Nutrient Profile and In Vitro Digestibility of Thirty Indonesian Soybean Genotypes Grown at Two Different Soil pH for Selection as Ruminant Feed
Research Article
Nutrient Profile and In Vitro Digestibility of Thirty Indonesian Soybean Genotypes Grown at Two Different Soil pH for Selection as Ruminant Feed
Taufiq Bachtiar1, Muftia Hanani2, Anisiyah2, Winda Puspitasari2, Wahidin Teguh Sasongko3, Teguh Wahyono4*
1Research Center for Environmental and Clean Technology, National Research and Innovation Agency (BRIN), West Java, 40135, Indonesia; 2Research Center for Radiation Process Technology, BRIN, South Jakarta 12440, Indonesia; 3Research Center for Animal Husbandry, BRIN, Bogor 16915, Indonesia; 4Research Center for Food Technology and Processing, BRIN, Gunungkidul 55861, Indonesia.
Abstract | This work investigates the effects of different soil pH and different genotypes on the nutrient profiles and in vitro digestibility of soybeans. Thirty soybean genotypes were evaluated for nutrient composition, fiber content, and in vitro digestibility after cultivation in pH 4.0 and 5.4 soils. The experiment was arranged factorially in a completely randomized block design. The experimental factors were soybean genotype and soil pH. Results showed significant (p < 0.001) differences in fiber content, non-fiber carbohydrate content, and in vitro dry matter digestibility (IVDMD) parameters among studied genotypes. There were significant differences (p < 0.05) between the two soil pH conditions for ash, organic matter, ether extract, neutral detergent fiber, acid detergent fiber, hemicellulose, and non-fiber carbohydrate. Organic matter, crude protein, ether extract, and non-fiber carbohydrate content in soil with pH 5.4 ranged between 91.94–94.67, 27.78–38.20, 13.99–22.78, and 16.31–33.82% DM, respectively. Meanwhile, in soil pH of 4.0, ranges were 92.24–94.80, 23.07–39.99, 14.88–22.82, and 12.69–38.20% DM, respectively. The Deja 2 genotype had the highest (p < 0.01) IVDMD value (84.07 %) in pH 5.4 soil. However, in pH 4.0 soil, the highest (p < 0.01) IVDMD was found in the Burangrang genotype (83.32%). Our results showed that IVDMD in soil with pH 4.0 was weakly correlated (p > 0.05) to NDF and ADF (R2 = -0.126 and R2 = -0.144, respectively). Despite the differences in soil pH affecting nutrient and fiber composition in soybeans, IVDMD value was not found to be significantly different. Due to its high and stable crude protein and IVDMD production, Gamasugen 2 was identified as having potential for development in the two different soil pH conditions.
Keywords | Acid soil, In vitro digestibility, Nutrient, Genotypes, Soybean
Received | May 11, 2022; Accepted | July 01, 2022; Published | August 01, 2022
*Correspondence | Teguh Wahyono, Research Center for Food Technology and Processing, BRIN, Gunungkidul 55861, Indonesia; Email: [email protected]
Citation | Bachtiar T, Hanani M, Anisiyah, Puspitasari W, Sasongko WT, Wahyono T (2022). Nutrient profile and in vitro digestibility of thirty Indonesian soybean genotypes grown at two different soil pH for selection as ruminant feed. Adv. Anim. Vet. Sci. 10(8):1818-1826.
DOI | https://dx.doi.org/10.17582/journal.aavs/2022/10.8.1818.1826
ISSN (Online) | 2307-8316
Copyright: 2022 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
INTRODUCTION
Ruminants are the main suppliers of animal protein for humans, in the form of dairy and meat products. Livestock production plays a primary role in providing high-nutrient foodstuffs for many people, particularly in developed countries (Moorby and Fraser, 2021). High-quality feed ingredients are necessary to improve livestock productivity. A more appropriate way of converting energy from feedstuffs to livestock is to take into account the proportion of feed drawn from potential human food sources (Broderick, 2018). One source that has high development potential as a feed ingredient is the soybean (Glycine max L.). Besides being a source of high-quality vegetable oil for humans, soybeans are also a high-quality vegetable protein source for animals around the world (Dei, 2011). In recent years, soybean meal has been a part of rations used for high-producing beef and dairy cattle in developed countries (Jeong et al., 2015). Soybeans are an excellent source of protein, fat, and vitamin E for ruminants (Chouinard et al., 1997; Lee et al., 2007). As protein sources, soybeans are well degraded in the rumen, producing ammonia, amino acids, and peptides for microbial protein synthesis (Jeong et al., 2015). Soybean meal (SBM), as a main by-product of soybeans, has been widely used as protein supplement due to its rich content of essential amino acids such as threonine, tryptophan, and lysine (Jayanegara et al., 2017). Primary soybean products for animal feeding are full-fat soybeans, SBM, soybean oil, and soybean protein concentrate (Dei, 2011). Full-fat soybeans are widely used as part of ruminant diets because of their high energy and protein content.
