Nematode Community Structure in Musa acuminata Colla (Lakatan) Farms with Continuous Cropping System
Nematode Community Structure in Musa acuminata Colla (Lakatan) Farms with Continuous Cropping System
Baby Nhor K. Ambel1*, Nneka Djen A. Matandog1, Neil Pep Dave N. Sumaya2, Florence Roy P. Salvaña1, Bryan Lloyd P. Bretaña1 and Ma. Teodora N. Cabasan1*
1Department of Biological Sciences, College of Science and Mathematics, University of Southern Mindanao, 9407 Kabacan, Cotabato, Philippines; 2Division of Plant Pathology, College of Agriculture, University of Southern Mindanao, 9407 Kabacan, Cotabato, Philippines.
Abstract | Monocropping is commonly practiced by small-scale banana farmers for ease of management; however, concerns arise regarding its long-term effects on soil health and ecosystem stability. In order to ascertain the fact, this study investigated the impact of different durations of monocropping Lakatan banana on soil health as reflected by nematode community structure. For the extraction of nematodes, soil and root samples were collected from various banana farms practicing monocropping for 2-4, 5-9 and 10-15 years. Farms with prolonged monocropping showed an increased population of plant-parasitic nematodes compared to free-living nematodes. Nematode family Hoplolaimidae was recorded as the most dominant (59.59%-80.49%) across all monocropping durations. Shannon and Simpson’s diversity indices were lowest at 10-15 years of continuous monocropping period, as compared to 2-4 years and 5-9-years. Maturity index (MI, 2.26) was highest at 5-9 years of continuous cultivation while structure index (SI, 68.93), and enrichment index (EI, 73.74) were highest in banana farms with monocropping periods of 2-4 years, and lower in banana farms with longer monocropping periods. Higher plant parasite index (PPI, 2.89-2.93) and PPI/MI (1.37) ratios were observed in monocropping periods of 10-15 years. Analysis of the c-p data indicated that soil conditions approach stress levels when farms are cultivated under 10-15 years of monoculture. Soil food web analysis revealed disturbances in the soil ecosystem, particularly in banana monocropping farms used for 5-15 years. Continuous monocropping for 5-15 years could disrupt the soil conditions, as evidenced by changes in the nematode community structure. These findings highlight that nematode communities can serve as sensitive indicators of soil quality and have the potential to elucidate the impact of farming management on soil health.
Received | April 10, 2024; Accepted | May 24, 2024; Published | June 10, 2024
*Correspondence | Baby Nhor Ambel and Ma. Teodora Cabasan, Department of Biological Sciences, College of Science and Mathematics, University of Southern Mindanao, Kabacan, Cotabato, Philippines; Email: bnkambel@usm.edu.ph, mtncabasan@usm.edu.ph
Citation | Ambel, B.N.K., Matandog, N.D.A., Sumaya, N.P.D.N., Salvaña, F.R.P., Bretaña, B.L.P. and Cabasan, M.T.N., 2024. Nematode community structure in Musa acuminata colla (Lakatan) farms with continuous cropping system. Pakistan Journal of Nematology, 42(1): 66-80.
DOI | https://dx.doi.org/10.17582/journal.pjn/2024/42.1.66.80
Keywords | Agroecosystem, Banana farms, Diversity, Monocropping, Nematode community structure, Soil food web
Copyright: 2024 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Sustainable agriculture emphasizes the long-term stewardship of natural resources over short-term economic gain. Its fundamental concept includes conservation of natural resources, maintaining soil fertility, and protection of biodiversity (Gomiero, 2021). Various crops, including bananas, are subjected to sustainable agricultural practice as it has become the fourth most important crop in various countries, particularly in Southeast Asia (CropTrust, 2022). Globally, banana production reached approximately 135 million tons in 2022 (Shahbandeh, 2024). Furthermore, bananas not only provide small-scale producers with adequate income but also serve as a crucial food source for over 400 million consumers (Rojas-Flores et al., 2022). In the Philippines, Lakatan is one of the most popular dessert banana varieties grown for domestic market and ranks 2nd after Cavendish in terms of production in the country. Region XII (SOCCSKARGEN) is among the top banana producers in the Philippines (ProMusa, 2020).
Like other staple commodities, lakatan banana plants are often cultivated using monoculture methods. This practice seeks to increase the production of high-yielding crops through the adaptation of new agricultural techniques (John and Babu, 2021). However, Zhong et al. (2014) reported that long term continuous cropping of banana is contentious due to its potential to reduce crop productivity and degrade soil fertility. The result is a more delicate environment reliant on insecticides and artificial fertilizers. The concentrated presence of a single cultivar renders the crop vulnerable to rapid devastation even caused by single opportunistic species or pathogen (Drenth and Kema, 2021).
Intensive management of agroecosystems, such as monocropping, could significantly affect soil biodiversity and alter the structure and complexity of food webs in the below-ground communities including nematodes, potentially compromising the ecosystem functions and services (Lazarova et al., 2021). Monocrop banana farms contain diverse nematodes; however, nematode population fluctuates in response to several environmental factors, such as variations in soil moisture, temperature and pH. Any modifications in soil fertility are mirrored in the structure of the nematode community (Bongers and Ferris, 1999). Therefore, the nematode community structure is deemed an appropriate bioindicator of soil conditions and its preservation is crucial for maintaining soil quality that supports plant growth (Bünemann et al., 2018). While plant-parasitic nematodes are becoming less vulnerable to disturbances (Aleuy and Kutz, 2020), community of free-living nematodes in banana farms, responsible for nutrient cycling and maintaining soil fertility, may be diminished due to monoculture practices (Zhong et al., 2012).
Globally, of over 150 species of nematodes, 51 genera are found in monoculture banana farms (Nimisha and Nisha, 2019), and changes in the nematode population reflects the conditions of the agroecosystem. Other studies on nematodes in banana focused on the interaction between the host plant and pathogen (Herradura et al., 2012; Lara Posadas et al., 2016; Olivares et al., 2021; Sousa, 2024). Nematodes were also used as bioindicator of soil quality in banana plantations. For instance, it was examined in semi-conventional monocrop banana farms in Indonesia treated with manure, lime, and fungicides (Yogaswara et al., 2021), in banana plantations in Uganda compared with coffee plantations (Bell et al., 2021), in banana farms with soil properties (Al-Ghamdi, 2021), and banana farms with vegetative ground covers (Pattison et al., 2020). The response of nematode communities to monoculture and mixed culture was also studied, but on legumes and grasses (Yingying et al., 2020). Thus, this study assessed the soil conditions of banana farms in relation to continuous cropping system, as reflected by the nematode community structure.
Materials and Methods
Description of the sampling site
Lakatan banana farms at Pigcawayan (7°16’39.612”N, 124°26’33.936”E) and Libungan (7°15’ 12.853”N, 124°30’25.2”E), province of Cotabato in Region XII (SOCCSKARGEN), at the Southern part of the Philippines were considered as the sampling sites. The average annual temperature of the study sites was 26.7°C with average precipitation of 2,132 mm. Banana planted in the study sites have identical soil type (clay loam) and were maintained under the same management practices. Overall, a total of 9 Lakatan farms consisting of three fields as replicate per treatment, with a minimum of one hectare each, were divided into three equal subplots. These farms had varying durations of continuous monocropping as follows: 2-4 years, 5-9 years and 10 to 15 years (Figure 1).
