Observation of Embryonic and Larval Developmental Stages in Endangered Nona Tengra (Mystus gulio) Induced with S-GnRHa
Observation of Embryonic and Larval Developmental Stages in Endangered Nona Tengra (Mystus gulio) Induced with S-GnRHa
Md. Alal Hossen1, Mohammad Amzad Hossain1*, A.K.M. Munzurul Hasan1,2, Bipresh Das1, Sohel Mian1, Mohammed Mahbub Iqbal1
1Department of Fish Biology and Genetics, Faculty of Fisheries, Sylhet Agricultural University, Sylhet-3100, Bangladesh.
2Department of Boreal Ecosystems and Agricultural Sciences, School of Science and the Environment, Memorial University of Newfoundland, Corner Brook, Newfoundland, Canada.
Abstract | Mystus gulio is a commercially important estuarine small catfish in Bangladesh and it is prone to decreasing due to natural and anthropogenic changes. Information of induced breeding of this catfish and its embryonic development could contribute to database and conservation approach. The S-GnRHa dose optimization and embryonic-larval development study were conducted in a freshwater condition in order specify optimal dose of synthetic hormones in induced breeding. The synthetic GnRHa was used as induction agent where male injected half of the doses of female and a control without S-GnRHa was assigned. The stages of embryonic development were observed under a photomicroscope. Average fertilization and hatching rates varied from 74.33% to 83.89% and from 72.33% to 85.11% respectively among different treatments and control females did not ovulate. The eggs of M. gulio was firmly adhesive and immediately after spawning the average diameter of fertilized eggs were 0.48±0.01 mm. After fertilization, the first cleavage stage of M. gulio occurred within 40 min. The distinctiveness of blastomeres was progressively dropped and morula, blastula, and gastrula stages were found at 3:40 h, 4:20 h and 5:00 h, respectively after fertilization. The larvae-initiated hatching at around 21:00 h after fertilization and total length of larvae were computed 1.11±0.01 mm while the temperature of water was 30±1°C. The present study revealed that application of S-GnRHa hormone at dose of 0.5 ml kg-1 body weight of female could be efficacious for production of M. gulio seed in freshwater successfully.
Novelty Statement | This research work is very first approach to breed Mystus gulio by using S-GnRHa in freshwater environment and to observe their early development stages.
Article History
Received: March 11, 2020
Revised: January 15, 2021
Accepted: March 28, 2021
Published: June 15, 2021
Authors’ Contributions
MAH did field and laboratory works, report writing and reviewing literature. MAH instructed the field and laboratory assays, produced manuscript and revised for publication. AKMMH and BD helped in field and laboratory works. SM and MMI planned and conceptualized the methodology and supervised the works academically.
Keywords
Mystus gulio, S-GnRHa, Induced breeding, Embryonic and larval development
Corresponding author: Mohammad Amzad Hossain
To cite this article: Hossain, M.A., Hossain, M.A., Hasan, A.K.M.M., Das, B., Mian, S., Iqbal, M.M., 2021. Observation of embryonic and larval developmental stages in endangered Nona Tengra (Mystus gulio) induced with S-GnRHa. Punjab Univ. J. Zool., 36(1): 91-99. https://dx.doi.org/10.17582/journal.pujz/2021.36.1.91.99
Introduction
Induced breeding is a method whereby using pituitary hormone or any other synthetic hormone ripe fish breeders are induced to breed in captivity (Bailung and Biswas, 2014; Kumar et al., 2018). Induced breeding technique is advantageous because it provides pure good quality spawn as well as ensures availability of fish seed, whereas availability of seed from natural source is contingent and depends on several environmental factors (Wahab et al., 2003; Mollah et al., 2008). Artificial propagation of breeding provides key information for the conservation of threatened species, management of cultivation and high productivity (Sahoo et al., 2008; Dhara and Saha, 2013; Nakaghi et al., 2014). Embryonic development observation will help to acquire knowledge on early biological characteristics of any fish which could be beneficial for hatchery manager and production programmers of this species (Kohinoor et al., 1991; Bromage et al.,2001).
The M. gulio contributes a lot to coastal fishery both in commercial aspects and in providing food and nutrition for local community (Kumar et al., 2018; Sakthivel et al., 2013). It is a popular and commercial small indigenous fish species, so now a days its demand is rapidly increasing in Bangladesh (Gupta, 2014; Sarker et al., 2002), but their seed is not available in anywhere. Some initial breeding trial had been approached previously only for brackishwater or salinity prone areas (Sarker et al., 2002; Mijkherjee et al., 2002; Alam et al., 2006; Hossain et al., 2014). Therefore, it is not cost effective to carry brackishwater far away from that of the coastal region for artificial breeding of M. gulio and using artificial saltwater in the hatchery might not be economically effective. However, beside the development of induced breeding and culture technique, it is obvious to draw out the embryonic development of M. gulio (Kimmel et al., 1995; Korzelecka-Orkisz et al., 2010; Olaniyi et al., 2013). With a view to the management and conservation of M. gulio, and to establish seed production technique and culture potentials in freshwater, the present exploration has been held out using S-GnRHa in freshwater and embryonic stages of M. gulio were observed as well.
