116
Mccarthy, E., 2015. The flesh-eating beetles that work at natural history museums. Animals: Mental Floss. https://www.mentalfloss.com/article/68184/beetles-work-natural-history-museums
Muñoz-Saba, Y., Sánchez-Nivicela, J.C., Sierra-Durán, C.M., Vieda-Ortega, J.C., Amat-García, G., Munoz, R., Casallas-Pabón, D., and Calvo-Roa, N., 2020. Cleaning osteological specimens with beetles of the genus Dermestes Linnaeus, 1758 (Coleoptera: Dermestidae). J. Natl. Sci. Collect., 7: 72-82.
Oliveira, M.B.d., 2018. Methods of biological maceration in the preparation of bat skulls: Benefits and limitations. Pap. Avuls. Zool., 58: e20185844. https://doi.org/10.11606/1807-0205/2018.58.44
Onwuama, K.T., Salami, S.O., Ali, M. and Nzalak, J.O., 2012. Effect of different methods of bone preparation on the skeleton of the african giant pouched rat (Cricetomys gambianus). J. Morphol., 30: 425-427. https://doi.org/10.4067/S0717-95022012000200011
Özdemir, S.T., 2003. Tıp eğitimi ve yetişkin öğrenmesi. Uludağ Üniv. Tıp Fak. Derg., 29: 25-28.
Pahl, A., 2020. Skeleton preparation best practices in the modern museum: The dermestid approach. Curator Mus. J., 63: 99-113. https://doi.org/10.1111/cura.12349
Rowley, B., 2015. Protocols for cleaning and articulating large mammal skeleton. In: Symposium. pp. 3. https://doi.org/10.15368/symp.2015v2n1.5
Russell, R.C., Otranto, D., and Wall, R.L., 2013. Beetles (Coleoptera: Meloidae, Oedemeridae, Staphylinidae and others). CABI, Wallingford: pp. 41-45. https://doi.org/10.1079/9781780640372.0041
Russell, W.C., 1947. Biology of the dermestid beetle with reference to skull cleaning. J. Mammal., 28: 284-287. https://doi.org/10.2307/1375178
Sanger-Ciarleglio, J.E., Perez, K.M., Motola, H.L. and DiGangi, E.A., 2020. Recommendations for maintaining a dermestid beetle colony (Dermestes maculatus) for processing human remains. J. Forensic Sci., 65: 1698-1703. Available from https://onlinelibrary.wiley.com/doi/pdfdirect/10.1111/1556-4029.14470?download=true, https://doi.org/10.1111/1556-4029.14470
Sarma, A., Baro, B., Das, G.C., Momin, A.D., and Bhuyan, M.B., 2017. Using of bleaching powder for cleaning embalmed cadaveric bones. J. med. Sci. clin. Res., 5: 15460-15465. Available from https://www.researchgate.net/publication/312177076, https://doi.org/10.18535/jmscr/v5i1.44
Simonsen, K.P., Rasmussen, A.R., Mathisen, P., Petersen, H., and Borup, F., 2011. A fast preparation of skeletal materials using enzyme maceration. J. Forensic Sci., 56: 480-484. Available from https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1556-4029.2010.01668.x, https://doi.org/10.1111/j.1556-4029.2010.01668.x
Sommer, H.G. and Anderson, S., 1974. Cleaning skeletons with dermestid beetles two refinements in the method. Curator, 17: 290-298. https://doi.org/10.1111/j.2151-6952.1974.tb01245.x
Tompsett, D.H., 1970. Anatomical techniques. Churchill Livingstone.
Xiang, J., Forrest, I.S. and Pick, L., 2015. Dermestes maculatus: An intermediate-germ beetle model system for evo-devo. EvoDevo, 6: 1-17. https://doi.org/10.1186/s13227-015-0028-0
Zanetti, N.I., Visciarelli, E.C., and Centeno, N.D., 2016. The effect of temperature and laboratory rearing conditions on the development of Dermestes maculatus (Coleoptera: Dermestidae). J. Forensic Sci., 61: 375-381. Available from https://onlinelibrary.wiley.com https://doi.org/10.1111/1556-4029.12965
To share on other social networks, click on any share button. What are these?