Soil sampling
In each sampling plot, ten soil cores from the rhizosphere (within 30-cm diameter from the pseudostem) were taken randomly and combined to obtain a composite soil sample. Plants during the sampling period were either at early flowering or at fruit bearing stage. Soil samples were obtained using a soil corer (5cm in diameter) down to a depth of 20cm.
Root examination and extraction of nematodes from root samples
A spade was used to collect the root portion from the plant base, on one side of the bunching sucker away from the mother plant and the following sucker. The roots were carefully examined for the occurrence of galling, lesions, and rotting symptoms caused by plant parasitic nematodes (Figure 2). Nematodes in the 10 g roots were extracted by maceration (Coyne et al., 2007) using a blender, and placed in extraction trays. Nematode suspension was collected after 48 hours.
Extraction of nematodes from soil samples
Nematodes were extracted from 200 g fresh soil using modified Baermann tray method (Whitehead and Hemming, 1965) for 48 hours. Nematode suspension was decanted into a 38 µm sieve. The residue was collected in containers and was added with 4% formaldehyde.
Counting and picking of nematodes
Nematodes in formaldehyde were rinsed off with tap water. Under the stereoscope, all the nematodes were counted with the use of the counting dish to obtain the nematode population and were then picked out using a nematode picking tool.
Fixing of nematodes
For fixing of nematodes, series of glycerol solutions (De Grisse, 1969) were used: solution 1 (4% formalin in 99 parts: glycerin in 1 part), solution 2 (95% ethanol in 95 parts: glycerin in 5 parts) and solution 3 (95% ethanol in 50 parts: glycerin in 50 parts). Picked out nematodes from the fixative were transferred to a dish containing 1 ml of solution 1. The dish with nematodes was placed in a closed glass vessel containing about 1/10 of its original volume of 95% ethanol. The dish was left in a saturated atmosphere for 12 hours in an incubator at 40-45 oC. Solution 2 was added and the dish was partially closed to allow slow evaporation of ethanol. This was kept in the oven for 3 hours at 37 oC. Solution 3 was added until nematodes are in pure glycerin.
Mounting and slide preparation
In mounting the nematodes, microscope slides were prepared with the paraffin wax ring. A paraffin ring was placed in the middle of the glass slide, and a small drop of glycerin was added (Ryss, 2017). The nematodes were then placed on it and a cover slip was placed at the top of the ring and was heated on a hot plate until the paraffin melts allowing the cover slip to settle down.
Identification of nematodes
Nematodes were characterized based on their morphological characteristics. Nematodes were identified to family and genus level. Reference books, identification guides, manual, and pictorial keys such as Jairajpuri and Khan (1982, 1992); Bongers (1994) were utilized to identify the nematodes.
Measurement of nematode diversity and indices
Diversity of nematodes from soil and roots of banana plants under different cropping periods were determined using the dominance, Simpson’s and Shannon-Wiener diversity indices. Maturity index (MI), plant parasitic index (PPI), channel index (CI), enrichment index (EI), structure index (SI), and PPI/MI ratio computed by utilizing the formula below. Dominance, Simpson’s and Shannon-Weiner indices were calculated using Paleontological Statistics (PAST) software. MI, PPI, EI, and SI were computed using the Nematode Indicator Joint Analysis (NINJA; Sieriebriennikov et al., 2014).
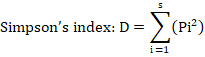
Where: D= Value of Simpson’s diversity index; Pi= proportion of individuals in the ith species; s= number of species.
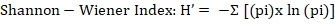
Where: H’= Shannon-Wiener index; pi = number of individuals of species; ln(pi) = total number of individuals belonging to species.
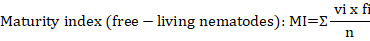
Where: vi = the c-p value for nematode family i; fi = is the frequency of nematode family i, n = is the total number of individual nematodes in the sample.
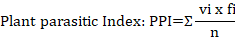
Where: vi= the c-p value for plant parasitic nematodes family i; fi= is the frequency of plant parasitic nematodes family i; n = is the total number of individual nematodes in the sample.
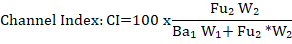
Where; CI= channel index; Ba₁= opportunistic bacterivorous nematodes; Fu₂= generalist fungivorous nematodes W₁=3.2; W₂= 0.8
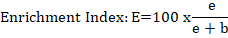
Where; EI= enrichment index; e = (Ba₁ W₁) + (Fu₂ W₂); b = (Ba ₂ + Fu ₂).
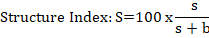
Where; SI= structure index; s= (Ban Wn)+ (Can Wn) + (Fun Wn) + (Omn Wn); b = (Ba₂ + Fu₂).
Statistical analysis
Values from the computed indices were compared in the three farms with varying durations of monocropping system using nonparametric Kruskal-Wallis. Differences at p < 0.05 was considered as significant. Dunn’s test was used as post-hoc analysis and was computed using Paleontological Statistics (PAST) software. Similarity percentages (SIMPER; Clarke, 1993) was used to assess the contribution of a particular taxa towards the observed similarity (dissimilarity) between the banana farms with different durations of continuous cultivation. The observed dissimilarity was then evaluated using Analysis of Similarity (ANOSIM) to determine the significant differences between the identified taxa from continuous cultivated banana farms.
Results and Discussion
Nematode population
The mean nematode population density in the rhizosphere was highest (178.7 individuals per 200 g soil) in banana farms with 10-15 years of continuous cropping (Table 1). In contrast, the lowest nematode density (81.9 individuals per 200 g soil) was recorded in farms with 5-9 years of continuous cropping. The average population count of nematodes in banana roots were comparable (87.8-103.8 individuals per 10 g roots) in the banana farms with different durations of monocropping. Normally, in a favorable environment, nematode populations are expected to increase as the banana plantation age (Davide, 1980). This is evident in the banana roots in this study, as the population density increased with the years of continuous cultivation. Variability in the nematode population in farms practicing different periods of monocropping was observed. Nematodes are diverse and abundant in soil and the fluctuations in their populations are attributed to their sensitivity to changes within the soil ecosystem driven by various agricultural practices (Yeates and Bongers, 1999) including continuous cultivation system.
Table 1: Mean nematode population density in the roots (10 g) and rhizosphere (200 g) of Lakatan (Musa acuminata) cultivated across varying periods of continuous cropping system.
|
Cropping duration (years) |
Nematode density |
|
|
Rhizosphere |
Roots |
|
|
2-4 |
126.8 ± 49.1 |
87.8 ± 26.7 |
|
5-9 |
81.9 ± 59.6 |
91.3 ± 85.3 |
|
10-15 |
178.7 ± 67.5 |
103.8± 95.1 |
Mean ± standard deviation.
Nematodes from monoculture banana farms with varying monocropping durations
Free-living and plant parasitic nematodes observed in the banana farms with different cultivation periods are presented in Table 2. There were 9.38% of free-living and 90.62% plant parasitic nematodes in 200 g of rhizosphere from banana farms under 2-4 years of monocropping. The relative abundance of free-living nematodes gradually decreased with years of continuous cultivation while plant parasitic nematodes showed otherwise. The lowest (2.18%) relative abundance of free-living nematodes from 10 g of roots was observed at 10-15 years of continuous cultivation while the highest (96.57%) plant parasitic nematodes was also observed in a similar duration. These findings are in accordance with the study of Chen et al. (2021) on monoculture cotton farms, indicating that longer periods of monoculture are detrimental to free-living nematodes while it increased the population of plant-parasitic nematodes. Long term monocropping have the potential to disrupt the natural diversity of the soil ecosystem and the complexity of available nutrients. This often creates conditions favorable to plant-parasitic nematodes, as resources fluctuates for other free-living nematodes. Furthermore, nematodes exhibit food and host specificity, and the continuous cultivation of banana could promote the proliferation of plant-parasitic nematodes that specifically feed on this host plant thereby limiting the access of other free-living nematodes to nutrients (Li et al., 2016a).