Materials and Methods
Study site and duration
The research was conducted for a three-month duration from June 2017 to August 2017 at a privately owned “Alalpur Hatchery and Fisheries Ltd.”, Mymensingh. Major treatment and pond research work of induced breeding was performed at the hatchery facilities of Alalpur Hatchery while observation of embryonic development was carried out at the nearby laboratory facility of Fisheries Biology and Genetics laboratory in Bangladesh Agricultural University, Mymensingh.
Nurturing and selection of brood fish
The experimental pond was 0.2 acre (1 acre = 4046.86 m2) having 0.9-1.1 meters in depth and brood fish was stocked at intensity of 2500-3000 per acre. During rearing period lime, fertilizer, and cow dung were properly applied. Urea and Triple Super Phosphate fertilizer were used at the level of 20 kg acre-1 and 10 kg acre-1 respectively at 15 days’ interval, then lime was used at the rate of 100 kg/acre and 5-7 days after application of cow dung at 600-700 kg/acre rate for one time. Broodfish were fed with supplementary feed according to the description of Siddiky et al. (2015), briefly 5-6% of body weight with feed (moisture 10%, protein 62%, fat 8%, carbohydrate 20%, ash 15%, fiber 1.5%, calcium 2.1%, and phosphorus 2.5%) were given twice a day. Good appearing, healthy sexually mature, and ready to spawn broods were chosen as breeders. Adult male and female brood fishes were recognized by prominent secondary sexual features (Seethal et al., 2016). The mature males were identified by their flat abdomens and protruded pointed genital opening, whereas the female’s broods were distinguished by presence of puffy belly as well as round-enlarged urogenital opening. The weight of the matured male and female were ranged between 10-20 g and 17-30 g, respectively.
Hormonal induction of brood fish
The breeding induction were performed by using synthetic S-GnRHa commercially produced by Ningbo Sansheng Pharmaceutical Co. Ltd. and liquid solution were injected beneath the dorsal side and above the lateral line with a 1ml syringe. Then brood fishes were kept simultaneously into the breeding tank with continuous water showering. Each of the tanks were 1.5×0.5×1 m3 in size and four treatments were assigned as T1, T2, T3 and T4 each with three replications as well (Table 1). The ratio of male and female was 1:1 in all treatments and fertilization were performed naturally.
Table 1: Induced breeding trial of M. gulio with S-GnRHa hormone.
|
Treatment |
Hapa |
Dose for female fish (ml kg-1bw) body weight |
Dose for male fish (ml kg-1bw) body weight |
|
T1 |
H1, H2, H3 |
0.25 |
0.125 |
|
T2 |
H1, H2, H3 |
0.5 |
0.25 |
|
T3 |
H1, H2, H3 |
1.0 |
0.5 |
|
T4 (Control) (0.9% NaCl) |
H1, H2, H3 |
1 |
0.5 |
Identification of fertilized eggs
The embryonic development had started immediately after fertilization followed by the subsequent hormone treatment and the fertilized eggs appeared wet, bulging as well as somewhat transparent, however, the color of unfertilized eggs was whitish and opaque in appearance (Figure 1).
Calculating the rate of ovulation, fertilization, and hatching
The ovulation rate was determined by using below formula from Legendrea et al. (2000).
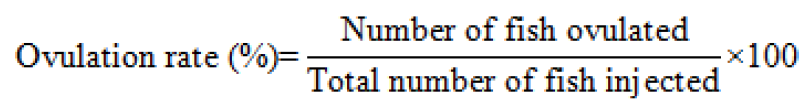
A random sample of 100 eggs were taken from the hatching jar in a dish to determine the fertilization rate. and the rate was calculated by using the description of Unuma et al. (2004).
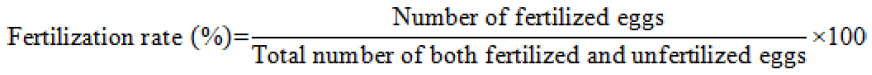
To calculate the rate of hatching, 100 fertilized eggs were putted in a distinct jar with unceasing water supply. Hatchling numbers were counted manually immediately after completion of hatching and the hatching rate was assessed using following formula (Unuma et al., 2004):
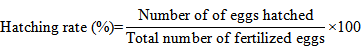
Observation of embryonic development
The fishes were ovulated at about 7-8 hs of the hormone injection and a few eggs were plotted in a Petridish with water. Study of embryonic development was performed under a dissecting microscope and for that only the fertilized eggs were chosen which contained uniform as well as round yolk sphere with smooth perivitelline space. At regular interval, egg was monitored to document the timing of each embryonic development stage and each stage was captured by using a camera (Rigla-32, Optikam B3 Digital camera, Italy) attached with the microscope (OLYMPUS CX21). The diameter of egg was measured in mm scale by using live sample on “ImageJ” software. Embryonic stages were characterized by following the description of Kumar et al. (2018).