As a developing country, Indonesia needs a large supply of soybeans to meet demand in the livestock sector, but remains dependent on other countries to supply most of its soybean needs. The USA and Canada were the largest soybean exporters to Indonesia in 2020, providing 2,238,480 and 229,644 tons, respectively (Statistics Indonesia, 2022). Indonesia has many genotypes that need to be optimized in moving away from reliance on imported soybean supplies. The challenge of soybean cultivation in Indonesia is the variation in agroecosystems, especially in some marginal areas. Marginal land in Indonesia consists of acid, saline, dry, and peat areas that have physical and chemical properties unfavorable for plant growth (Puspitasari et al., 2021). Opportunities for soybean development in areas of acid soil are extensive both because of soybean’s ability to grow in acid conditions and because of the large area of acid land available (Kuswantoro, 2016). In Indonesia, wet conditions and intensive precipitation cause alkaline washing, leading to approximately 70% of the country’s land being acidic (Sumiahadi and Acar, 2019). The use of land for growing feedstuffs must however be effectively considered to avoid conflicts with land use for bioenergy production and human food crops (Moorby and Fraser, 2021).
The chemical composition and nutrient value of soybean products depend on seed genotype, environmental conditions, harvesting age, and storage of beans (Ibáñez et al., 2020). For soybean development, soil pH, temperature and NO3-N concentration under minimum tillage are favorable for nodulation supporting higher microbial activities (Farhangi-Abriz et al., 2021). Problems affecting crops growing on acid soils include proton rhizotoxicity (low pH), nutrient deficiency (P, K, and Ca), and metal toxicity (Al and Mg) (Liang et al., 2013). In light of these problems, an initial screening of Indonesian soybean genotypes is needed to identify varieties that deliver good nutrient composition when cultivated in acid soils. Puspitasari et al. (2021) reported that soybean genetic diversity adaptive to acid soils remains limited. The Indonesian Legumes and Tuber Crops Research Institute (ILETRI) has developed soybean genotypes that are tolerant of acid soils, namely Tanggamus and Demas 1. Furthermore, there are several genotypes obtained from radiation mutation breeding that need to be tested for nutrient quality. To our knowledge, no study so far has attempted to investigate the influence of different soil pH on soybean nutrient characteristics, especially from the point of view of feed. Therefore, the objectives of this research are to measure the effects of different soil pH and genotypes on the nutrient profiles and in vitro digestibility of soybeans.
MATERIALS AND METHODS
Experimental site and soybean genotypes
The research was conducted from February to June 2021 in the glasshouse laboratory of the National Research and Innovation Agency of Indonesia, Pasar Jumat Area, Jakarta. The site is located at 6o17’ S and 106o46’ E and at 38 m above sea level. The genetic materials used in the experiments were viable seeds of thirty soybean genotypes. The varieties were provided by the ILETRI Ministry of Agriculture, Indonesian Center for Agricultural Biotechnology and Genetic Resource Research and Development (ICABIOGRAD) Ministry of Agriculture, and the Center for Isotope and Radiation Application (CIRA) National Research and Innovation Agency of Indonesia (Table 1).
Experimental soil preparation, cultivation, and sample collection
Soil samples at depths of 0–20 cm of the surface layer were collected from Pasar Jumat Area, Jakarta, and Jasinga Bogor, West Java. The physicochemical characteristics of the soil are described in Table 2. The soil was air dried and milled to pass through a 10-mesh filter. Pot experiments consisting of 5 kg of soil in 30 x 30 cm polybags were carried out in a greenhouse from October 2020 to April 2021. The pots were arranged factorially in a completely randomized block design. The factors were soybean genotypes and soil pH. Two soybean seeds were sown in each polybag at 3 cm depth. Plants were allowed to grow under normal environmental conditions (dark at night and sunlit during the day) and irrigated twice daily. Nitrogen fertilizer was applied using urea (46.72% N) on days 7 and 35 after planting at about 10 ppm per polybag. Potassium and phosphorus fertilizers of 50 ppm SP-36 (33.59% P2O5) and 37.5 ppm KCl (55.06% K2O) were applied at time of sowing. During plant growth, seedlings were thinned so only one plant remained in each polybag. Soybean seeds were harvested according to the ideal harvesting time for each genotype, approximately 68–92 days after planting (Table 1). The seeds were sorted and cleaned manually to remove foreign materials, placed into individual paper bags, and dried at 60oC for 72 h until a constant weight was achieved. Dry seeds were then ground and passed through a 1 mm sieve and kept in a refrigerator maintained at 4oC for proximate, fiber, carbohydrate, and in vitro digestibility analysis.
