Meat Quality of Commercial Spent Hens and Broilers
Research Article
Meat Quality of Commercial Spent Hens and Broilers
Shirina Akter Toma1, Shah Ahmed Belal2, Md Abdul Baset3, Md Nazim Uddin3*
1Department of Animal Science, Habiganj Agricultural University, Habiganj-3300, Bangladesh; 2Department of Poultry Science, Sylhet Agricultural University, Sylhet-3100, Bangladesh; 3Department of Livestock Production and Management, Sylhet Agricultural University, Sylhet-3100, Bangladesh.
Abstract | Meat quality is an important topic and a fundamental requirement for consumers. We aimed to assimilate the physicochemical properties of drumstick, wing, thigh, and breast meat of commercially spent hens and broilers. The commercial spent hens (n=30) and broilers (n=30) were slaughtered at market age (306 days spent hen and 28 days broiler). The thigh, drumstick, breast, and wing muscles from both sides of the carcasses were separated. The right thigh, drumstick, breast, and wing meat was vacuumed pack and kept at 4°C until it was examined for cooking loss, drip loss, pH, color, and nutritional qualities. Meat from the breast, and thighs of spent hens contained more protein than broiler meat. The spent hen’s breasts had a pH that was noticeably higher yet the broiler’s cooking loss was greater. Overall collagen content was highest in the thigh and breast muscles of the spent hen but the concentration of soluble collagen in broiler meat was greater. The spent hen was distinguished from broilers by having more moisture and ash contents (except wing meat of broiler), better pressing loss and Warner-Bratzler shear force value, and less intramuscular fat. Results revealed that broilers had the highest level of potassium except drumstick while spent hens had the highest concentration of microelements. Broiler’s thigh and breast meat had greater amino acid composition but wing and drumstick meat of spent hen had higher concentration of amino acid. Spent hen meat has several distinctive qualities and shows greater advantages over conventional broilers. The broiler’s meat compared to spent hens has a variety of unique qualities. The current information can be useful for consumers’ healthy lifestyles for the selection of desired muscle meat from commercial spent hen and broiler.
Keywords | Broilers, Spent hen, Meat, Muscles, Nutritional properties, Quality traits
Received | June 26, 2023; Accepted | December 10, 2023; Published | February 15, 2024
*Correspondence | Md Nazim Uddin, Department of Livestock Production and Management, Sylhet Agricultural University, Sylhet-3100, Bangladesh; Email: uddinmn.alm@sau.ac.bd
Citation | Toma SA, Belal SA, Baset MA, Uddin MN (2024). Meat quality of commercial spent hens and broilers. Adv. Anim. Vet. Sci., 12(4):647-656.
DOI | https://dx.doi.org/10.17582/journal.aavs/2024/12.4.647.656
ISSN (Online) | 2307-8316
Copyright: 2024 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
INTRODUCTION
The poultry industry has made a noticeable development in Bangladesh over the last few decades. At present, the poultry industry is considered a highly commercial business. The chicken industry contributes 22-27% of the total protein needed by human and offers noteworthy job opportunities (Moula et al., 2020). Meat quality attributes physicochemical characteristics (pH, cooking loss, color, water holding capacity, drip loss, moisture content, muscle fiber diameter, myoglobin content, collagen content, extractable proteins, shear force value, and proximate composition) and sensory characteristics (Polizer et al., 2019). Spent hens are the impressive poultry industry by-products, which typically sell for less money than broiler meat. Chicken meat is one of the most palatable worldwide because of its carbohydrates, high protein, minerals, polyunsaturated fatty acids (PUFA), amino acids, and lower fat content (Kamboh and Zhu, 2013). Jayasena et al. (2013) reported, The cost of poultry meat is significantly lower than that of cattle, buffalo, lamb, goat, and pork meat which greatly encourages manufacturing and intake of poultry meat. Chicken meat is preferred over red meat due to its many health benefits, lower cholesterol, and lower fat content. Chicken meat is easy to handle and has no religious restrictions for consumption (Jaturasitha et al., 2008).
The breed and location of the muscles in chicken meat samples also affected their physicochemical characteristics (Choe and Kim, 2020). Physicochemical properties and nutritional value are typically regarded as the key important, fundamental factors in purchaser attitudes toward meats and meat products. Meat’s physicochemical properties such as its pH, color, cooking loss value, shear force value, drip loss, collagen content, and ability to hold water are crucial for processing meat and influencing consumer acceptance. The spent hens (80-100 weeks) are a probable source of chicken meat, but their meat was unacceptably tough due to the abundance of collagen which was heat-stable (Kang et al., 2009). The majority of commercial broilers are raised for four to seven weeks, although slower-growing breeds rear their young for about 14 weeks (https://en.wikipedia.org). However, despite their potential as protein sources, their poor organoleptic properties and low edible meat production result in low market value (Reddy et al., 2016). The meat is extremely chewy and tough that is why, spent hen meat is more compatible with the production of convenience meat products (Petek and Cavusoglu, 2021).