Five orders of nematodes were observed in banana farms with 2-4 years of monocropping period, namely: Dorylaimida, Mononchida, Monhysterida, Rhabditida, and Tylenchida (Table 3). All of these nematode taxa were also found in Lakatan farms with 5-9 and 10-15 years of monocropping duration except for order Monhysterida.
Table 2: Relative abundance (%) of free-living and plant parasitic nematodes in the roots (10 g) and rhizosphere (200 g) of Lakatan farms under various monocropping periods.
|
Cropping duration (years) |
Rhizosphere |
Root |
Total |
|||
|
Free-living (%) |
Plant-parasite (%) |
Free living (%) |
Plant-parasite (%) |
Free living (%) |
Plant-parasite (%) |
|
|
2-4 |
9.38 |
90.62 |
14.68 |
85.32 |
11.55 |
88.45 |
|
5-9 |
6.25 |
93.75 |
8.27 |
91.73 |
7.31 |
92.69 |
|
10-15 |
2.18 |
97.82 |
3.43 |
96.57 |
2.64 |
97.36 |
Table 3: Nematodes identified in the roots and rhizosphere of banana farms under three different monocropping durations.
|
Order |
Family |
Genus |
Trophic classification |
c-p value |
p-p values |
Cropping duration (years) |
||
|
2-4 |
5-9 |
10-15 |
||||||
|
Dorylaimida |
Aporcelaimidae |
Aporcelaimellus |
Predator |
5 |
N/A |
+ |
- |
- |
|
Dorylaimidae Nygolaimidae |
Dorylaimus |
Omnivore |
4 |
N/A |
+ |
+ |
+ |
|
|
Nygolaimus |
Predator |
5 |
N/A |
+ |
+ |
+ |
||
|
Qudsianematidae |
Eudorylaimus |
Predator |
4 |
N/A |
+ |
+ |
+ |
|
|
Mononchida |
Mononchidae |
Clarkus |
Predator |
4 |
N/A |
+ |
+ |
+ |
|
Iotonchus |
Predator |
4 |
N/A |
+ |
+ |
- |
||
|
Monhysterida |
Monhysteridae |
Eumonhystera |
Bacterivore |
2 |
N/A |
+ |
- |
- |
|
Rhabditida |
Cephalobidae |
Cephalobus |
Bacterivore |
2 |
N/A |
+ |
+ |
+ |
|
Panagrolaimidae |
Panagrolaimus |
Bacterivore |
1 |
N/A |
+ |
+ |
+ |
|
|
Rhabditidae |
Mesorhabditis |
Bacterivore |
1 |
N/A |
+ |
+ |
+ |
|
|
Tylenchida |
Hoplolaimidae |
Helicotylenchus |
Semi-endoparasite |
N/A |
3 |
+ |
+ |
+ |
|
Hoplolaimus |
Semi-endoparasite |
N/A |
3 |
+ |
+ |
+ |
||
|
Rotylenchus |
Semi-endoparasite |
N/A |
3 |
+ |
+ |
+ |
||
|
Heteroderidae |
Meloidogyne |
Sedentary endoparasite |
N/A |
3 |
+ |
+ |
+ |
|
|
Pratylenchidae |
Pratylenchus |
Migratory-endoparasite |
N/A |
3 |
+ |
+ |
+ |
|
+present; - absent; N/A – not applicable; c-p-colonizer-persister; p-p- plant-parasite.
Table 4: Mean nematode abundance and relative abundance (%) obtained from Lakatan farms with varying monocropping durations.
|
Nematode taxa |
Cropping durations |
|||||
|
2-4 years |
5-9 years |
10-15 years |
||||
|
Abundance |
Relative abundance (%) |
Abundance |
Relative abundance (%) |
Abundance |
Relative abundance (%) |
|
|
Aporcelaimidae |
0.11 |
0.05 |
0.00 |
0.00 |
0.00 |
0.00 |
|
Dorylaimidae Nygolaimidae |
0.22 |
0.05 |
0.11 |
0.19 |
0.22 |
0.08 |
|
2.00 |
0.90 |
0.11 |
0.19 |
0.22 |
0.12 |
|
|
Qudsianematidae |
1.11 |
0.52 |
0.44 |
0.26 |
0.22 |
0.08 |
|
Mononchidae |
1.89 |
0.80 |
0.44 |
0.26 |
0.22 |
0.08 |
|
Monhysteridae |
0.11 |
0.05 |
0.00 |
0.00 |
0.00 |
0.00 |
|
Cephalobidae |
11.33 |
5.18 |
8.11 |
4.68 |
5.00 |
1.77 |
|
Panagrolaimidae |
3.11 |
1.33 |
1.89 |
1.10 |
1.22 |
0.43 |
|
Rhabditidae |
5.00 |
2.30 |
1.11 |
0.64 |
0.22 |
0.08 |
|
Hoplolaimidae |
149.33 |
70.02 |
103.22 |
59.59 |
238.44 |
80.49 |
|
Heteroderidae |
6.78 |
3.10 |
1.44 |
0.83 |
11.22 |
3.97 |
|
Pratylenchidae |
33.56 |
15.70 |
55.89 |
32.26 |
36.44 |
12.90 |
On the nematode family level, 12 families were recorded in areas with 2-4 years of monocropping period. Similar nematode families (except for Aporcelaimidae and Monhysteridae) were also observed at 5-9 years and 10-15 years of banana cultivation. Commonly, the abundance of Aporcelaimidae and Monhysteridae indicates the presence of varied food sources capable of sustaining their development and reproduction. The decline in population during the succeeding years of monocropping could possibly be due to fluctuations in nutrients availability within the banana farms as the period of continuous cropping persists (Tabarant et al., 2011).
A total of 15 genera were identified in this study in which bacterial feeders Mesorhabditis and Panagrolaimus are classified under cp-1 while Cephalobus and Eumonhystera are categorized as cp-2. On the other hand, Clarkus, Iotonchus, and Eudoryolaimus fall into cp-4, and Nygolaimus along with Aporcelaimellus, are designated as cp-5 indicating their role as predators. Nematodes with omnivorous tendencies, such as Dorylaimus are characterized as cp-4. Meanwhile, herbivorous nematodes, including Meloidogyne (sedentary parasites), Pratylenchus (migratory endoparasites), Rotylenchus, Helicotylenchus, and Hoplolaimus (semi-endoparasites) are classified under p-p 3 (Bongers, 1999).