Data analysis
A one-way analysis of variance (ANOVA) was followed to determine the significance (p<0.05) level of hormonal treatments. The level of significance of the results was tested following Tukey HSD using SPSS (IBM version 26) programming and by using Microsoft Excel 2016.
Results and Discussion
Optimal dose of S-GnRHa and rate of ovulation, fertilization, and hatching
A synthetic gonadotropin-releasing hormone S-GnRHa was used at three distinct doses. For female, doses were 0.25, 0.5 and 1.0-ml kg-1bw, whereas for male were injected as 0.125, 0.25-, and 0.5-ml kg-1bw (Table 2). Among the doses, 0.5 ml kg-1bw for female showed the maximum fertilization rate (83.89%) and hatching rates (85.11%), and this is statistically different from the other doses in female (P<0.05) (Table 2). All females were ovulated within 12 hs for all treatment tanks excluding control (Table 2) and the latency period was accounted as 7-8 h. All hormone dose recipient females spawned successfully and hatched between 18:00-21:00 h after spawning. However, statistically there was no significant difference in fertilization rate and hatching percentage among hormone treated groups. The highest fertilization rate was significantly different at P<0.05 in T2 (83. 89%) followed by T3 (78. 56%) and T1 (74.33%) as well as the highest hatching rate was significantly different at P<0.05 in T2 (85.11%) followed by T3 (74.66%) and T1 (72.33%) (Table 2).
Embryonic development
The fertilized eggs of M. gulio were firmly glued. Detailed discloser of embryonic development stages and time interval has been presented in Table 3. The embryonic development stages took place within the chorion and end up with hatching. Membrane of the ovum was disconnected from the rest of the ovum cell by a tiny perivitelline surface. The unfertilized eggs of M. gulio were globular, demersal, opaque, and whitish (Figure 2a). They were slightly adhesive. The mean size of unfertilized egg diameter was measured 0.48±0.00 mm. The color of fertilized eggs was brownish, and it appeared as spherical, demersal as well as adhesive to the substratum (Figure 2b). The common diameter of the fertilized egg was observed as 0.48±0.00 mm.
Table 2: Performance of different doses of S-GnRHa hormone on ovulation, fertilization, and hatching rate of M. gulio.
|
Parameter (Mean ± SD) |
Treatment |
|||
|
T1 (0.25 ml kg-1bw) |
T2 (0.50 ml kg-1 bw) |
T3 (1.00 ml kg-1bw) |
T4 (1.00 ml kg-1bw) (.9%NaCl) |
|
|
Total length (cm) Body weight (gm) Ovulation rate (%) Fertilization rate (%) Hatching rate (%) |
11.66 ± 0.75 20.89 ± 4.075 100 74.33 ±1.658c 72.33 ± 1.5 c |
12.5 ± 0.866 23.33 ± 2.5 100 83.89 ±1.364a 85.11 ±2.088a |
12.33 ± 1 22.66 ± 2.179 100 78.55 ±1.810b 74.66±2.121b |
12.00±.866 21.77±1.855 - - - |
Note: Values are means of data obtained ± Std. Deviation (mean ± SD) of determinations. Values in the same row with different superscripts are significantly different (P>0.05). The absence of superscripts indicates no significant difference between treatments.
Table 3: Different stages of embryonic development of the fertilized egg and their respective time in M. gulio.
|
Time after spawning (h: min) |
Development stage |
Egg size (mm) (mean±sd) |
Key description |
|
|
Unfertilized egg |
0.486±0.0036 |
Opaque and whitish in color |
a |
|
|
00:00 |
Fertilized egg |
0.486±0.0038 |
Spherical, demersal, adhesive, watery in color |
b |
|
00:15 |
Blastodics formation |
0.488±0.00057 |
Blastodisc formed at the pole |
c |
|
00:40 |
2 cell stage |
0.488±0.00057 |
First cleavage |
d |
|
00:55 |
4 cell stage |
0.489±0.00057 |
Second cleavage |
e |
|
01:15 01:40 02:00 02:30 |
8 cell stage 16 cell stage 32 cell stage Multi-cells |
0.489±0.00029 0.489±0.00034 0.490±0.00042 0.490±0.00057 |
Third cleavage Fourth cleavage Fifth cleavage Sixth cleavage |
f g h i-n |
|
03:40 |
Morula stage |
0.491±0.00079 |
Blastulation progresses to form a multicellular blastodisc. |
o |
|
04:20 |
Blastula stage |
0.653±0.0021 |
A third of egg space occupied with blastoderm cells. |
p |
|
05:00 |
Gastrula stage |
0.675±0.0023 |
Blastoderm spread on both the side which covering about 60-70% area and generating a thread like germinal ring |
q-r |
|
06:40 |
Yolk plug stage |
0.702±0.0018 |
Yolk generation complete and cephalic region gets thicker. |
s |
|
08:00 |
Kidney shaped embryo |
0.735±0.0025 |
Elongated embryo with distinct notochord. |
u |
|
10:15 |
Enlarged embryo |
0.756±0.00094 |
The cephalic and caudal end become prominent and visible |
v |
|
11:15 |
Kupffer’s vesicle formed |
0.797±0.001 |
Observation of an oval area at the base of the caudal region forming kupffer’s vesicle |
w |
|
12:30 |
Optic vesicle developed |
0.898±0.0015 |
The tail becomes separated and optic vesicle fully built. |
x |
|
15:30 |
Rapid twisting movement |
0.902±0.0025 |
Yolk mass segregated into yolk bulb and caudal region become highly active. |