Table 1: Information of thirty Indonesian soybean genotypes with regards growth duration, production, and release year.
|
Genotype |
Growth duration (days) |
Release year |
Production (ton/ha) |
Source |
|
Anjasmoro |
82-92 |
2001 |
2,25 |
ILETRI |
|
Argo mulyo |
80-82 |
1998 |
2.0 |
ILETRI |
|
Biosoy 1 |
83 |
2018 |
2.71 |
ICABIOGRAD |
|
Biosoy 2 |
84 |
2018 |
2.63 |
ICABIOGRAD |
|
Burangrang |
80-82 |
1999 |
2.5 |
ILETRI |
|
Dega 1 |
71 |
2016 |
2.78 |
ILETRI |
|
Deja 1 |
79 |
2017 |
2.87 |
ILETRI |
|
Deja 2 |
80 |
2017 |
2.75 |
ILETRI |
|
Demas 1 |
84 |
2014 |
2.5 |
ILETRI |
|
Dena 1 |
78 |
2014 |
2.9 |
ILETRI |
|
Dena 2 |
81 |
2014 |
2.8 |
ILETRI |
|
Derap 1 |
76 |
2018 |
2.82 |
ILETRI |
|
Dering 1 |
81 |
2012 |
2.8 |
ILETRI |
|
Detam 3 |
75 |
2013 |
2.9 |
ILETRI |
|
Detam 4 |
76 |
2013 |
2.5 |
ILETRI |
|
Detap 1 |
78 |
2017 |
2.7 |
ILETRI |
|
Devon 1 |
83 |
2015 |
2.75 |
ILETRI |
|
Devon 2 |
77 |
2017 |
2.67 |
ILETRI |
|
Gepak Kuning |
73 |
2008 |
2.22 |
ILETRI |
|
Gamasugen 2 |
68 |
2013 |
2.4 |
CIRA |
|
Grobogan |
76 |
2008 |
2.77 |
ILETRI |
|
Kemuning 1 |
79-80 |
2019 |
3.5 |
CIRA |
|
Meratus |
73-77 |
1998 |
1.4 |
CIRA |
|
Mitani |
82-90 |
2008 |
3.2 |
CIRA |
|
Muria |
83-88 |
1987 |
1.8 |
CIRA |
|
Mutiara 2 |
87 |
2014 |
2.4 |
CIRA |
|
Mutiara 3 |
84 |
2014 |
2.4 |
CIRA |
|
Panderman |
85 |
2003 |
2.11 |
ILETRI |
|
Rajabasa |
- |
2004 |
2.05 |
CIRA |
|
Tengger |
73-79 |
1991 |
1.4 |
CIRA |
Indonesian Legumes and Tuber Crops Research Institute (ILETRI). Indonesian Center for Agricultural Biotechnology and Genetic Resource Research and Development (ICABIOGRAD). Center for Isotope and Radiation Application (CIRA). Source: Mastur (2018); CIRA (2020); ILETRI (2022a); ILETRI (2022b).
Table 2: Physicochemical profile of soil for soybean cultivation.
|
Soil properties |
Jasinga |
Criteria |
Pasar Jumat |
Criteria |
|
Type |
Ultisol |
- |
Latosol |
- |
|
pH |
4.0 |
Very acidic |
5.4 |
Acidic |
|
N total |
0.02% |
Very low |
0.19% |
Low |
|
P total |
111.1 ppm |
Very high |
78.1 ppm |
Very high |
|
C-organic |
1.73% |
Low |
1.51% |
Low |
|
Al |
11.52 cmol/kg |
Very high |
1.22 cmol/kg |
High |
Analysis of proximate, fiber, and carbohydrate compositions
The proximate components of ash, organic matter (OM), crude protein (CP) and ether extract (EE) were determined following the official methods of analysis of AOAC (2005). Neutral detergent fiber (NDF) and acid detergent fiber (ADF) content were measured using Van Soest et al. (1991) methods. Hemicellulose and non-fiber carbohydrate (NFC) were calculated as follows:
Hemicellulose (%) = NDF (%) - ADF (%)
NFC(%) = OM (%) - CP (%) - NDF (%) - EE (%)
(Kondo et al. 2015; Wahyono et al. 2019).
Determination of in vitro digestibility
To determine in vitro dry matter digestibility (IVDMD), this experiment was carried out in accordance with Animal Ethics Committee of Research CIRA Technology (001/KEPPHP-BATAN/X/2021). IVDMD was determined using the in vitro Ankom Daisy technique (Ankom Technology Corp, Fairport, New York, USA) (Ayaşan et al., 2020). Rumen fluids were collected from three local cattle (approximate live weight 280 kg) slaughtered at a local abattoir in South Tangerang, Banten, Indonesia. Buffer solutions were prepared according to Ankom Daisy procedure. Approximately 450 g samples (DM basis) were inserted into filter bags (F57 Ankom, Ankom Technology Corp, NY, USA) and placed into digestion jars (24 samples per jar). One blank filter bag was also placed in each jar for correction factor calculation. Buffer solutions (1200 ml) and rumen liquor (400 ml) were mixed into each jar and incubated for 48 h at 39oC. After incubation, the bags were rinsed with tap water and dried at 105oC for 12 h. IVDMD was determined by the following equation:
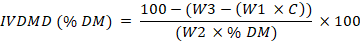
Where; IVDMD= in vitro dry matter digestibility; W1= filter bag weight; W2= sample weight; W3= sample weight after Daisy incubation; C1= correction factor of blank filter bag.