Numerous studies on chicken meat quality over the last few decades have primarily concentrated on carcass features, color, pH, and water-holding capacity (Petek and Cavusoglu, 2021). Additionally, there is little research on physicochemical properties of meat and the nutritional value of some old hens has not been assessed by scientific investigations. Thus, the purpose of the present work was to identify the physicochemical composition of spent hen and broiler drumstick, breast, thigh, and wing muscles and to compare physicochemical characteristics among the thigh, breast, drumstick, and wing muscles of broilers and spent hen.
MATERIALS AND METHODS
Birds and meat sample preparation
A standard marketable spent hen (n= 30, 1.5-2.0 Kg) and broilers (n= 30, 1.5-2.0 Kg) were collected from the local market near SAU, Sylhet. After starving the birds for eight hours, live weight was recorded. The spent hen and broilers were slaughtered using the traditional neck cut procedure and eviscerated 2 minutes post-bleeding (Chen et al., 2016). The biological sample was buried in the ground. Using the water immersion procedure, the eviscerated carcass was refrigerated before being chopped and deboned. The thigh, drumstick, breast, and wing muscles from both sides of the carcasses were separated. Excess fat, visible skin, and connective tissue were clipped from the thigh, breast, drumstick, and wing muscle and weighted. The right thigh, drumstick, breast, and wing meat was vacuumed pack and kept at 4°C until it was examined for cooking loss, drip loss, pH, color, and nutritional qualities (Amino acid and Minerals). The remaining halves of each bird were minced separately, vacuum-packed, and kept in the freezer at -20°C till additional nutritional examination. Before any research, the meat was melted overnight at 4℃. The samples were analyzed immediately for various physicochemical and nutritional characteristics.
Proximate analysis
The trimmed separated, boneless, fatless, breast, thigh, drumstick, and wing meat samples were used to determine the proximate configuration (moisture, crude protein, and ash content) according to the standard procedures of the Association of Official Analytical Chemists (AOAC, 2007).
Identifying the physicochemical and nutritive characteristics of meat
pH measurement
The meat samples’ pH was measured in a duplicate manner using a portable pH meter (Orion model 301; Orion, Beverly, MA, USA) prepared with a glass electrode in the form of a probe. That electrode was calibrated in 4.00 and 7.01 pH values of calibration buffers at room temperature (Choe and Kim, 2020).
Determination of color
Meat color was determined by using a colorimeter (Chroma meter CR-210; Minolta, Tokyo, Japan). The color was measured after a 25-minute bloom period. The diameter of lighting area 50 mm and measurement area 8 mm. CIE L* (Lightness), a* (redness), and b* (yellowness) color values were determined on the exterior of triplicate meat samples (Choe and Kim, 2020).
Measurement of cooking loss
Around 100 g blocks of flesh from the thigh, breast, drumstick, and wing meat were used to measure the meat samples cooking loss. The sample blocks were sealed in plastic bags and cooked in a preheated water bath until the internal core temperature reached 70°C. The digital needle-tipped thermometer was used to check the interior temperature (H 1145, Hanna Instruments, Italy). After the sample was taken out of the water bath and the water was drained from the bag, the prepared samples were promptly chilled in running water at a temperature of 18°C for 30 minutes. Paper towels were used to absorb any extra moisture before samples were eventually weighed. According to Vargas-Ramella et al. (2022), the cooking loss was measured as the percentage of initial weight lost and expressed using the equation below.
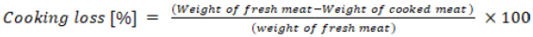
Measurement of drip loss
Drip loss was determined by EZ-Drip loss methods of Kaic et al. (2023) with some modification. Muscle samples were individually weighed, packed, and suspended in plastic bags at 4℃ for 24 h, and the weight loss percentage during storage was expressed as drip loss.
Measurement of pressing loss
The sample’s pressing loss was determined using the technique of Li et al. (2012). A compression machine (YYW-2, Nanjing Soil Instrument, Nanjing, Jiangsu, China) was used to evaluate pressing loss. The weight loss percentage both prior to and following compression of flesh was used to determine pressing loss, and the findings were represented as a percentage.
Determination of moisture content
Halogen moisture analyzer (HR73, Mettler Toledo, Switzerland) was use to determine the moisture. The moisture contents of fresh and frozen meat samples were assessed. Each meat sample, weighing 2.5 g, was placed in the dish of aluminum and dried at 105°C. The final result expresses a moisture content % of the meat sample.