y |
|
17:30 |
Fully active embryo |
0.999±0.0015 |
The egg membrane become decomposed and lost its shape. |
z |
|
18:30 |
Just before hatching |
1.00±0.0015 |
Embryo with the prominent eye and maxillary barbells. |
i |
|
21:00 |
Hatching |
1.114±0.0026 |
Hatching of embryos start. |
ii |
Blastodisc stage was recognized on the presence of brownish spot on the animal pole in fertilized eggs which appeared within 00:15 h of post-fertilization. The cytoplasm of the egg cell was entirely segregated from the yolk and appeared as a distinct cap or blast disc at the animal (Figure 2c). The average diameter of this stage was 0.48±0.00 mm. At the initial of cleavage stage, the blastodisc was separated into doubled cells within 00:40 h after fertilization (Figure 2d) and the mean diameter was still 0.48±0.00 mm. The second cleavage (4-cell stage) appeared approximately 00:55h after fertilization and average diameter was 0.48±0.00 mm (Figure 2e). Third cleavage stage generated 8 blastomere cells within 1:15 h of fertilization and diameter was recorded as 0.48±0.00 mm (Figure 2f). Construction of blastomere cells increased as time progressed and attained 16 cells, 32 cells and multi cells within 1:40 h, 2:00 h and 2:30 h respectively after fertilization and mean diameter were observed 0.48±0.00, 0.49±0.00, and 0.49±0.00 mm, respectively (Figure 2g, h and i-n).
The morula stage characterized irregular and continuous cell divisions with numerous reduced sized cells. The cells were highly compacted, and thus, cell number counting was next to impossible. As blastomere cells were reduced in extent and the morula stage was attained within 3:40 h after fertilization and average diameter was recorded 0.49±0.00 mm (Figure 2o). Blastula stage of M. gulio appeared at about 4:20 h of post-fertilization. The blastula phase was typified by squeezing and compaction of the blast dermal cells (Figure 2p). The blastomeres cells were completely lost their distinctiveness and transferred in both the periphery of animal pole occupying 30% area over the yolk and mean diameter was recorded as 0.65±0.00 mm. In this study, blastomeres started to invade the yolk by proliferating across the yolk in shape of a thin sheet, then 5.00 h after fertilization the gastrulation ring was appeared. At this stage, the blastoderm further spread in both the side which covered about 60-70% area (Figure 2q) and poduce a structure of germinal ring (Figure 2r). The “C” shape embryonic stage appeared at gastrula stage (Figure 2r) and average diameter was observed 0.67±0.00 mm.
The earliest sign of the embryo was visible, and showed beginnings of optic cups after 10:15 h of fertilization (Figure 2v), and average diameter was observed 0.756±0.00094 mm. In about 11:15 h from fertilization, the somites started to appear and the head and tail end was differentiated (Figure 2w). At that moment, the terminal part of anterior and posterior region looked somewhat round and somites number increased gradually (Figure 2x-z). In this stage, the average diameter was detected 0.898±0.0015 to 0.999±0.0015 mm. At the 15:30 h from fertilization, both tail, and head end were evidently noticeable. The embryo turns out to be lengthened circling around the yolk sphere. The cardiac beating was activated perfectly. Hollow pole of notochord surfaced and some of the embryos shown slight winding movement and the blood circulation could be observed (Figure 2i). After 18:30 h of fertilization, end point of both head and tail region were distinctly recognizable, embryo became elongated and it encircled the sphere of yolk as well as active heart beating continued.Hollow poleof notochord emerged and a few of the embryos showed unusual faint bending movement and the blood circulation was observable (Figure 2i).
The embryonic development progresses continued, and its movement became stronger gradually, and ultimately the embryo was able to be out of the encompassed membrane. Just before the hatching, embryo pounded speedily by its tail and it’s became free, whereas yolk sac was attached to the head portion of embryo. The larvae started hatching at 21:00 h of fertilization. After hatching the larvae was straight and can be differentiated by head, trunk as well as tail and its length was 1.114±0.0026 mm (Figure 2ii).
The size of twenty-four-h larvae calculated typically 3.13±0.054 mm. Three sets of barbells arrived, and the maxillary couple was noticeably distinguished. The average lengths of 24 hs old larvae were 3.13±0.054 mm and three pair of barbells was emerged and among them, maxillary pair was distinctly visible. At this stage, total numbers of myotomes were 35-40 and mouth and anus of the larvae had started to open as well. Lateral line of the larvae was discernible while the swim bladder as well as nostril also formed already (Figure 2iii-vii).