Among all the 12 families identified, Hoplolaimidae was observed to be the most abundant (149.33 individuals or 70.02%) under 2-4 years of Lakatan monoculture (Table 4). This was followed by Pratylenchidae (33.56 or 15.70%), Cephalobidae (11.33 or 5.18%), and Heteroderidae (6.78 or 3.10%), while the other nematode taxa appeared in very low frequencies (0.11-5.00 individuals or 0.05%-2.30%). Similar trend was observed under 5-9 years of monocropping period and 10-15 years of continuous cultivation except that the abundance of Cephalobidae decreased under 10-15 years whereas the abundance of Heteroderidae increased in the same period of monocropping. Notably, along with Pratylenchus and Meloidogyne, several genera of Hoplolaimidae such as Rotylenchulus, Helicotylenchus, and Hoplolaimus were frequently encountered in banana farms in West Bengal, India (Khan and Hasan, 2010). Among those aforementioned species of nematodes, Helicotylenchus are found to be dominant in banana farms in tropical areas like Tamil Nadu, India (Tharani et al., 2021). Their frequencies may suggest a shift towards a soil ecosystem dominated by plant-parasitic nematodes (Dutta and Phani, 2023). Aside from plant parasitic nematodes, c-p 2 Cephalobidae, were also influenced by varying continuous cropping periods. The abundance of Cephalobidae in this study, indicates a stressed or polluted soil ecosystem. However, the gradual decline in their population, coupled with the sudden prevalence of plant-parasitic nematodes during monocropping, mirrors a shift towards a less bacterial-prevalent ecosystem (Ferris and Bongers, 2006). Omnivorous nematodes were also
Table 5: Distribution of various feeding types of nematodes identified from Lakatan farms with various periods of monocropping.
|
Trophic classification |
Cropping durations |
p value |
|||||
|
2-4 years |
5-9 years |
10-15 years |
|||||
|
Abundance |
Relative abundance (%) |
Abundance |
Relative abundance (%) |
Abundance |
Relative abundance (%) |
||
|
Bacterivores |
176 |
9.11 |
100 |
6.41 |
58 |
2.28 |
0.30 |
|
Predators |
46 |
2.38 |
11 |
0.71 |
7 |
0.28 |
0.23 |
|
Omnivores |
1 |
0.05 |
3 |
0.19 |
2 |
0.08 |
0.43 |
|
Herbivores |
1708 |
88.46 |
1445 |
92.69 |
2475 |
97.36 |
0.82 |
|
Plant parasitic nematode |
|||||||
|
Semi-endoparasite |
1345 |
78.75 |
929 |
64.29 |
2046 |
82.67 |
0.01 |
|
Sedentary parasite |
61 |
3.57 |
13 |
0.90 |
101 |
4.08 |
0.18 |
|
Migratory parasite |
302 |
17.68 |
503 |
34.81 |
328 |
13.25 |
0.18 |
observed in lower frequencies. The lower population or the absence of omnivorous nematodes could reflect a soil ecosystem heavily exposed to contaminants and disturbances, further reflecting a disruption of food resources and less diverse soil ecosystem (Stirling and Linsell, 2014; Sánchez-Moreno and Ferris, 2018).
In 2-4 years of cultivation, plant parasitic nematodes dominated which accounts for 88.46% (1,708 individuals) of the population, followed by bacterial feeders at 9.11% (176 individuals), and few predators (46 individuals or 2.38%) (Table 5). Similarly, 10-15 years of monocropping exhibited a prevalence of plant parasites at 97.36% (2,475). No significant difference in the relative abundance of nematode trophic classification was observed between the different monocropping periods, except on semi-endoparasitic nematodes. Among all the plant-parasites, semi-endoparasites thrive the most with the highest abundance (2,046 or 82.67%) at 10-15 years of monocropping cultivation. Migratory plant-parasitic nematodes showed a gradual increase in population over time. Plant-parasitic nematodes are host-specific nematodes, therefore, continuous cultivation of banana could increase the intensity of resource competition between plant-parasitic nematodes (Stefanovska et al., 2023).
The proliferation of plant parasitic nematodes poses significant threat to agricultural crops like banana. By feeding on the roots of banana, these nematodes damage root tissues, directly impeding water and nutrient uptake crucial for plant development. In line with the findings of this study, Ozarslandan et al. (2019) reported the predominance of Helicotylenchus (Hoplolaimidae), Meloidogyne (Heteroderidae), as well as Pratylenchus (Pratylenchidae), identifying them as the major causes of root diseases in bananas farms in Turkey. Additionally, the high frequencies of plant-parasitic nematodes in soil may induce bacterial and fungal infections in crops, as it provides entry for other pathogens like Fusarium, Rhizoctonia solani and Thielaviopsis basicola (Manzanilla-Lopez and Starr, 2009; Olivares et al., 2021), that could potentially lead to greater economic losses in banana plantations.
Table 6: Nematode diversity indices calculated in the rhizosphere of Lakatan farms under different cultivation periods.
|
Cropping duration |
p value |
|||
|
2-4 years |
5-9 years |
10-15 years |
||
|
Taxa |
12 |
10 |
9 |
0.91 |
|
Individuals |
1141 |
737 |
1608 |
0.97 |
|
Dominance |
0.77 |
0.82 |
0.75 |
0.94 |
|
Simpson |
0.23 |
0.18 |
0.25 |
0.94 |
|
Shannon |
0.62 |
0.47 |
0.49 |
0.91 |
|
MI |
2.25 |
2.26 |
2.14 |
0.82 |
|
PPI |
2.72 |
2.82 |
2.93 |
0.88 |
|
PPI/MI |
1.20 |
1.25 |
1.37 |
0.90 |
Nematode diversity in banana farms with varying periods of continuous cropping
On farms with 2-4 years of monocropping, highest number (12) of nematode taxa was recorded, but not significantly different in farms with 10-15 years of continuous cultivation (Table 6). In contrast, dominance was most pronounced (0.82) in monocropping durations of 5-9 years, and less (0.75) in 10-15 years monocropping durations. The high dominance observed on continuous monocropping could possibly be due to the high density of nematodes obtained per periods of monoculture; although it is not always the case, lower density may favor the dominance of a particular species as there is lesser intensity for resource competition (Hillebrand et al., 2008).
Simpson’s diversity index exhibited its lowest value in 5-9 years of monoculture at 0.18. Accordingly, Shannon diversity index was highest (0.62) at 2-4 years but decreased (0.47-0.49) at 5-15 years of monocropping banana. Similar observations were recorded in rubber plantations by Panklang et al. (2022) where the Shannon and Simpson’s diversity indices decreased at 10 years of monoculture, leaving particular species to proliferate as the years of continuous cultivation of rubber increases. This could possibly arise when the soil environment can no longer sustain varied species, instead promoting the growth of only those that can readily adapt towards the prevailing conditions (Nielsen et al., 2015). However, compared to the computed diversity indices in this study, monocropped rubber, as reported by Panklang et al. (2022) have a higher value of Shannon (1.4) and Simpson’s (7.8) diversity indices, indicating a more diverse nematode community than those that were observed in continuous cultivated Lakatan farms.
The maturity index (MI) was highest at monocropping duration of 5-9 years at 2.26 and lowest (2.14) at 10-15 years of banana monoculture. MI values obtained in this study signifies a polluted environment across banana farms under varying monocropping periods. This further corresponds to the prevalence of pollution- or stress- tolerant bacterivores nematodes (c-p2) recorded in this study. Moreover, the recorded MI is notably lower compared to the findings of Hoang et al. (2021), who noted a computed maturity index ranging from 3.48-3.39 over a span of 2-12 years of monocropping Robusta coffee. The differences between the values of diversity indices computed across different types of continuous cultivated crops could be due to the variations in farming managements such as utilization of different fertilizers, pesticides and other cultural practices. Other than that, each crops secrete different types of nutrients available and favorable for certain types of nematodes (Ortiz et al., 2016).