Statistical analysis
The experiment was arranged factorially in a completely randomized block design. The factors were thirty soybean genotypes and two soil pH conditions (4.0 and 5.4). All statistical analyses were conducted using IBM SPSS Statistics 25.0 (IBM, Armonk, NY, USA). Means were compared using one-way analysis of variance (ANOVA). Duncan’s multiple range test (DMRT) was also used to identify significant differences among means, at p < 0.05. A simple linear correlation analysis was performed to determine the relationship between parameters.
RESULTS and DISCUSSION
Characterization of thirty Indonesian soybean genotypes
Table 1 presents information for the thirty soybean genotypes studied. In the study, thirty well-known soybean genotypes from three research institutions were investigated. Nineteen genotypes were released from ILETRI, nine from CIRA, and two from ICABIOGRAD. The nine genotypes obtained from CIRA were soybeans that had been bred with radiation mutations. Average harvesting age ranged from 68 to 92 days. Muria is a mutant soybean genotype that was originally developed by CIRA in 1987. Furthermore, Kemuning is the newest genotype, released in 2019. Gamasugen 2 is the earliest ripening type (68 days harvesting age).
Soil properties
Table 2 shows the physicochemical profiles of the different soil samples used for the cultivation of soybeans in this study. Samples from Jasinga had lower pH and N total than Pasar Jumat soil. In contrast, Jasinga soil had higher Al concentration, P total, and C-organic than Pasar Jumat soil. The soil types of Pasar Jumat and Jasinga are latosol and ultisol, respectively.
Nutrient profile
The nutrient, fiber, and NFC compositions in soybeans grown on soils with different pHs are summarized in Table 3. Ash, OM, EE, NDF, ADF, hemicellulose, and NFC content showed significant (p < 0.001) differences among the genotypes. However, CP content did not significantly differ (p > 0.05). Except for CP, soil pH gave significant (p < 0.05) differences in all nutrient and fiber contents. There was a significant (p < 0.001) interaction between soil pH and genotypes for ash, OM, EE, NDF, ADF, hemicellulose, and NFC content. The OM, CP, EE, and NFC content in soil with pH 5.4 were in the ranges 91.94–94.67, 27.78–38.20, 13.99–22.78, and 16.31–33.82%, respectively. Meanwhile, in soil with pH 4, the ranges were 92.24–94.80, 23.07–39.99, 14.88–22.82, and 12.69-38.20 %, respectively. Soil at pH 5.4 showed higher average values for ash, NDF, ADF, and NFC compared with pH 4.0. Conversely, soil at pH 4.0 showed higher average values for OM, EE, and hemicellulose. The CP content in Gamasugen 2 tended to be higher than other genotypes, both at pH 5.4 and 4.0. The highest EE content in pH 5.4 and 4.0 was produced by Biosoy 2 and Detap 1 genotypes. Mitani had higher NFC content than other genotypes, both at pH 5.4 and 4.0.
In vitro digestibility
The IVDMD of soybeans is shown in Figure 1. The IVDMD differed significantly (p < 0.001) among the studied genotypes. The differences in soil pH did not affect the IVDMD value (p > 0.05). The interaction of soybean genotypes with soil pH was not significant (p > 0.05). The IVDMD value ranged from 70.85 to 84.07 and 68.83 to 83.32 for all studied genotypes in pHs 5.4 and 4.0, respectively. The Deja 2 genotype had the highest (p < 0.01) IVDMD value (84.07 %) in soil pH 5.4. However, in soil pH 4.0, the highest (p < 0.01) IVDMD was found in the Burangrang genotype (83.32 %).
Correlation
Figure 2 shows the correlations in nutrient composition, fiber content, and IVDMD, revealing differences in the relationships between parameters in the two different soil pH conditions. At soil pH 5.4, correlation between CP content and ADF was significantly negative (p < 0.05; R2= -0.440). CP and NFC content also had strong negative correlation (p < 0.01; R2= -0.737). There was medium correlation between EE and ADF (p < 0.01; R2= -0.505), as well as EE and NFC (p < 0.01; R2= -0.590). A very strong correlation was reported in this study between NDF
and ADF content (p < 0.01; R2= 0.821). Although not significant (p > 0.05), we found a weak correlation of IVDMD with the NDF and hemicellulose content of soybeans (R2= -0.263 and R2= -0.257, respectively). Furthermore, at soil pH 4.0, the correlation between CP and NFC content was very strong (p < 0.01; R2= -0.874). We found a medium correlation (p < 0.01) of EE with the ADF and NFC content (R2 = -0.442 and R2 = -0.423, respectively). In similar results at pH 5.4, a positive correlation was reported in this study between NDF and ADF content (p < 0.01; R2 = 0.551). Our results showed that IVDMD in soil with pH 4.0 was weakly correlated (p > 0.05) to NDF and ADF (R2 = -0.126 and R2 = -0.144, respectively).