The latency period with S-GnRHa of M. gulio was begin at 7-8 h of hormone treatment and completed at 12 h of post hormone treatment. This finding was found to be little different to some previous research conducted with other hormone at different dosages as well as different environmental conditions (Alam et al., 2006; Begum et al., 2009). The duration of latency period were counted as 7–8 h for Ompok bimaculatus (Raizada et al., 2013), 14- to 17h (Sahoo et al., 2005) and 10-12 h (Müller et al., 2020) for Clarias batrachus with carp pituitary (CPE) hormonal administration. Catfishes are seemed to have large gap in their latency period with synthetic hormonal injection (Sahoo et al., 2005, 2008). The rate of fertilization have been accounted as 77.5 % with 0.6mg/Kg ovaprim treatment in Mystus dibrugarensis (Biswas, 2014), 77.33% with 5 μg g−1 of Human Chorionic gonadotropins in M. gulio (Kumar et al., 2021). In current study, fertilization rates of T1, T2 and T3 groups were 74.33%, 83. 89% and 78.56%, respectively and this finding is coherent with the research of Alam et al. (2006) who found around 85% fertilization rate of M. gulio females with ovaprim induction. Some other studies in catfishes also found similar observation as 75% in Pseudopimelodus charus, and 80% in Rhamdia quelen with synthetic hormone induction (Sampaio and Sato, 2006). The highest and the lowest hatching rate were in present research recorded as 85.11% and 72.33% at the dose of 0.5 ml kg-1 bw and 0.25 ml kg-1bw of S-GnRHa hormone respectively. These figures are also supportive to previous study by Kumar et al. (2021) who observe hatching rates 71% for same species whereas Srivastava et al. (2012) found 55-60% hatching rate for female Clarius batachus with ovaprim treatment. The current study showed that the fertilized egg cell of M. gulio were strongly glued due to the presence of sticky jelly on the egg surface and brownish in color which aligned with the annotation from Arockiaraj et al. (2003), Puvaneswari et al. (2009), and Ferosekhan et al. (2015) for catfish ovum. The average thickness of the fertilized egg of M. gulio was noted as 0.486 mm in the present study. The average thickness of fertilized eggs of M. cavasious was 0.50 mm (Rahman et al., 2004), 3.1 mm for Pimelodus maculatus (Sato et al., 2003), 3.6 mm for Zungaro jahu (Nogueira et al., 2012) and 1.0 to 1.3 mm for Ompok pabo (Sarma et al., 2012). Therefore, egg diameter size is seeming to be species specific and varied among fish to fish.
A prominent reddish spot-on fertilized eggs of M. gulio had been reported by several researchers on different fish species (Rahman et al.,2004; Ferosekhan et al., 2015). Rahman et al. (2004) reported 0.61 mm in diameter of blastodisc in M. cavacius which was lower than that of the present study as 0.48 mm. The cleavage stage of M. cavasius had attained after 00:45 h to 2:00 h (Rahman et al., 2004) while this stage in P. pangasius occurred after 1:07-2:32 h (Ferosekhan et al., 2015), 00:24 h to 1.1 h in Botia lohachata (Dey and Barat, 2015). The size of different cleavage stages were varied between 0.48-0.490mm in diameter at present study and this might be happened because of the variation of species and difference in environmental condition at the hatchery. Morula phase in the present study was emerged by 3:40 h post fertilization whereas same stage found at 3:40 h post fertilization in M. cavasius (Rahman et al., 2004), at 03:43 h post fertilization in Pangasius pangasius (Ferosekhan et al., 2015) and at 2h post fertilization in Botia lohachata (Dey and Barat, 2015). This difference may be because of environmental factors as well as different species. Blastula stage of M. gulio was appeared at 4:20 h post fertilization, with a mean diameter 0.65 mm in current research, whereas the same stage found at 3-3:30 h post fertilization in O. pabo (Chakrabarti et al., 2009; Sarma et al., 2012), 05:12 h post fertilization in P. pangasius (Ferosekhan et al., 2015) and at 1.11-3.05 h post fertilization in B. lohachata (Dey and Barat, 2015). The “C” shape embryo stages have been reported at 07:27 h after fertilization in P. pangasius (Ferosekhan et al., 2015), 3.05 to 6.33 h after fertilization in B. lohachata (Dey and Barat, 2015), 5:00 h after fertilization in M. cavasius (Rahman et al., 2004) and in between 4 hs for Giant catfish Heterobranchus bidorsalis (Olaniyi and Omitogun, 2014). Dey and Barat (2015) reported that somites formed at 6.46 to 14.27 h post-fertilization, however, this stage appeared at 9:00-10:00 h in O. pabo (Sarma et al., 2012), at 11:00 h in P. sutchi (Islam, 2005). The current research showed that at 11:15 h postfertilization, the somites started to appear and the head and tail end became differentiate. After 15:30 h of fertilization, end of both head and tail of embryo appeared to be distinctly recognizable. Embryo of M. gulio became elongated and it encircled the sphere of yolk as well as heart was actively beating. Ferosekhan et al. (2015) recorded this stage at 21:30 h post fertilization in P. pangasius, Rahman et al. (2004) at 15:00 h post fertilization in M. cavasius, Khan and Mollah (1998) at 18-22 h post fertilization in Clarias gariepinus and Kohinoor et al. (1997) at 14 h post fertilization in O. pabo. The morula, blastula, gastrula and neurula and organogenesis stages were found to be completed at 1:30, 3:00, 5:30, 7:30 and 17:15 h post-spawning respectively for induced breeding of Mystus gulio using human chorionic gonadotropin (Kumar et al., 2018).