The plant parasitic index (PPI) of the banana farms in the rhizosphere in this study increased over time, reaching 2.93 at 10-15 years of monocropping. The PPI value exceeding 2.0 suggests an assemblages dominated by semi-endoparasitic nematodes (Du Preez et al., 2022). This is supported by the gradual increase of semi-endoparasitic nematodes recorded in this study. Similarly, PPI/MI was lowest (1.20) at 2-4 years monocropping and it progressively increased to 1.37 at 10-15 years of monocropping. No significant differences were observed between nematode community diversity indices across the different monocropping periods. However, the PPI/MI observed is lower than what Chen et al. (2021) documented in 10-15 years of monocropping cotton, having 1.56 PPI/MI ratio. The rising value of PPI/MI indicates a community prevalent of plant-parasitic nematodes over free-living nematodes.
Table 7: Nematode diversity indices calculated from roots of Lakatan banana under different cultivation periods.
|
Cropping duration |
p value |
|||
|
2-4 years |
5-9 years |
10-15 years |
||
|
Taxa |
3 |
3 |
3 |
0.95 |
|
Individuals |
674 |
754 |
902 |
0.93 |
|
Dominance |
0.32 |
0.45 |
0.54 |
0.96 |
|
Simpson |
0.68 |
0.56 |
0.46 |
0.91 |
|
Shannon |
1.40 |
1.03 |
0.92 |
1.00 |
|
PPI |
2.56 |
2.75 |
2.89 |
0.92 |
The diversity of plant parasitic nematodes identified from the root samples associated with different monocropping periods is presented in Table 7. In contrast to the nematode community observed in the rhizosphere, dominance values increased proportionally with the duration of cultivation but not significantly with 10-15 years period showing the highest dominance value of 0.54. Meanwhile, Simpson and Shannon indices decreased with prolonged continuous cultivation. Similar to the nematode community in the rhizosphere, the PPI observed in root samples was lowest (2.56) at initial years of monocropping but gradually increased (2.89) at 10-15 years of monocropping. The recorded PPI of nematodes in the roots was lower than what was observed in the rhizosphere. Since plant-parasitic nematodes are host-specific nematodes, continuous cultivation of banana could increase the intensity of resource competition between plant-parasitic nematodes (Stefanovska et al., 2023). Endoparasitic nematodes penetrate root cells, damaging the tissues and subsequently reducing root exudates, which in turn affects the nutrient acquisition of other plant-parasitic nematodes (Liu and Park, 2018). This study further suggests that continuous cultivation could lead to the disruption of nematode diversity in banana farms in the long run, promoting the accumulation of stress-tolerant and semi-endoparasitic nematodes (Tian et al., 2020).
The similarity percentages (SIMPER) were calculated to assess the degree of dissimilarities between the various periods of monocropping and to determine which nematode taxa contribute the most to the dissimilarity between the communities across the three monocropping durations (Table 8). The average dissimilarity between 2-4 years and 5-9 years monocropping durations was 38.86%. Out of all the nematode taxa identified, Hoplolaimidae had the highest contribution to the dissimilarity accounting for 50.04 followed by Pratylenchidae (31.0), and Cephalobidae (5.47). Conversely, Aporcelaimidae and Monhysteridae have the lowest (0.08) contribution to the dissimilarity. The dissimilarity observed between the taxa from 2-4 years and 5-9 years of monocropping are not significantly different except for Rhabditidae (p = 0.04). Furthermore, the calculated R value (0.03) indicates a difference between monocropping periods but with some overlap.
On the other hand, the average dissimilarity between 2-4 years and 10-15 years of monocropping is 34.42%. Accordingly, Hoplolaimidae contributed the most to the dissimilarity (64.63%). Of all the 12 identified nematode taxa, Hoplolaimidae, Rhabditidae, Panagrolaimidae, Mononchidae, and Nygolaimidae are significantly different (p < 0.05) between 2-4 years and 10-15 years of monocropping banana. The R value recorded between the similar periods of cultivation is 0.19, suggesting an observable difference (with some overlaps) between the two periods of monocropping.
Dissimilarity between 5-9 years and 10-15 years of monoculture is the highest observed dissimilarity in this study, accounting for 45.12%, on average. All the nematode taxa are not significantly different except for Rhabditida (p= 0.02) and Hoplolaimidae (p=0.01). Moreover, based on the computed analysis of similarity (ANOSIM), 5-9 years and 10-15 years of cultivation has the highest R value (0.33), indicating weak variation between similar periods of monoculture.
Table 8: Similarity percentages of nematodes (roots and rhizosphere) under different monocropping durations.
|
Order |
Family |
Contribution (%) |
Significance |
|
2-4 years vs 5-9 years of monocropping (average dissimilarity = 38.86%; R = 0.03) |
|||
|
Dorylaimida |
Aporcelaimidae |
0.08 |
1.00 |
|
Dorylaimidae |
0.18 |
0.81 |
|
|
Nygolaimidae |
1.44 |
1.00 |
|
|
Qudsianematidae |
0.70 |
0.30 |
|
|
Mononchida |
Mononchidae |
1.17 |
0.07 |
|
Monhysterida |
Monhysteridae |
0.08 |
1.00 |
|
Rhabditida |
Cephalobidae |
5.47 |
0.54 |
|
Panagrolaimidae |
1.49 |
0.21 |
|
|
Rhabditidae |
3.58 |
0.04 |
|
|
Tylenchida |
Hoplolaimidae |
50.04 |
0.24 |
|
Heteroderidae |
4.76 |
0.76 |
|
|
Pratylenchidae |
31.0 |
0.81 |
|
|
2-4 years vs 10-15 years of monocropping (average dissimilarity = 34.42%; R =0.19) |
|||
|
Dorylaimida |
Aporcelaimidae |
0.00 |
1.00 |
|
Dorylaimidae |
0.20 |
1.00 |
|
|
Nygolaimidae |
1.20 |
0.03 |
|
|
Qudsianematidae |
0.61 |
0.15 |
|
|
Mononchida |
Mononchidae |
1.01 |
0.04 |
|
Monhysterida |
Monhysteridae |
0.00 |
1.00 |
|
Rhabditida |
Cephalobidae |
4.93 |
0.06 |
|
Panagrolaimidae |
1.48 |
0.03 |
|
|
Rhabditidae |
3.12 |
0.02 |
|
|
Tylenchida |
Hoplolaimidae |
64.63 |
0.02 |
|
Heteroderidae |
7.03 |
0.70 |
|
|
Pratylenchidae |
15.66 |
0.07 |
|
|
5-9 years vs 10-15 years of monocropping (average dissimilarity = 45.12%; R = 0.33) |
|||
|
Dorylaimida |
Aporcelaimidae |
0.00 |
1.00 |
|
Dorylaimidae |
0.13 |
1.00 |
|
|
Nygolaimidae |
0.12 |
1.00 |
|
|
Qudsianematidae |
0.23 |
0.62 |
|
|
Mononchida |
Mononchidae |
0.23 |
0.62 |
|
Monhysterida |
Monhysteridae |
0.00 |
1.00 |
|
Rhabditida |
Cephalobidae |
3.17 |
0.32 |
|
Panagrolaimidae |
0.91 |
0.24 |
|
|
Rhabditidae |
0.51 |
0.02 |
|
|
Tylenchida |
Hoplolaimidae |
67.25 |
0.01 |
|
Heteroderidae |
4.80 |
0.07 |
|
|
Pratylenchidae |
22.66 |
0.40 |
|
Nematode community structure in banana farms with different periods of continuous cropping
The structure index was highest (68.93) at 2-4 years of monocropping and was lowest (46.22) at 5-9 years of continuous monocropping cultivation (Table 9). Enrichment index on the other hand, decreased (73.74-53.69) with the increasing period of monocropping. This reflects a less structured, less complex and unbalanced community, and an agroecosystem becoming lesser enriched over the years of continuous cultivation. Having a similar variety of crop planted over time within a specific field could disrupt the soil and the availability of nutrients. This is because monoculture farms typically utilize a limited variety of fertilizers and promotes a less varied influx of organic matter, leading to imbalances in soil health. These imbalances could adversely affect the proliferation of bacterial and fungal feeder nematodes that contribute to nutrient cycling (Yogaswara et al., 2021).