Determination of protein
The determination of protein involves the determination of the Nitrogen content of samples which is multiplied by 6.25. For determining the protein, a 1 gm sample was taken with nitrogen-free paper and placed into a Kjeldahl flask. Then 2 gm of mixed catalyzer was added into the flask. After that 25 ml of H2SO4 was added and heated by raising the temperature. When digestion was completed, the flask was removed from the digestion chamber and blown to cool, and 100 ml of distilled water were added. After digestion 20 ml 2% Boric acid solution were taken into a 300 ml conical flask and mixed with 1 drop indicator then placed into a distillation set. Secondly, 90 ml of 40% NaOH solution were taken into a Kjeldahl flask and some glass rod and zinc pieces were added into the flask to increase the chemical reaction. Finally, the Kjeldahl flask was placed into the upper portion of the distillation set and allowed to distill the sample. After the completion of distillation, the sample was allowed to be titered with 0.1N HCL drop by drop until the pale greenish color was removed; the titration value was calculated. According to Talpur et al. (2018), From the recorded data Protein value was calculated by using this formula:
Nitrogen content of the sample (%) = 0.1 × 0.014 × 100 × Titration value/ Wt. of the sample (gm)
Protein percentage = Nitrogen content×6.25
Determination of ash
Representative samples were analyzed for ash by using a muffle furnace (Muffle furnace LT 5/12 with lift door, Germany). Take the weight of the empty crucible. One g sample was kept in a crucible and reweighted. The crucible was kept in the oven at 105℃ for 24 h. The crucible was cooled for 10 minutes, kept (10-15 min) in the digestion plant to remove the foam, placed in a muffle furnace at 600℃ for 6h, and reweighted. According to Sarker et al. (2022), From the recorded data Ash content was calculated by using the following formula:
Ignitate sample weight = After muffle furnace wt. – Empty crucible wt.
Ash% = Ignitate sample weight /Dry sample wt. ×100
Determination of fat
Representative samples were analyzed for ether extract by using the Soxhlet apparatus (Xie et al., 2021). Five gm of sample were taken in a thimble and extracted continuously with Diethyl ether for a period of 6 h. The round bottom flask containing ether and ether extract was kept first in the fume cupboard and then in an oven at 60°C for 2 h to evaporate the rest of the ether. Cooled in desiccators and weighed.
Fat percentage = Wt. of fat / Wt. of sample ×100
Warner bratzler shear force measurement
The sample’s pressing loss was determined using the Vargas-Ramella et al. (2022) technique. The Warner-Bratzler shear force of a fresh meat sample was tested to determine tenderness. Following the assessment of cooking losses, shear force was obtained for each meat sample steak. Each meat sample steak was sliced into at least 5 cores with an average diameter of 0.5 inches and a direction parallel to the muscle fiber direction. The highest force necessary in each samples 5 cores were measured for using an Instron Universal Testing Machine (Model 3342; Instron Corporation, Norwood, MA, USA) with a V-shaped shear blade, a load cell of 50 kg, and a cross-head speed of 200 mm/min and expressed as Newton (N). In the statistical study, the average shear force value from each sample steak was employed.
Determination of amino acid composition
The amounts of amino acid in the samples of freeze-dried meat were measured following Huo et al. (2021) by utilizing the Amino Acid Analyzer (L-8900, HITACHI, Tokyo, Japan), and represented as a gram of amino acids (g/100 g) for each 100 g of freeze-dried meat samples.
Determination of mineral
The samples mineral element content was examined according to Lorenzo et al. (2019). Each 0.1 g of the sample was combined with 10 mL of 65% suprapure nitric acid from Merck in Germany to assess the mineral content. The sample was then processed using microwave digestion equipment (MARS 5, CEM Co., Matthews, NC, USA) at 1200 Watts, 150 pressure, and 150℃ for 30 min. An Optima 4300 DV inductively coupled plasma-optical emission spectrometer was used to evaluate the pretreatment sample (5 mL) after it had been combined with DDW (5 mL) (ICP-OES, Perkin-Elmer, Norwalk, NJ, USA). Phosphorus, copper, potassium, iron, calcium, magnesium, sodium, manganese, aluminum, zinc, and selenium were all found in the produced solutions.
Soluble collagen content and total collagen determination
The amount of soluble and total collagen was calculated with slight modifications from Biesek et al. (2020). For total collagen content, 0.5 g samples were hydrolyzed with 5 ml of 6 N HCl at 110°C for 24 hours. The hydrolysate was filtered, neutralized with 10 M NaOH, and cleared with activated carbon. The neutralized hydrolysate was then made into a final amount of 50 ml with distilled water. A spectrophotometer (DU 530, Beckman Instruments Inc., Fullerton, CA, USA) operating at a wavelength of 550 nm was used to measure the quantity of hydroxyproline. The factor of 7.25 was used to translate the concentration of hydroxyproline to the collagen content.