The current study appealed that the average hatching period in M. gulio accounted as 21 h after fertilization in between 29 to 31°C water temperature, whereas Alam et al. (2006) reported the hatching period of M. gulio was 18 to 20 h after fertilization, temperature range was 30.2 to 32.8°C and Rahman et al. (2004) stated the hatching period at 19 to 21 h after fertilization in M. cavasius at a water temperature 27-29.5°C. Ferosekhan et al. (2015) found the hatching period at 25:27 h after fertilization in P. pangasius with a temperature range from 27.5 to 28.5°C. Incubation temperature and time of egg hatching was inversely correlated. Rahman et al. (2004) observed the barbell partially appeared of M. cavasius in 06 h old larvae and it took to 12 h for a clear appearance. The average length of one-day-old larvae was measured 3.13 mm and clearly visible 3 pairs of barbells appeared in current work. However, this is supportive to previous finding of the length of one-day-old larvae of M. gulio was 3.39-3.98 mm (Kumar et al., 2021; Rahman et al., 2004; Alam et al., 2006) and 2.0 mm in Rita rita (Mollah et al., 2011). Slight variation may be due to the quality and environments of brood stock.
Conclusions and Recommendations
Findings of present study revealed that M. gulio can be induced successfully by using 0.5 ml kg-1 dose of S-GnRHa hormone under captivity condition in freshwater. Further research is recommended for development and extension of mass breeding initiatives in freshwater condition with commercial aquaculture practices of this species.
Acknowledgements
Authors express earnest appreciation to Alalpur Hatchery and Fisheries Ltd., Mymensingh, Fisheries Biology and Genetics laboratory, Bangladesh Agricultural University, Mymensingh, for their logistic and technical support and Fish Biology and Genetics department, Sylhet Agricultural University for the financial support to conduct this study. All author declares no conflict of interests.
Conflict of interest
The authors have declared no conflict of interest.
References
Alam, M.J., Begum, M., Islam, M.A. and Pal, H.K., 2006. Spawning behaviour and induced breeding of an estuarine catfish, Mystus gulio (Ham.). Bangladesh J. Fish. Res., 10: 101-109.
Arockiaraj, A.J., Haniffa, M.A., Seetharaman, S. and Sing, S.P., 2003. Early development of a threatened freshwater catfish Mystus montanus (Jerdon). Act. Zool. Taiw., 14: 23–32.
Bailung, B. and Biswas, S.P., 2014. Successful Induced Breeding of a Bagrid Catfish. Mystus dibrugarensis in Captive Condition. https://doi.org/10.4172/2155-9546.1000281
Begum, M., Pal, H.K., Islam, M.A. and Alam, M.J., 2009. Embryonic and larval development of Mystus gulio (Ham.) Bangladesh J. Fish Res., 13: 169-177.
Biswas, S.P.B.B., 2014. Successful induced breeding of a bagrid catfish, Mystus dibrugarensis in captive condition. J. Aquact. Res. Dev., 5: 7–9. https://doi.org/10.4172/2155-9546.1000281
Bromage, N.R., Porter, M.J.R. and Randall, C.F., 2001.The environmental regulation of maturation in farmed finfish with special reference to the role of photoperiod and melatonin. Aquatic, 197: 63-98. https://doi.org/10.1016/B978-0-444-50913-0.50008-4
Chakrabarti, P.P., Chakrabarty, N.M. and Mondal, S.C., 2009. Breeding and seed production of butter catfish, Ompok pabda (Siluridae) at Kalyani Centre of CIFA, India. Aquat. Asia, 14: 33-35.
Dey, A. and Barat, S., 2015. Standardization of proper dose of synthetic hormone for induced breeding of three loaches of genus Botia. Int. J. Mult. Res. Dev., 2: 507-509.
Dhara, K. and Saha, N.C., 2013. Controlled breeding of asian catfish Clarias batrachus using pituitary gland extracts and ovaprim at different temperatures, latency periods and their early development. J. Aquat. Res. Dev., 4: 1-9. https://doi.org/10.4172/2155-9546.1000186
Ferosekhan, S., Sahoo, S.K., Giri, S.S., Saha, A. and Paramanik, M., 2015. Embryonic and larval development of yellow tail catfish, Pangasius pangasius. J. Aquat. Res. Dev., 6: 341-343. https://doi.org/10.4172/2155-9546.1000343
Gupta, S., 2014. Morphology, growth pattern, feeding and reproductive biology of Mystus gulio (Hamilton-Buchanan, 1822) (Siluriformes: Bagridae). Intl. J. Aquat. Biol., 2: 201–205.