Table 9: Structure and enrichment indices of Lakatan farms (roots and rhizosphere) under different continuous monocropping cultivation periods.
|
Index |
Cropping duration |
p-value |
||
|
2-4 years |
5-9 years |
10-15 years |
||
|
Structure |
68.93 |
46.22 |
48.72 |
0.11 |
|
Enrichment |
73.74 |
59.67 |
53.61 |
1.00 |
Notably, no channel index was calculated since fungal-feeder nematodes were not observed. The utilization of chemical pesticides with fungicidal properties in farming practices could possibly affect the fungal community, further reducing the number of fungal-feeding nematodes (Zhang et al., 2020). Nonetheless, different monocropping periods exhibit no significant variation in nematode community’s structure and enrichment indices.
In addition, the nematode community structure and soil stability were assessed by utilizing the c-p group succession, as shown by the constructed c-p triangle (Figure 3). Among all the various monocropping periods, farms under 10-15 years of monocropping were the closest to a stressed condition, followed by 5-9 years, and lastly, the 2-4 years being the least stressed environment. As reflected by the succession of c-p groups in the c-p triangle, 10-15 years of continuous cultivation was the closest to having a stress soil condition. This was evident by the increase in c-p 2 nematodes (general opportunists, stress tolerant) and gradual decrease in c-p1 (enrichment opportunists) and c-p 3 -5 (persisters) (de Goede et al., 1993). This further suggests that long term continuous cropping could result in a more stressful environment and a gradual shift from a more enriched opportunist community to general opportunists-dominated community (Berkelmans et al., 2003).
Food web analysis (Figure 4) underscore the soil health status of farms with varying monocropping durations as reflected by the nematode communities. Initial (2-4) years were found to have a maturing food web condition, low C:N, N-enriched with bacterial feature environment, while 5-9 years and 10-15 years of continuous cultivation of banana were placed at quadrant A, indicating a community with high disturbance, N-enriched, and a disturbed food web condition. Food web analysis in this study further suggests that long-term mono-cropping system causes the nematode community to have a less complex food web interactions and will result to a disturbed soil environment (Leiririo et al., 2022). This observation aligns to the findings of Li et al. (2016b) where long-term monocropping of strawberry caused the soil food web to be disturbed as reflected by the nematode community structure.
Disturbed food web and soil ecosystem encompasses various imbalances and disruptions within an ecosystem’s food chain and the physico-chemical properties of the soil. It is an indicative of changes within nematode’s population dynamics, involving a sudden increase or decrease in specific populations such as predators, and decomposers (Ferris, 2010). This is evident from the lower abundance of the predators and bacterivores nematodes recorded in the study.
Conclusions and Recommendations
The varying periods of continuous cultivation of banana plants have contributed to the variations in the nematode communities observed in both the roots and rhizosphere of banana farms. Nematode diversity is adversely influenced by prolonged periods of monocropping, leading to a noticeable prevalence of plant-parasitic nematodes. Differences in food web complexity, maturity, and structure of nematode communities are also associated with the varying periods of continuous banana cropping. The 5-9-year and 10-15-year periods present a stressed and disturbed soil environment. Longer years of monocropping have exerted a negative influence on soil health conditions, as reflected by the nematode community. This study highlights importance of sustainable farming management and underscores the use of nematode community structure in understanding soil conditions and soil biodiversity as influenced by several agricultural practices including monocropping.
Acknowledgement
The authors would like to thank the University of Southern Mindanao for supporting the implementation of this research through the facilities provided by the Department of Biological Sciences, College of Science and Mathematics.
Novelty Statement
Monitoring of nematode communities in agroecosystems particularly in high-value crop plantations can improve conventional practices and management strategies. The use of nematodes as indicators of environmental changes due to agricultural practices in banana plantation such as in this study can guide farmers in managing nematode-related diseases and improve soil health.
Author’s Contribution
Baby Nhor K. Ambel: Conceptualization, investigation, methodology, data analysis, and manuscript writing.
Ma. Teodora N. Cabasan and Nneka Djen A. Matandog: Conceptualization, investigation, methodology.
Bryan Lloyd P. Bretaña and Ma. Teodora N. Cabasan: Conceptualization, supervision, validation of nematode identification.
Neil Pep Dave N. Sumaya: Methodology, and editing.
Florence Roy P. Salvaña: Editing and supervision.
All authors read and approved the final manuscript.
Abbreviations
PPNs, Plant parasitic nematodes; SI, Structure index; CI, Chanel index; MI, Maturity index; c-p, Colonizer-persisters; p-p, plant-parasite.
Funding
Not applicable.
Ethics approval and consent to participate
Not applicable.
Conflict of interest
The authors have declared no conflict of interest.
References
Aleuy, O.A. and Kutz, S., 2020. Adaptations, life-history traits and ecological mechanisms of parasites to survive extremes and environmental predictability in the face of climate change. Int. J. Parasitol. Parasites Wildl., 12: 308–317. https://doi.org/10.1016/j.ijppaw.2020.07.006
Al-Ghamdi, A.A.M., 2021. Relationship between nematodes and some soil properties in the rhizosphere of banana plants. Int. Lett. Nat. Sci., 82. https://doi.org/10.56431/p-o5usqt
Bell, C.A., Namaganda, J., Urwin, P.E. and Atkinson, H.J., 2021. Next-generation sequencing of the soil nematode community enables the sustainability of banana plantations to be monitored. Appl. Soil Ecol., 166: 103999. https://doi.org/10.1016/j.apsoil.2021.103999
Berkelmans, R., Ferris, H., Tenuta, M. and Bruggen, A., 2003. Effects of long-term crop management on nematode trophic levels other than plant feeders disappear after 1 year of disruptive soil management. Appl. Soil Ecol., 23: 223–235. https://doi.org/10.1016/S0929-1393(03)00047-7
Bongers, T., 1994. The reaction of nematodes to manuring. In: Kok, O. (Ed.), Report 4th Int. Biology Olympiad. SLO Inst. Curr. Development. pp. 100-105. https://doi.org/10.1159/000236810
Bongers, T., 1999. The maturity index, the evolution of nematode life history traits, adaptive radiation and cp-scaling. Plant Soil, 212: 13–22. https://doi.org/10.1023/A:1004571900425
Bongers, T. and Ferris, H., 1999. Nematode community structure as a bioindicator in environmental monitoring. Trends Ecol. Evol., 14(6): 224–228. https://doi.org/10.1016/S0169-5347(98)01583-3
Bünemann, E.K., Bongiorno, G., Bai, Z., Creamer, R. E., De Deyn, G., de Goede, R., Fleskens, L., Geissen, V., Kuyper, T. W., Mäder, P., Pulleman, M., Sukkel, W., van Groenigen, J. W. and Brussaard, L., 2018. Soil quality. A critical review. Soil Biol. Biochem.,120(120): 105–125. https://doi.org/10.1016/j.soilbio.2018.01.030
Chen, H., Yang, L. and Zhang, F., 2021. Effects of continuous cotton monocropping on soil physicochemical properties and nematode community in Xinjiang, China. J. Appl. Ecol., 32(12): 4263-4271.