7 g of meat samples were homogenized with 28 ml of 25% Ringer’s solution to extract the soluble collagen. The homogenate sample was centrifuged for 30 minutes at 1600×g and 4°C, and heated for 70 min at 77°C. The supernatant was collected and hydrolyzed for 24 hours at 110°C in 10 liters of 6N HCl. The methodology for calculating total collagen was then followed to calculate the amount of soluble collagen. Milligrams of collagen per gram of dry meat were used to indicate the amounts of soluble and total collagen. The solubility of collagen was computed as follows:
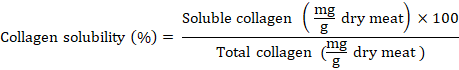
Statistical analysis
To assess the data, a one way ANOVA was performed. Duncan’s multiple range tests were used to determine significant differences at p = 0.05. The statistical analysis was performed using the SPSS version 17.0 (SPSS Inc., Chicago, IL, USA) software program for Windows, and the findings were given as mean ± standard deviation deviation of triplicate determination.
RESULT AND DISCUSSION
Proximate composition of spent hen and broiler meat
The proximate compositions of Spent hen and broiler breast, thigh, drumstick, and wing meat are presented in Table 1. The proximate components of meat were influenced by the breeds.
Additionally, Table 1 demonstrated the protein content of both broiler and Spent hen meat was substantially greater (P<0.05) than that of the meat from the thigh, drumsticks, and wings. Similar results were found in broilers, crossbred chickens, and spent hens (Chen et al., 2016). The spent hen’s breast meat had a considerably (P<0.05) higher protein content than the breast meat from broilers. Polish, Danish, and English Pekin breast muscles contain a considerably (P<0.05) greater protein content than the Spent hen and broiler evaluated by Kokoszynski et al. (2020). Spent laying ducks (breast and thigh muscles) had higher protein content as evaluated by Qiao et al. (2017). Talpur et al. (2018) found that broiler’s breast meat had a significantly (P<0.05) higher protein content than the research being done now.
Table 1: Proximate composition and collagen content of breast, thigh, drumstick, and wing meat of spent hens and broilers.
|
Parameters |
Spent hen (n=30) |
Broiler (n=30) |
||||||
|
Breast |
Thigh |
Drumstick |
Wing |
Breast |
Thigh |
Drumstick |
Wing |
|
|
Protein (g/100g) |
22.32a ±0.48 |
19.47c ±0.37 |
20.42b± 0.40 |
19.37d± 0.57 |
21.55a± 0.84 |
18.56d± 0.44 |
21.20b± 0.40 |
19.42c± 0.57 |
|
Intramuscular fat(g/100g) |
1.92c ±0.13 |
3.42a ±0.16 |
2.72b± 0.23 |
1.52d± 0.29 |
2.82b± 0.31 |
3.54a± 0.88 |
2.56c± 0.03 |
2.25d± 0.29 |
|
Ash (g/100g) |
1.42a ±0.10 |
0.98c ±0.03 |
0.96d± 0.09 |
1.02b± 0.08 |
1.32a± 0.35 |
0.94c± 0.03 |
0.92d± 0.09 |
1.05b± 0.08 |
|
Moisture (g/100) |
75.17b ±0.53 |
76.46a ±0.61 |
74.54c± 0.67 |
73.87d± 0.64 |
74.81a± 0.17 |
73.52b± 0.91 |
66.55c± 0.96 |
61.69d± 0.57 |
|
Total collagen (mg/g) |
3.89c ±0.17 |
5.39a ±0.26 |
4.19b± 0.16 |
3.36d± 0.31 |
2.25d± 0.67 |
4.25a± 0.67 |
3.29c± 0.34 |
3.52b± 0.16 |
|
Soluble collagen (mg/g) |
0.97d ±0.06 |
1.67a ±0.09 |
1.49b± 0.13 |
1.11c± 0.04 |
0.93d± 0.20 |
2.00a± 0.14 |
1.52b± 0.13 |
1.29c± 0.06 |
|
Collagen solubility (%) |
30.67b ±0.86 |
32.7a ±0.76 |
29.27c± 0.96 |
25.74d± 0.84 |
30.24a± 1.42 |
32.71b± 1.93 |
19.42d± 5.96 |
25.14c± 0.38 |
Mean values are presented as mean±SD. Means with different small letters in the same row indicated significantly different (p< 0.05).
A significant (P<0.05) highest protein content was noted in spent breeder hen meat compared to Spent layer meat and Spent broiler meat (Reddy et al., 2016). Park and Kim (2021) revealed that commercial broilers had significantly (P<0.05) higher protein content than the present study. Despite the fact that there was no discernible variation between the four genotypes of duck, the Nageswari duck had a greater protein content than the others (Sarker et al., 2022). According to Kokoszynski et al. (2020), the leg and breast muscles of the compared ducks of various genotypes displayed a significant concentration of Protein content. Compared to leg muscles, the breast muscles had a higher content of protein. The content of pH was significantly (P<0.05) higher in broiler thigh and lower in spent hen breast (Lee and Kim, 2021).