Hossain, M.M., Hassan, M.M., Ahamed, S., Mostafiz, M., Akter, T., Islam, M.M. and Kabir, M.A., 2014. In relation to climate change adaptation: culture potential of long whiskers catfish, Mystus gulio in greater Noakhali region, Bangladesh. SAU Res. Prog. Rep., 2: 57-63.
Islam, A., 2005. Embryonic and larval development of Thai Pangas (Pangasius sutchi Fowler, 1937). Dev. Grow. Diff., 47: 1-6. https://doi.org/10.1111/j.1440-169x.2004.00773.x
Khan, M.M.R. and Mollah, M.F.A., 1998. Embryonic and larval development of African catfish, Clarias gariepinus (Burchell). Bangladesh J. Fish., 21: 91-97.
Kimmel, C.B., Ballard, W.W., Kimmel, S.R., Ullmann, B. and Schilling, T.F., 1995. Stages of embryonic development of the Zebrafish. Dev. Dyn., 203: 253–310. https://doi.org/10.1002/aja.1002030302
Kohinoor, A.H.M., Akhteruzzaman, M., Hussain, M.G. and Shah, M.S., 1991. Observations on the induced breeding of koi fish, Anabas testudineus (Bloch) in Bangladesh. Bangladesh J. Fish., 14: 73-77.
Kohinoor, A.H.M., Islam, M.S., Jahan, D.A. and Hussain, M.G., 1997. Embryonic and larval development of Ompok pabda (Hamilton). Prog. Agric., 8: 37-41.
Korzelecka-Orkisz, A., Smaruj, I., Pawlos, D., Robakowski, P., Tanski, A., Szulc, J. and Formicki, K., 2010. Embryogenesis of the stinging catfish, Heteropneustes follilis (Actinopterygii: Siluriformes: Heteropneustidae). Acta Ich. Pisca., 40: 187–197. https://doi.org/10.3750/AIP2010.40.2.12
Kumar, P., Biswas, G., Ghoshal, T.K., Kailasam, M. and Vijayan, K.K., 2018. Embryonic and larval developments of brackish water catfish, Mystus gulio (Hamilton and Buchanan, 1822) induced with human chorionic gonadotropin and consequent larval rearing. Aquact. Res., 49: 2466–2476. https://doi.org/10.1111/are.13706
Kumar, P., Kailasam, M., Biswas, G., Christina, L. and Ghoshal, T.K., 2021. Effect of different inducing hormones, sex ratio and oocyte diameter on breeding performance of brackish water catfish, Mystus gulio. Aquaculture, 530: 735821. https://doi.org/10.1016/j.aquaculture.2020.735821
Legendrea, M., Jacques S., Jojo S. and Anang, H.K., 2000. Ovulation rate, latency period and ova viability after GnRH- or HCG-induced breeding in the Asian catfish Pangasius hypophthalmus (Siluriformes, Pangasiidae). Aquat. Liv. Resour. 13: 145−151. https://doi.org/10.1016/S0990-7440(00)00148-0
Mijkherjee, M., Praharaj, A. and Das, S., 2002. Conservation of endangered fish stocks through artificial propagation and larval rearing technique in West Bengal, India. Aquat. Asia, 7: 8-11.
Mollah, F.A.M., Taslima, K., Rashid, H., Hossain, Z., Nasif, S.M. and Khan, R.K.M., 2011. Embryonic and larval development of critically endangered riverine catfish Rita rita. EurAsian J. Biosci., 118: 110–118. https://doi.org/10.5053/ejobios.2011.5.0.13
Mollah, M.F.A., Amin, M.R., Sarowar, M.N. and Muhammadulla, M., 2008. Induced breeding of the riverine catfish Rita rita. J. Bang. Agric. Univ., 6: 361–366. https://doi.org/10.3329/jbau.v6i2.4835
Müller, T., Ács, É., Beliczky, G., Makk, J., Földi, A., Kucska, B., Horváth, L., Ittzés, Á., Hegyi, Á., Szabó, T., Urbányi, B., Quyen, N.N., Orbán, L. and Havasi, M., 2020. New observations about the fertilisation capacity and latency time of sperm inseminated into the ovary of african catfish (Clarias gariepinus), an oviparous modelfish. Aquaculture, 522: 735109. https://doi.org/10.1016/j.aquaculture.2020.735109
Nakaghi, L.S.O., Neumann, E., Faustino, F., Mendes, J.M.R. and Braga, F.M., 2014. Moments of induced spawning and embryonic development of Bryconama zonicus (Teleostei, Characidae). Zygote, 22: 549-557. https://doi.org/10.1017/S0967199413000130
Nogueira, L.B., Azevedo, P.G., Canelhas, M.R., Bedore, A.G., Lopes, J.M. and Godinho, H.P., 2012. Induced spawning and early ontogeny in hatchery-reared catfish Zungaro jahu (Siluriformes: Pimelodidae). Neot. Ich., 10: 89-98. https://doi.org/10.1590/S1679-62252012000100009
Olaniyi, W.A. and Omitogun, O.G., 2013. Stages in the early and larval development of the African catfish Clarias gariepinus (Teleostei, Clariidae). Zygote, 22: 314–330. https://doi.org/10.1017/S0967199413000063