Clarke, K.R., 1993. Non-parametric multivariate analyses of changes in community structure. Austral. J. Ecol., 18: 117–143. https://doi.org/10.1111/j.1442-9993.1993.tb00438.x
Coyne, D.L., Nicol, J.M. and Claudius-Cole, B., 2007. Practical plant nematology: A field and laboratory guide. SP-IPM Secretariat, International Institute of Tropical Agriculture (IITA), Cotonou, Benin. pp. 40.
CropTrust., 2022. Banana Plantain. https://www.croptrust.org/pgrfa-hub/crops-countries-andgenebanks/cro-ps/banana-/-plantain/ Accessed December 23, 2022
Davide, R., 1980. Influence of cultivar, age, soil texture, and pH on Meloidogyne incognita and Radopholus similis on banana. Plant Dis., 64(6): 571. https://doi.org/10.1094/PD-64-571
de Goede, R.G.M., Bongers, A.M.T. and Ettema, C.H., 1993. Graphical presentation and interpretation of nematode community structure: c-p triangles. Med. Fac. Landbouww. Univ. Gent, 58/2b.
De Grisse, A.T., 1969. Redescription ou modification de quelques techniques utilissée dans l’étude des nematodes phytoparasitaires. Mededelingen Rijksfaculteti der Landbouveten Gent: 351–369.
Drenth, A. and Kema, G., 2021. The vulnerability of bananas to globally emerging disease threats. Phytopathology, 111(12): 2146–2161. https://doi.org/10.1094/PHYTO-07-20-0311-RVW
Du Preez, G., Daneel, M., De Goede, R., Du Toit, M.J., Ferris, H., Fourie, H., Geisen, S., Kakouli-Duarte, T., Korthals, G., Sánchez-Moreno, S. and Schmidt, J.H., 2022. Nematode-based indices in soil ecology: Application, utility, and future directions. Soil Biol. Biochem., 169: 108640. https://doi.org/10.1016/j.soilbio.2022.108640
Dutta, T.K. and Phani, V., 2023. The pervasive impact of global climate change on plant-nematode interaction continuum. Front. Plant Sci., 14. https://doi.org/10.3389/fpls.2023.1143889
Ferris, H., 2010. Contribution of nematodes to the structure and function of the soil food web. J. Nematol., 42(1), 63–67. https://www.ncbi.nlm.nih.gov/pmc/ articles/PMC3380510/
Ferris, H. and Bongers, T., 2006. Nematode indicators of organic enrichment. J. Nematol., 38(1): 3–12.
Gomiero, T., 2021. Organic agriculture: Impact on the environment and food quality. Environmental impact of agro-food industry and food consumption. Academic Press. pp. 31–58. https://doi.org/10.1016/B978-0-12-821363-6.00002-3
Herradura, L.E., Lobres, M.A.N., De Waele D., Davide R.G. and Van Den Bergh I., 2012. Yield response of four popular banana varieties from southeast Asia to infection with a population of Radopholus similis from Davao, Philippines. Nematology, 14(7): 889–897. https://doi.org/10.1163/156854112X632196
Hillebrand, H., Bennett D.M. and Cadotte, M.W., 2008. Consequences of dominance: A review of evenness effects in local and regional ecosystem. Ecology, 89(6): 1510-1520. https://doi.org/10.1890/07-1053.1
Hoang, H., Pham, H., Chu, C., Nguyen, T., Tran, L., Trinh, Q., de Boer, T., Brouwer, A., Nguyen, D. and Chu, H., 2021. Investigation of the soil nematode community composition in a monoculture Robusta coffee plantation in Dak Lak, Vietnam. Glob. Ecol. Conserv., 32: e01932. https://doi.org/10.1016/j.gecco.2021.e01932
Jairajpuri, M.S. and Ahmad, W., 1992. Dorylaimida free-living, predaceous and plant-parasitic nematodes. Leiden, Holland: E.J. Brill, xv + 458 pp. https://doi.org/10.1163/9789004630475
Jairajpuri, M.S. and Khan, W.U., 1982. Predatory nematodes (Mononchida) with special reference to India. Associate Publishing Company, New Delhi, India, pp. 131.
John, D.A. and Babu, G.R., 2021. Lessons from the aftermaths of green revolution on food system and health. Front. Sustain. Food Syst., 5(1). https://doi.org/10.3389/fsufs.2021.644559
Khan, M. and Hasan, M., 2010. Nematode diversity in banana rhizosphere from West Bengal, India. J. Plant Prot. Res., 50(3): 263-268. https://doi.org/10.2478/v10045-010-0046-9
Lara Posadas, S.V., Núñez Sánchez, Á.E., López-Lima, D., and Carrión, G., 2016. Plant parasitic nematodes associated to banana roots (Musa acuminata AA) in central Veracruz, México. Rev. Mex. fitopatol., 34(1): 116-130.
Lazarova, S., Coyne, D., Rodríguez, M.G., Peteira, B., and Ciancio, A., 2021. Functional diversity of soil nematodes in relation to the impact of agriculture. A review. Diversity, 13(2): 64. https://doi.org/10.3390/d13020064
Leiririo, J., Karuri, H. and Nyaga, N., 2022. Nematode-based soil food web condition in mung bean under semi-arid conditions. J. Agric. Food Res., 10: 100465. https://doi.org/10.1016/j.jafr.2022.100465
Li, N., Pan, F., Han, X. and Zhang, B., 2016a. Development of soil food web of microbes and nematodes under different agricultural practices during the early stage of pedogenesis of a mollisol. Soil Biol. Biochem., 98: 208–216. https://doi.org/10.1016/j.soilbio.2016.04.011
Li, X., Lewis, E., Liu, Q., Li, H., Bai, C. and Wang, Y., 2016b. Effects of long-term continuous cropping on soil nematode community and soil condition associated with replant problem in strawberry habitat. Sci. Rep., 6(1): 30466. https://doi.org/10.1038/srep30466
Liu, W. and Park, S., 2018. Underground mystery: Interactions between plant roots and parasitic nematodes. Curr. Plant Biol., 15: 25–29. https://doi.org/10.1016/j.cpb.2018.11.004
Manzanilla-López, R.H. and Starr, J.L., 2009. Interactions with other pathogens. Root-Knot Nematodes, pp. 223–245. https://doi.org/10.1079/9781845934927.0223
Nielsen, N., Wall, D. and Six, J., 2015. Soil biodiversity and the environment. Annu. Rev. Environ. Resour., 40(1): 63–90. https://doi.org/10.1146/annurev-environ-102014-021257
Nimisha, A. and Nisha M., 2019. Effect of biofumigation for the management of nematodes in banana. J. Entomol. Zool. Stud., 7(5): 951–956.