This study investigated the effects of different soil pHs (5.4 vs 4.0) and different genotypes on the nutrient profile and in vitro digestibility of soybeans. Furthermore, we wanted to identify soybean genotypes that are acid tolerant and produce good nutritional content. In a preliminary study, Puspitasari et al. (2021) reported that soil with pH 4.0 would result in shorter plants, and fewer branches and nodes compared to pH 5.4 (p < 0.05). In contrast, Nadeem et al. (2019) reported that acidic soils (6.0 and 5.1) could enhanced forage production (38% and 39%, respectively) of soybean compared to soil of pH 6.8. This might be due to differences in the genotypes and soil pH used in both studies. In our study, nutrient value and digestibility characteristics are the aspects discussed. The plant genotype, the soil, the climate, and the rhizobia involved in nitrogen fixation are known to influence nutrient content, especially protein and oil in soybean seeds (BFAP, 2021).
We assume that the nutrient content and digestibility produced in this study are lower than in previous studies conducted at neutral soil pH. The majority of crop plants prefer neutral soil pH due to the micro- and macronutrients more available in soil pH range 6.0–7.5 (Nadeem et al., 2019). Aluminum (Al) toxicity in acid soil causes a reduction in crop and seed yields due to lower absorption of water and nutrients (Joris et al., 2013). In the present study, Al toxicity was very high at pH 4.0 (Table 2). However, there are several soybean genotypes that are tolerant of Al toxicity and can maintain nutritional quality (Kang et al., 2011). Demas 1 was released as a genotype tolerant of acid soil and experimental results for soybean yields strengthen the view of Demas 1 as tolerant genotype (Puspitasari et al., 2021).
The average ash content of soybeans grown at pH 5.4 was higher than those grown at pH 4.0 (6.79 vs 6.55%). The ash content of the Mitani genotype grown in soil with pH 5.4 was the highest (p < 0.05), while the lowest was obtained from Derap 1 grown at soil pH of 4.0 (p < 0.05). The ash content, which tends to be high in soybeans grown at pH 5.4, represents a high mineral uptake in the seeds. Ash content is used to determine mineral content of samples (BFAP, 2021). High ash content in a soybean sample is an indication that it could be an essential source of minerals (Alamu et al., 2019). High Al toxicity in acid soil could reduce uptake of macronutrients (Kang et al., 2011). Ash content ranged from 5.20 to 8.06% for all studied genotypes. The average ash content was 6.67, which is quite similar to the ash content of soybean genotypes reported in other studies (4.49–6.19 %) (Dei, 2011; Golshan et al., 2019; Venturelli et al., 2015). Higher ash content in the present study can be attributed to the application of P fertilizer during cultivation (Alamu et al., 2019).
The average CP content in samples from soils with pHs 5.4 and 4.0 were 33.06 and 34.78 %, respectively. Although not significant (p > 0.05), Gamasugen 2 had the highest CP value in both soil pHs, while the lowest value was obtained from Mitani. We assume that the CP content from soybeans grown in two different soil types (ultisol vs latosol) would be different. BFAB (2021) reported that genotype, type of soil, harvesting seasons, and environmental conditions during management influence the proximate analyses of soybeans. However, in the present study, differences in soil type and soil pH did not affect the CP content of any of the genotypes. This might be due to all soybean genotypes having the same ability in both soil pHs. Nevertheless, the results were slightly lower than the values reported by Jayanegara et al. (2017) (43.8–44.8 %), Ishler and Varga (2015) (40.9%), and BFAB (2021) (34–40%). Alemayehu et al. (2021) reported that the minor difference in CP content could be related to the genetic differences among seed types studied and agronomic practices applied during cultivation. Substantial impact of cultivars on CP percentages of soybean indicated that these parameters were only controlled by the genetic constitution of plants (Farhangi-Abriz et al., 2021). Due to their high CP content, soybean seeds are a valuable high-protein feed in livestock rearing (Niwińska et al., 2020).
In contrast to CP, the average EE content produced by soybeans grown at pH 4.0 was higher than those grown in pH 5.4 soils, with a range of 18.91 to 19.34%. We assume that with the same fertilization and irrigation treatment, all Indonesian soybean genotypes have the ability to produce a fairly stable EE content. This implies that the soybeans used in this study could have good potential as energy sources for livestock. Due to the high content of fat and EE, soybean seeds are a valuable high-energy feed source in cattle rations (Alamu et al., 2019). Whole raw soybean seeds are often used as a component of the daily ration for beef and dairy cattle. However, a large amount of soybean fat in the ration could have a negative effect on rumen fermentation due to the toxic effect of unsaturated fatty acids on rumen microbes (Niwińska et al., 2020). Increasing levels of raw soybean (0–27%) in the diet of lactating cows linearly decreases milk yield but linearly increases unsaturated fatty acids and milk fat content (Venturelli et al., 2015). The range of EE content of samples from different pH soils was similar to those reported in earlier works of 17–20% (Van Eys, 2015), 19.2–20.4% (Jayanegara et al., 2017), 17.7% (Ishler and Varga, 2015), and 15–18% (BFAP, 2021).