The intramuscular fat (IMF) contents of thigh meat were significantly (P<0.05) higher than that of the wing, breast, and drumstick meat in both broiler and Spent hen. Similar results were found in Polish Pekin, Danish Pekin, and English Pekin (Kokoszynski et al., 2020). Intramuscular fat (IMF) contents of broiler meat were significantly (P<0.05) higher than the Spent hen meat except drumstick. Jung et al. (2011) indicated that the comparatively low intramuscular fat content of wing, breast, and thigh meat is possibly due to the sole compositional physiognomies. According to studies done in 2019 by Ismoyowati and Sumarmonoal, duck meat has an average fat level of 1-2% more than that of chicken and turkey.
The highest amount of fat content was found in the Spent quail than the young quail (Boni et al., 2010). The intramuscular fat (IMF) contents of the South and North Korean native chickens (Jeon et al., 2010) were lower than in the present study. Ostrich, Turkey, and Broiler had a low amount of intramuscular fat found by Jukna et al. (2012). The moisture content of the Spent hen was significantly (P<0.05) higher in the thigh than in the wing meat. The moisture content of broilers was significantly (P<0.05) higher in the breast than in the thigh meat. The overall moisture content of the Spent hen meat was suggestively (P<0.05) higher than the broiler meat. The meat of Spent quail showed lower moisture content than young quail meat (Boni et al., 2010). No difference in moisture content was observed in the breast and thigh meat of commercial broilers, North and South Korean native chickens reported by Jeon et al. (2010). The ash content was considerably (P<0.05) higher in the breast meat than the drumstick. The ash content was almost similar in the thigh and drumstick meat of the Spent hen and broiler while the wing muscle contained slightly higher ash content. Similar results were found in the thigh meat of the Mini chicken (Talpur et al., 2018). Broiler meat had significantly (P<0.05) lowest ash content compared to the Spent layers meat and breeder meat (Reddy et al., 2016). Boni et al. (2010) reported that the Young quail contained more ash than the Spent quail. The ash content of Ostrich (Jukna et al., 2012) was significantly (P<0.05) higher than the spent hen and broiler.
The collagen characteristics of Spent hen and broiler breast, thigh, drumstick, and wing meat are presented in Table 1. The total, soluble, and collagen solubility of the thigh meat were considerably (P<0.05) higher than that the meat of wing, drumstick, and breast. Discrepancies in the collagen properties among the four muscles could be recognized due to dissimilarities in the position of muscles in the body. It has also been revealed that collagen solubility declined with augmented collagen cross-linking, and crosslinking escalations as the animal ages and the activity of muscles in the body. Therefore, thigh meat had higher total collagen content compared with other muscles.
Color and physicochemical characteristics of spent hen and broiler meat
Color and Physicochemical properties of Spent hen and broiler breast, thigh, drumstick, and wing meat are presented in Table 2. Several muscles affected the color of the meat, which is a crucial characteristic in the production of chickens. Compared to drumstick, thigh, and wing meat, the L* and b* values of the breast meat were significantly greater (p<0.05). Thus, the thigh meat was significantly (P<0.05) redder than the breast, drumstick, and wing meat. Due to its significant and direct influence on consumers’ purchasing rates and preferences, color is a crucial component (Kim et al., 2020).
As shown in Table 2, the pH, cooking loss, and pressing loss were significantly (P<0.05) higher (p<0.05) in the thigh than in the breast and drumstick while the wing showed the lowest values of pH, the cooking loss, and pressing loss. The drip loss was significantly (P<0.05) higher in the breast meat than in the thigh, drumstick, and wing whereas the WBSF was significantly (P <0.05) higher in the drumstick and lower in the breast muscle. Reduced loss of meat from cooking and pressing is beneficial as it will prevent weight loss, especially when handling processed meat products. This outcome may have resulted from variations in the muscles’ soluble and total collagen contents. The thigh meat contains the highest amount of soluble and total collagen than the other muscles were revealed in Table 3.
Amino acid composition of spent hen and broiler meat
The amino acid profiles of breast meat, thigh, drumstick, and wing meat are shown in Table 4. The meat portions of Spent hen muscle types of chicken revealed significant (P<0.05) variances in their amino acid contents except for arginine, methionine, and tyrosine in the breast.