Olivares, B.O., Rey, J.C., Lobo, D., Navas-Cortés, J.A., Gómez, J.A., and Landa, B.B., 2021. Fusarium wilt of bananas: A review of agro-environmental factors in the Venezuelan production system affecting its development. Agronomy, 11(5): 986. https://doi.org/10.3390/agronomy11050986
Ortiz, V., Phelan, S. and Mullins, E., 2016. A temporal assessment of nematode community structure and diversity in the rhizosphere of cisgenic Phytophthora infestans-resistant potatoes. BMC Ecol., 16(1): 1–23. https://doi.org/10.1186/s12898-016-0109-5
Ozarslandan, A., Dinçer, D. and Unlu, M., 2019. Nematode damage and management in banana in Turkey. Turkish J. Entomol., 44(12): 3–12. https://doi.org/10.16970/entoted.597606
Panklang, P., Philippe T., Thoumazeau, A., Chiarawipa, R., Sdoodee, S. and Brauman, A., 2022. How 75 years of rubber monocropping affects soil fauna and nematodes as the bioindicators for soil biodiversity quality index. Acta Agric. Scand., B Soil Plant Sci., 72(1): 612–622. https://doi.org/10.1080/09064710.2022.2034930
Pattison, A.B., Limbaga, C., Gervacio, T., Notarte, A., Juruena, M., Dennis, P.G., Lindsay, S.J. and Molina, A., 2020. Integrated management of Fusarium wilt of bananas in the Philippines and Australia. Final Report. Australian Centre for International Agricultural Research (ACIAR), Australia.
ProMusa, 2020. A project to improve the understanding of banana and to inform discussions in this atypical crop. https://www.promusa.org/Philippines Accessed January 11, 2023.
Rojas-Flores, S., De La Cruz-Noriega, M., Nazario-Naveda, R., Benites, S.M., Delfín-Narciso, D., Angelats-Silva, L. and Murga-Torres, E., 2022. Use of banana waste as a source for bioelectricity generation. Processes, 10(5): 942. https://doi.org/10.3390/pr10050942
Ryss, A., 2017. A simple express technique to process nematodes for collection slide mounts. J. Nematol., 49(1): 27–32. https://doi.org/10.21307/jofnem-2017-043
Sánchez-Moreno, S. and Ferris, H., 2018. Nematode ecology and soil health. In: Plant parasitic nematodes in subtropical and tropical agriculture. https://doi.org/10.1079/9781786391247.0062
Sánchez-Moreno, S., and Ferris, H., 2018. Nematode ecology and soil health. In: Plant parasitic nematodes in subtropical and tropical agriculture. Sikora, R.A., Coyne, D., Hallmann, J., and Timper, P. CAB International, Wallingford. pp. 62-68. https://doi.org/10.1079/9781786391247.0062
Shahbandeh, M., 2024. Volume of bananas produced worldwide 2010-2022. https://www.statista.com/statistics/716037/global-banana-market-volume. Accessed March 29, 2024
Sieriebriennikov, B., Ferris, H. and de Goede, R.G., 2014. NINJA: An automated calculation system for nematode-based biological monitoring. Eur. J. Biol., 61: 90–93. https://doi.org/10.1016/j.ejsobi.2014.02.004
Sousa, A.B.P., Rocha, A.D.J., Oliveira, W.D.D.S., Rocha, L.D.S. and Amorim, E.P., 2024. Phytoparasitic nematodes of Musa spp. with emphasis on sources of genetic resistance: A systematic review. Plants, 13: 1299. https://doi.org/10.3390/plants13101299
Stefanovska, T., Skwiercz A., Pidlisnyuk, V., Zhukov, O. and Shapoval, P., 2023. Can nematode communities work as an indicator of soil health in a multiyear miscanthus × miganteus plantation growing in lead-contaminated soil? Agronomy, 13(6): 1620. https://doi.org/10.3390/agronomy13061620
Stirling, G. and Linsell, K., 2014. Nematodes as a biological indicator. https://www.soilquality.org.au/factsheets/nematodes-as-a-biological indicator Accessed January 13, 2023.
Tabarant, P., Villenave, C., Risede, J., Estrade, J., Thurier, J. and Dorel, M., 2011. Effects of four organic amendments on banana parasitic nematodes and soil nematode communities. Appl. Soil Ecol., 49: 59–67. https://doi.org/10.1016/j.apsoil.2011.07.001
Tharani, G., Alagesan, A., Jawahar, S., Saranya, S., Balakrishnan, A., Padmanaban, B. and Manivannan, S., 2021. Distribution and molecular characterization of Helicotylenchus multicinctus (Tylenchida: Hoplolaimidae) from banana roots in Namakkal region, Tamil Nadu. Int. Bot. Stud., 6(2): 965–971.
Tian, X.L., Zhao, X.M., Zhao, S.Y., Zhao, J.L. and Mao, Z.C., 2020. The biocontrol functions of Bacillus velezensis strain Bv-25 against Meloidogyne incognita. Front. Microbiol., 13. https://doi.org/10.3389/fmicb.2022.843041
Whitehead, A.G. and Hemming, J.R., 1965. A comparison of some quantitative methods extracting small vermiform nematodes from the soil. Ann. Appl. Biol., 55: 25–38. https://doi.org/10.1111/j.1744-7348.1965.tb07864.x
Yeates, G. and Bongers, T., 1999. Nematode diversity in agroecosystems. Invertebrate Biodiversity as Bioindicators of Sustainable Landscapes. pp. 111–135. https://doi.org/10.1016/B978-0-444-50019-9.50010-8
Yingying, Y.E., Yichao, R.U.I., Zhaoxia, Z., Xunyang, H.E., Kelin, W. and Jie, Z., 2020. Responses of soil nematode community to monoculture or mixed culture of a grass and a legume forage species in China. Pedosphere, 30(6): 791–800. https://doi.org/10.1016/S1002-0160(20)60039-X
Yogaswara, D.A., Kasmara, H. and Hermawan, W., 2021. Using nematode community to evaluate banana soil food web in Mekargalih, Cianjur, West Java. Pertanika J. Trop. Agric. Sci., 44(2): 465–483. https://doi.org/10.47836/pjtas.44.2.12
Zhang, Y., Li, S., Li, H., Wang, R., Zhang, Q. and Xu, J., 2020. Fungi–nematode interactions: Diversity, ecology, and biocontrol prospects in agriculture. J. Fungi, 6(4): 206. https://doi.org/10.3390/jof6040206
Zhong, S., He, Y., Han, N., Zhou, Z., Ma, W., Zeng, H., and Jin, Z., 2012. Effect of continuous cropping of banana on soil nematode community structure and diversity. Chin. J. Eco-Agric., 20(5): 604–611. https://doi.org/10.3724/SP.J.1011.2012.00604
Zhong, S., Mo, Y., Guo, G., Zeng, H. and Jin, Z., 2014. Effect of continuous cropping on soil chemical properties and crop yield in banana plantation. J. Agr. Sci. Tech., 16: 239–250. https://jast.modares.ac.ir/article-23-3022-en.pdf
To share on other social networks, click on any share button. What are these?