In our findings, NDF had negative correlation with EE, so that the NDF and ADF content in soybeans grown at pH 4.0 was lower than at pH 5.4. This may be related to the better rate of fiber formation at pH 5.4 due to better nutrient uptake. However, this needs further investigation. NDF content represents most of the cell wall/fiber of soybeans seeds (Van Eys, 2015), while ADF content consists of primary lignocellulose structure and is resistant to microbial fermentation in the rumen (Jayanegara et al., 2017). NDF levels have a negative correlation with feed intake, while ADF has a negative correlation with digestibility values of feedstuffs (Wahyono et al., 2021). The mean NDF content (19.97 %) of the soybean genotypes was higher than that reported in other literature (Dei, 2011; Ishler and Varga, 2015; Van Eys, 2015). However, the obtained results were quite similar to Jayanegara et al. (2017) findings (21.8%). The mean value of ADF (12.22 %) in this study was also higher than the values reported by Van Eys (2015) (6.4 %) and Dei (2011) (7.2 %). This suggests that the soybeans used in this study were high in fiber fractions (NDF and ADF). Highest NFC content, both at pH 5.4 and 4.0 was reported for the Mitani genotype (p < 0.05). The cotyledons of soybean are the main storage area for carbohydrates (starch and sugars) (Van Eys, 2015). Glucose, fructose, sucrose, stachyose, and raffinose are the principal sugars present in soybean seeds (Alamu et al., 2019). High carbohydrate soybeans are also a valuable feed ingredient due to their high energy content.
It is interesting to note that IVDMD was not significantly different between soybeans grown on pH 5.4 and 4.0 soils, although there was a significant difference in fiber fraction. This may be explained by the weak correlation between fiber fractions (NDF and ADF) and soybean digestibility. A high negative relationship between fiber and digestibility may be found in forage, but not in soybeans. Previous studies reported that high fiber fraction will reduce the digestibility of the feed (Wahyono et al., 2019, 2021). In present study, EE was negatively correlated with IVDMD. In fact, EE has a positive relationship to digestible energy and metabolizable energy (Li et al., 2015). The negative correlation may be caused by an indirect path of decreasing NFC content due to an increase in EE value (Romero et al., 2014). Another possibility is due to the limited capacity of microorganisms to digest EE (McDonald et al., 2010). We assume that although IVDMD did not differ protein digestibility could be different, but this opinion should be studied further. In addition, we identified that several studied soybean genotypes have high digestibility (> 80%) even though they are grown in acid soils, namely Burangrang, Gamasugen 2, Kemuning 1, Rajabasa, and Anjasmoro.
CONCLUSIONs and Recommendations
In conclusion, despite the differences in soil pH affecting nutrient (except CP) and fiber composition in soybeans, IVDMD value were not significantly different. Gamasugen 2 has the capability to be developed in two different soil pH conditions, due to it producing high and stable CP and IVDMD. The Ash, OM, EE, NDF, ADF, hemicellulose, NFC, and IVDMD differed among the studied genotypes. Ether extract content of soybeans have negative correlation with NDF, ADF, and NFC.
ACKNOWLEDGEMENTS
We wish to thank Mr. Dedi Ansori for his guidance in laboratory analysis, and Mr. Firsoni, MP, for his assistance with the in vitro analysis. This research was funded by The Indonesia Endowment Funds for Education (LPDP) through Prioritas Riset Nasional, 2020, contract number 019/E1/PRN/2020.
Novelty Statement
Soybean cultivation in Indonesia is challenged by the variety of agroecosystems used, especially in acid soil areas. An initial screening of Indonesian soybean genotypes is needed to identify soybeans characterized by good nutrient composition when grown in acid soils. Our findings report that the differences in soil pH affecting nutrient and fiber composition in soybeans. However, IVDMD value was not significantly different. Our results shows that Gamasugen 2 having potential for development in the two different soil pH conditions.
AUTHOR’S CONTRIBUTION
Bachtiar designed the experiment, supervised and revised the manuscript; Hanani and Anisiyah collected the data and performed laboratory analysis; Puspitasari prepared raw sample, designed and revised the manuscript; Sasongko collected the data and performed in vitro digestibility analysis; Wahyono designed the experiment, conducted in vitro analysis, analyze the data; and wrote the first-draft article.
Conflict of interest
The authors have declared no conflict of interest.