Table 2: Color and physicochemical properties of breast, thigh, drumstick, and wing meat of spent hens and broilers.
|
Parameters |
Spent hen (n=30) |
Broiler (n=30) |
||||||
|
Breast |
Thigh |
Drumstick |
Wing |
Breast |
Thigh |
Drumstick |
Wing |
|
|
Color L* |
53.28a± 0.92 |
49.79b± 0.81 |
48.71c± 0.63 |
47.69d±0.62 |
53.81a±0.92 |
49.23b±1.11 |
48.07±0.37c |
45.96d±0.56 |
|
Color a* |
7.07d± 0.82 |
14.97a± 0.42 |
12.57b± 0.58 |
10.77c±0.52 |
5.37d±0.02 |
11.58a±0.85 |
10.87b±0.81 |
10.17c±0.12 |
|
Color b* |
12.23a± 0.91 |
9.43b± 0.21 |
5.43c± 0.71 |
4.33d±0.49 |
3.81d±0.36 |
5.12b±0.25 |
5.31a±0.88 |
4.30c±0.46 |
|
pH |
5.80b± 0.19 |
6.10a± 0.29 |
5.20c± 0.33 |
4.90d±0.36 |
5.73d±0.23 |
6.30a±0.33 |
5.92c±0.29 |
6.20b±0.19 |
|
Cooking loss (%) |
22.24b± 0.76 |
23.44a± 0.86 |
21.64c± 0.66 |
19.20d±0.63 |
25.19c±0.12 |
33.33a±0.29 |
31.20b±0.61 |
23.61d±0.31 |
|
Drip loss (%) |
4.80a± 0.39 |
4.30b± 0.49 |
3.80c± 0.59 |
2.80d±0.79 |
2.95b±0.15 |
3.93a±0.69 |
2.70c±0.29 |
2.25d±0.52 |
|
Pressing loss (%) |
26.87a± 0.89 |
26.54b± 0.33 |
24.67c± 0.69 |
23.77d±0.83 |
26.12b±1.75 |
26.89a±0.77 |
24.09c±0.30 |
23.57d±0.85 |
|
WBSF (N) |
54.47c± 2.89 |
64.49b± 2.81 |
74.42a± 2.58 |
34.52d±2.88 |
41.29a±1.82 |
38.22b±7.81 |
32.42d±2.98 |
36.26c±2.76 |
Mean values are presented as mean±SD. Means with different small letters in the same row indicated significantly different (P < 0.05). WBSF = Warner-Bratzler shear force, N = Newton
With the exception of a few specific amino acids in the non-essential fraction, most likely cysteine, proline, and glycine, the breast meat showed considerably (P<0.05) higher total amino acid levels and the essential amino acid/non-essential ratios (E/NE) than the thigh, drumstick, and wing meat. Among all muscle types, lysine, leucine, arginine, and threonine were the main amino acids. The leucine and lysine contents were significantly (P<0.05) higher values in breast meat than those of other parts of spent hen. The leucine and lysine contents were higher in broiler found by Macelline et al. (2021). There were significant (P<0.05) differences among breast, thigh, drumstick, and wing meat. In the non-essential amino acid fraction, the glutamic acids were the amplest amino acid, followed by alanine, aspartic acid, and proline, whereas the lowermost contents were stated in cysteine for breast, thigh, drumstick, and wing meat of Spent hen. According to assessments of other red meats like cattle, buffalo, sheep, and goat as well as white meats like rooster and hen (Franco et al., 2012), duck, goose, and turkey meat (Geldenhuys et al., 2015), the main non-essential amino acids were glutamic and aspartic acid, while the essential amino acids were lysine and leucine. Moreover, the essential amino acid: Non-essential amino acid content of the breast, thigh, drumstick, and wing in the current study was significantly (P<0.05) higher than that found by Franco et al. (2012). The comparatively higher amount the essential amino acid: non-essential amino acid was recorded for the breast and drumstick as revealed in Table 4. Therefore, the result noted that muscle types of Spent hen considerably influenced the amino acid composition. Zhao et al. (2011) indicated that the intensity of meat amino acids was prejudiced by the muscle location and age of the slaughter birds. Therefore, the difference in amino acid existence may be due to the different muscle positions in the body. The World Health Organization’s study (WHO, 2007) lists the amino acid requirements for humans (g/100 g/d). Spent hens would be an excellent supply of these vital amino acids.