Olaniyi, W.A. and Omitogun, O.G., 2014. Embryonic and larval developmental stages of African giant catfish Heterobranchus bidorsalis (Geoffroy Saint Hilaire, 1809) (Teleostei, Clariidae). Springerplus, 3: 677. https://doi.org/10.1186/2193-1801-3-677
Puvaneswari, S., Marimuthu, K., Karuppasamy, R. and Haniffa, M.A., 2009. Early embryonic and larval development of Indian catfish, Heteropneustes fossilis. EurAsian J. Biol., 96: 84-96. https://doi.org/10.5053/ejobios.2009.3.0.12
Rahman, M.R., Rahman, M.A., Khan, M.N. and Hussain, M.G., 2004. Observation on the embryonic and larval development of silurid catfish, gulsha (Mystus cavasius Ham.). Pak. J. Biol. Sci., 7: 1070-1075. https://doi.org/10.3923/pjbs.2004.1070.1075
Raizada, S., Lal, K.K., Sarkar, U.K., Varshney, P.K., Sahu, V., Yadav, K.C., Agnihotri, P., Awasthi, A. and Jena, J.K., 2013. Captive breeding and embryonic development of butter catfish (Ompok bimaculatus, bloch 1794), a threatened fish of indian sub-continent in northern India. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci., 83: 333–339. https://doi.org/10.1007/s40011-013-0156-z
Sahoo, S.K., Giri, S.S., Chandra, S. and Mohapatra, B.C., 2008. Evaluation of breeding performance of Asian catfish Clarias batrachus at different dose of HCG and latency period combinations. Turk. J. Fis. Aquact. Sci., 8: 249- 251.
Sahoo, S.K., Giri, S.S. and Sahu, A.K., 2005. Induced spawning of Asian catfish, Clarias batrachus (Linn.): effect of various latency periods and SGnRHa and domperidone doses on spawning performance and egg quality. Aquat. Res., 36: 1273-1278. https://doi.org/10.1111/j.1365-2109.2005.01317.x
Sakthivel, M., Deivasigamani, B., Alagappan, K.M., Kumaran, S., Balamurugan, S. and Rajasekar, T., 2013. Seasonal changes in selected immune response of Mystus gulio and Mystus vittatus. J. Environ. Biol., 34: 37–42.
Sampaio, E.V. and Sato, Y., 2006. Biologia reprodutiva e desova induzida de duas espécies de bagres (Osteichthyes: Siluriformes) da bacia do rio São Francisco. Acta Sci. Biol. Sci., 28: 263-268. https://doi.org/10.4025/actascibiolsci.v28i3.227
Sarker, P., Pal, H.K., Rahman, M.M. and Rahman, M.M., 2002. Observation on the fecundity and gonado-somatic index of Mystus gulio in Brackishwaters of Bangladesh. J. Biol. Sci., 2: 235–237. https://doi.org/10.3923/jbs.2002.235.237
Sarma, D., Dutta, A.U.C. and Das, J.G., 2012. Early embryonic and development of Ompok pabo with notes on its nursery rearing. Eur. J. Exp. Biol., 2: 253-260.
Sato, Y., Fenerich-Verani, N. and Godinho, H.P., 2003. Reprodução induzida de peixes da bacia do São Francisco. In: H.P. Godinho and A.L. Godinho (Orgs.). Águas, peixes e pescadores do São Francisco das Minas Gerais. Belo Horizonte, Editora PUC Minas. pp. 275-289.
Seethal, L.S., Jaya, D.S. and Sherly, W.E., 2016. Reproductive biology of estuarine catfish, Mystus gulio (Hamilton-Buchanan). Int. J. Sci. Res., 5: 2015-2017. https://www.ijsr.net/archive/v5i11/v5i11.php
Srivastava, P.P.S., Raizada, R., Dayal, S., Chowdhury, S., Lakra, W.S., Yadav, A.K. and Gupta, J., 2012. Breeding and larval rearing of asian catfish, Clarias batrachus (Linnaeus, 1758) on live and artificial feed. J. Aqu. Res. Dev., 3: 3-6. https://doi.org/10.4172/2155-9546.1000134
Unuma, T., Shigenori, K., Hideki, T., Hirohiko, K., Kazuharu, N. and Hiromi, O., 2004. Determination of the rates of fertilization, hatching and larval survival in the Japanese eel, Anguilla japonica, using tissue culture microplates. Aquat., 241: 345–356. https://doi.org/10.1016/j.aquaculture.2004.08.005
Wahab, M.A., Thilsted, S.H. and Hoq, M.E., 2003. Small indigenous species of fish in Bangladesh. Proceeding of BAU-ENRECA/DANIDA Workshop on Potentials of Small Indigenous Species of Fish (SIS) in Aquaculture and Rice-field Stocking for Improved Food and Nutrition Security in Bangladesh, 30-31 October 2002, BAU, Mymensingh, Bangladesh and ENRECA/DANIDA., pp. 166.
To share on other social networks, click on any share button. What are these?