REFERENCES
Alamu EO, Gondwe T, Akinwale G, Suzuki K, Chisonga C, Chigeza G, Busie MD (2019). Impact of soil fertility management practices on the nutritional quality of Soybean (Glycine max L. Merr.) genotypes grown in Eastern Zambia. Cogent Food Agric., 5: 1–13. https://doi.org/10.1080/23311932.2019.1671117
Alemayehu GF, Forsido SF, Tola YB, Teshager MA, Assegie AA, Amare E (2021). Proximate, mineral and anti-nutrient compositions of oat grains (Avena sativa) cultivated in Ethiopia: Implications for nutrition and mineral bioavailability. Heliyon, 7: e07722. https://doi.org/10.1016/j.heliyon.2021.e07722
AOAC (2005). Official method of analysis. 18th Edition. Maryland: Association of official analytical chemists.
Ayaşan T, Cetinkaya N, Aykanat S, Celik C (2020). Nutrient contents and in vitro digestibility of different parts of corn plant. S. Afr. J. Anim. Sci., 50(2): 302–309. https://doi.org/10.4314/sajas.v50i2.13
BFAP (2021). Key drivers of quality of soybean products for feed use in South Africa (Issue March). SA: Bureau for Food and Agricultural Policy (BFAP).
Broderick GA (2018). Review: Optimizing ruminant conversion of feed protein to human food protein. Animal, 12(8): 1722–1734. https://doi.org/10.1017/S1751731117002592
Chouinard PY, Girard V, Brisson GJ (1997). Performance and profiles of milk fatty acids of cows fed full fat, heat-treated soybeans using various processing methods. J. Dairy Sci., 80: 334–342. https://doi.org/10.3168/jds.S0022-0302(97)75943-5
Dei HK (2011). Soybean as a feed ingredient for livestock and poultry. In: Recent trends for enhancing the diversity and quality of soybean products (ed. D. Krezhova). (Issue May). IntechOpen. https://doi.org/10.5772/17601
Farhangi-Abriz S, Ghassemi-Golezani K, Torabian S (2021). A short-term study of soil microbial activities and soybean productivity under tillage systems with low soil organic matter. Appl. Soil Ecol., 168(January): 104122. https://doi.org/10.1016/j.apsoil.2021.104122
Golshan S, Pirmohammadi R, Khalilvandi-Behroozyar H (2019). Microwave irradiation of whole soybeans in ruminant nutrition: Protein and carbohydrate metabolism in vitro and in situ. Vet. Res. Forum, 10(4): 343–350.
Ibáñez MA, de Blas C, Cámara L, Mateos GG (2020). Chemical composition, protein quality and nutritive value of commercial soybean meals produced from beans from different countries: A meta-analytical study. Anim. Feed Sci. Tech., 267: 114531. https://doi.org/10.1016/j.anifeedsci.2020.114531
Ishler V, Varga G (2015). Soybeans and soybean byproducts for dairy cattle. In Dairy and Animal Science. http://extension.psu.edu/animals/dairy/nutrition/nutrition-and-feeding/ration-ingredients. Accessed February,12th 2022.
Jayanegara A, Novandri B, Yantina N, Ridla M (2017). Use of black soldier fly larvae (Hermetia illucens) to substitute soybean meal in ruminant diet: An in vitro rumen fermentation study. Vet. World, 10(12): 1439–1446. https://doi.org/10.14202/vetworld.2017.1439-1446
Jayanegara A, Sari YC, Ridwan R, Diapari D, Laconi EB (2017). Protein fractionation and utilization of soybean and redbean as affected by different drying temperature. Bull. Peternakan, 41(1): 37–47. https://doi.org/10.21059/buletinpeternak.v41i1.13922
Jeong CD, Mamuad LL, Kim SH, Choi YJ, Soriano AP, Cho KK, Jeon CO, Lee SS, Lee SS (2015). Effect of soybean meal and soluble starch on biogenic amine production and microbial diversity using in vitro rumen fermentation. Asian-Australas. J. Anim. Sci., 28(1): 50–57. https://doi.org/10.5713/ajas.14.0555
Joris HAW, Caires EF, Bini AR, Scharr DA, Haliski A (2013). Effects of soil acidity and water stress on corn and soybean performance under a no-till system. Plant Soil, 365: 409–424. https://doi.org/10.1007/s11104-012-1413-2
Kang DJ, Seo YJ, Ujiie K, Vijarnsorn P, Ishii R (2011). Agronomic and tolerant performance of acid soil-tolerant wild soybean (Glycine soja Sieb. and Zucc.) in acid sulfate soil of Thailand. Plant Prod. Sci., 14(2): 156–163. https://doi.org/10.1626/pps.14.156
Kondo M, Yoshida M, Loresco M, Lapitan RM, Herrera JRV, Barrio AND, Uyeno Y, Matsui H, Fujihara T (2015). Nutrient contents and in vitro ruminal fermentation of tropical grasses harvested in wet season in the Philippines. Adv. Anim. Vet. Sci., 3(12): 694–699. https://doi.org/10.14737/journal.aavs/2015/3.12.694.699
Kuswantoro H (2016). Potential yield of acid-adaptive soybean promising lines in ultisols of tanah laut regency, South Kalimantan Province, Indonesia. Biotropia, 23(1): 52–57. https://doi.org/10.11598/btb.2016.23.1.561
Lee JH, Waller JC, Melton SL (2007). Distribution of fatty acids and effect of chemically treated ground, full-fat soybean supplements on tocopherols concentrations in crossbred (Dorset × Suffolk) lambs. Small Rumin. Res., 68: 269–278. https://doi.org/10.1016/j.smallrumres.2005.10.016
Liang C, Piñeros MA, Tian J, Yao Z, Sun L, Liu J, Shaff J, Coluccio A, Kochian LV, Liao H (2013). Low pH, aluminum, and phosphorus coordinately regulate malate exudation through GmALMT1 to improve soybean adaptation to acid soils. Plant Physiol., 161: 1347–1361. https://doi.org/10.1104/pp.112.208934
Li Z, Wang X, Guo P, Liu L, Piao X, Stein HH, Li D, Lai C (2015). Prediction of digestible and metabolisable energy in soybean meals produced from soybeans of different origins fed to growing pigs. Arch. Anim. Nutr., 69: 473-486. https://doi.org/10.1080/1745039X.2015.1095461
McDonald P, Edwards RA, Greenhalgh JFD, Morgan CA, Sinclair LA, Wilkinson RG (2010). The digestion and metabolism of nutrients. In: Animal nutrition. Pearson, London, UK. 7th Ed: 594-607.