Mineral composition of spent hen and broiler meat
The mineral composition of wing, thigh, breast, and drumstick meat of Spent hen and broiler are shown in Table 5. In the current research, macromineral potassium revealed the higher attention, followed by magnesium, phosphorous, sodium, and calcium. The outcome is parallel to the outcomes of Lorenzo et al. (2019) who stated that phosphorous and potassium were the major minerals in duck, chicken, and ostrich meat. The value of potassium was considerably (P<0.05) higher in drumstick meat than in the breast, thigh, and wing meat of Spent hen. The primary sources of potassium in the human diet are milk, fruits, and vegetables. However, the present research showed that 100 g of Spent hen drumstick flesh would provide around 20% of the daily recommended potassium intake. However, the potassium content was significantly (P<0.05) higher in breast meat than in the thigh, drumstick, and wing meat of the broiler. The micro minerals noted in the current study were aluminum, selenium, manganese, zinc, and iron. Like amino acid compositions, micro minerals were different with the different muscle types of Spent hen and broiler. In the current study, micro minerals in the thigh, drumstick, breast, and wing meat of zinc revealed the highest concentration, followed by iron, aluminum, manganese, and selenium. Additionally, the authors reported that the meat from spent hens had higher levels of zinc and iron than that from turkeys. This means that spent hen meat had the highest levels of micro minerals and could therefore be considered the most important nutritional contribution to the micro mineral requirements of human dietetics.
Table 4: Amino acid composition of breast, thigh, drumstick, and wing meat (g/100g dry weight) of spent hens and broilers.
|
Amino acids (AA) |
Spent hen (n=30) |
Broiler (n=30) |
||||||
|
Breast |
Thigh |
Drumstick |
Wing |
Breast |
Thigh |
Drumstick |
Wing |
|
|
Essential AA |
||||||||
|
Arginine (Arg) |
4.682a± 0.132 |
4.505b± 0.120 |
4.375c± 0.205 |
4.190d± 0.082 |
4.789a± 0.032 |
4.625a± 0.020 |
4.201b± 0.052 |
4.120b± 0.312 |
|
Histidine(His) |
2.724a± 0.113 |
2.004c± 0.042 |
1.960d± 0.070 |
2.030b± 0.042 |
3.137a± 0.103 |
2.844b± 0.612 |
1.890d± 0.071 |
2.190c± 0.054 |
|
Isoleusine (Ile) |
3.460a± 0.061 |
3.161b± 0.078 |
3.014c± 0.068 |
2.990d± 0.102 |
4.831a± 0.051 |
4.046b± 0.098 |
3.209c± 0.015 |
2.810d± 0.115 |
|
Leucine (Leu) |
6.126a± 0.144 |
5.711b± 0.117 |
5.404c± 0.201 |
5.402c± 0.120 |
7.171a± 0.104 |
6.281b± 0.118 |
5.342c± 0.102 |
5.292c± 0.150 |
|
Lysine (Lys) |
6.468a± 0.141 |
6.121b± 0.140 |
5.887c± 0.143 |
5.589d± 0.140 |
7.846a± 0.122 |
6.820b± 0.102 |
5.777c± 0.135 |
5.619c± 0.129 |
|
Methionine (Met) |
1.920a± 0.062 |
1.746b± 0.052 |
1.530± 0.030 |
1.504± 0.054 |
1.990a± 0.031 |
1.804b± 0.099 |
1.570c± 0.040 |
1.524c± 0.063 |
|
Phenylalanine(Phe) |
3.050a± 0.073 |
2.846b± 0.090 |
2.590c± 0.052 |
2.608c± 0.078 |
4.001a± 0.052 |
3.648b± 0.070 |
2.770c± 0.055 |
2.708c± 0.007 |
|
Threonine (Thr) |
4.174a± 0.080 |
3.582b± 0.101 |
3.448c± 0.070 |
3.105d± 0.042 |
4.191a± 0.710 |
3.728b± 0.063 |
3.588c± 0.050 |
3.195d± 0.094 |
|
Valine (Val) |
3.640a± 0.078 |
3.260b± 0.080 |
3.119c± 0.110 |
3.010d± 0.090 |
3.781a± 0.089 |
3.560c± 0.079 |
3.682b± 0.020 |
3.310d± 0.494 |