Moorby JM, Fraser MD (2021). Review: New feeds and new feeding systems in intensive and semi-intensive forage-fed ruminant livestock systems. Animal, 15: 100297. https://doi.org/10.1016/j.animal.2021.100297
Nadeem M, Pham TH, Nieuwenhuis A, Ali W, Zaeem M, Ashiq W, Gillani SSM, Manful C, Adigun OA, Galagedara L, Cheema M, Thomas R (2019). Adaptation strategies of forage soybeans cultivated on acidic soils under cool climate to produce high quality forage. Plant Sci., 283: 278–289. https://doi.org/10.1016/j.plantsci.2019.03.014
Niwińska B, Witaszek K, Niedbała G, Pilarski K (2020). Seeds of n-GM soybean genotypes cultivated in Poland and their processing products as high-protein feeds in cattle nutrition. Agriculture, 10: 1–13. https://doi.org/10.3390/agriculture10050174
Puspitasari W, Bachtiar T, Robifahmi N, Anisiyah, Iqbal M (2021). Acid soil tolerance of 28 soybean genotypes in hydroponics and soil-based evaluation. IOP Conf. Ser. Earth Environ. Sci., pp. 911: 012001. https://doi.org/10.1088/1755-1315/911/1/012001
Romero S, Rodrigues PHM, Marino CT, Pinedo LA, Martins MF, Cassiano ECO, Perna Jr F (2014). Effect of energy sources on the apparent total tract digestibility and excretion of nutrients by bovine cattle. Rev. MVZ Cordoba, 19: 4072-4085. https://doi.org/10.21897/rmvz.101
Statistics Indonesia (2022). Impor Kedelai Menurut Negara Asal Utama, 2010-2019. https://www.bps.go.id/statictable/2019/02/14/2015/impor-kedelai-menurut-negara-asal-utama-2010-2019.html. Accessed February, 2nd 2022.
Sumiahadi A, Acar R (2019). Forage crops in acid soils of Indonesia. In: Proceedings of international symposium for environmental science and engineering research (ISESER 2019), Konya, Turkey, pp. 529–543.
Van Eys JE (2015). Manual of quality analyses for soybean products in the feed industry (2nd Edition). U.S. Soybean Export Council, US.
Van Soest PJ, Robertson JB, Lewis BA (1991). Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci., 74: 3583–3597. https://doi.org/10.3168/jds.S0022-0302(91)78551-2
Venturelli BC, de Freitas Júnior JE, Takiya CS, de Araújo APC, Santos MCB, Calomeni GD, Gardinal R, Vendramini THA, Rennó FP (2015). Total tract nutrient digestion and milk fatty acid profile of dairy cows fed diets containing different levels of whole raw soya beans. J. Anim. Physiol. Anim. Nutr., 99: 1149–1160. https://doi.org/10.1111/jpn.12297
Wahyono T, Sasongko WT, Maharani Y, Ansori D, Handayani T, Priyoatmojo D, Trinugraha AC (2021). Investigation of eighteen Indonesian mutant rice straw genotypes as ruminant roughage. Adv. Anim. Vet. Sci., 9(11): 1757–1764. https://doi.org/10.17582/journal.aavs/2021/9.11.1757.1764
Wahyono T, Sugoro I, Jayanegara A, Wiryawan KG, Astuti DA (2019). Nutrient profile and in vitro degradability of new promising mutant lines sorghum as forage in Indonesia. Adv. Anim. Vet. Sci., 7(9): 810–818. https://doi.org/10.17582/journal.aavs/2019/7.9.810.818
To share on other social networks, click on any share button. What are these?