|
Total essential AA |
36.246b± 0.884 |
32.936a± 0.82 |
31.327c± 0.949 |
30.428d± 0.75 |
41.737± 1.294 |
37.356± 1.261 |
32.029± 0.54 |
30.768± 1.418 |
|
Non-essential AA |
||||||||
|
Alaline (Ala) |
4.660a± 0.105 |
4.276b± 0.091 |
3.780d± 0.086 |
4.106c± 0.061 |
4.610b± 0.017 |
4.883a± 0.040 |
3.691d± 0.103 |
4.190c± 0.057 |
|
Aspartic acid (Asp) |
6.260b± 0.290 |
6.575a± 0.180 |
5.840c± 0.208 |
5.208d± 0.125 |
6.350b± 0.150 |
6.680a± 0.264 |
5.681c± 0.120 |
5.388d± 0.027 |
|
Cysteine (Cys) |
0.544b± 0.012 |
0.610a± 0.045 |
0.450c± 0.033 |
0.624a± 0.054 |
0.792a± 0.068 |
0.651b± 0.020 |
0.544c± 0.063 |
0.639b± 0.083 |
|
Glutamic acid(Glu) |
11.030a± 0.261 |
10.459b± 0.192 |
9.70d± 0.310 |
9.980c± 0.212 |
11.532a± 0.294 |
10.506b± 0.115 |
9.870c± 0.011 |
9.955c± 0.249 |
|
Glycine (Gly) |
2.730b± 0.066 |
2.767a± 0.092 |
2.456c± 0.050 |
2.756a± 0.064 |
2.659b± 0.040 |
2.827a± 0.012 |
2.410c± 0.027 |
2.695b± 0.045 |
|
Proline (Pro) |
4.720b± 0.148 |
5.040a± 0.150 |
3.850d± 0.064 |
4.524c± 0.106 |
4.700± 0.180b |
5.141a± 0.105 |
3.810d± 0.064 |
4.424c± 0.115 |
|
Serine (Ser) |
3.010a± 0.065 |
2.820b± 0.076 |
2.482c± 0.042 |
2.792b± 0.087 |
3.030a± 0.024 |
2.865b± 0.055 |
2.400d± 0.042 |
2.780c± 0.050 |
|
Tyrosine (Tyr) |
2.560a± 0.067 |
2.400b± 0.099 |
2.257c± 0.070 |
2.194d± 0.215 |
2.581a± 0.076 |
2.435± 0.059b |
2.288c± 0.066 |
2.210c± 0.200 |
|
Total Non-essential AA |
35.514± 1.014 |
34.947± 0.925 |
30.815± 0.863 |
32.184± 0.924 |
36.254± 0.849 |
35.988± 0.67 |
30.694± 0.496 |
32.281± 0.826 |
|
Essential: Non-essential |
1.021± 0.871 |
0.942± 0.886 |
1.017± 1.099 |
0.945± 0.811 |
1.151± 1.524 |
1.038± 1.882 |
1.043± 1.00 |
1.169± 1.717 |
Mean values are presented as mean±SD. Different small letters in the same row indicated significantly different (P < 0.05).
Table 5: Mineral composition (mg/100 g dry weight) of breast, thigh, drumstick, and wing meat of Spent hens and broilers.
|
Mineral |
Spent hen (n=30) |
Broiler (n=30) |
||||||
|
Breast |
Thigh |
Drumstick |
Wing |
Breast |
Thigh |
Drumstick |
Wing |
|
|
Macro minerals |
||||||||
|
Calcium |
20.20d± 1.09 |
30.45a± 2.77 |
26.02c± 2.35 |
27.92b± 1.87 |
19.61d± 1.39 |
28.77a± 2.83 |
26.31c±1.80 |
27.55b±1.61 |
|
Potassium |
1232.15b± 10.36 |
1068c± 10.65 |
1312.60a± 8.97 |
955.21d± 7.94 |
1456.26a± 11.54 |
1365b± 10.09 |
1020c±10.38 |
977.44d±7.90 |
|
Phosphorus |
924.50a± 9.30 |
772.52c± 7.52 |
840.30b± 6.29 |
667.76d± 8.01 |
933.1a± 9.50 |
848.49b± 7.21 |
823.04c±7.44 |
627.64d±8.34 |
|
Magnesium |
122.60a± 5.48 |
104.32b± 4.21 |
93.37c± 6.40 |
79.05d± 5.96 |
115.71a± 5.29 |
98.22± 4.39b |
91.20c±7.23 |
77.03d±5.82 |
|
Sodium |
145.87d± 3.65 |
176.25a± 5.40 |
155.25c± 3.66 |
159.02b± 1.83 |
131.45d± 3.70 |
165.25a± 5.09 |
153.51c±3.37 |
160.21b±1.95 |
|
Micro-minerals |
||||||||
|
Selenium |
0.10b± 0.00 |
0.15a± 0.00 |
0.10b± 0.02 |
0.15a± 0.01 |
0.09d± 0.01 |
0.11c± 0.01 |
0.12b±0.01 |
0.16a±0.02 |
|
Zinc |
2.24d± 0.13 |
7.92a± 0.27 |
6.99b± 0.34 |
5.40c± 0.11 |
2.25d± 0.19 |
7.67a± 0.18 |
6.81b±0.40 |
5.52c±0.21 |
|
Iron |
3.05d± 0.21 |
4.13a± 0.29 |
3.89b± 0.13 |
3.71c± 0.34 |
2.79d± 0.38 |
3.99a± 0.42 |
3.93b±0.59 |
3.64c±0.24 |
|
Manganese |
0.37a± 0.03 |
0.36a± 0.00 |
0.33c± 0.02 |
0.35b± 0.03 |
0.26d± 0.01 |
0.37b± 0.03 |
0.32c±0.01 |
0.39a±0.02 |
|
Aluminium |
1.05d± 0.11 |
1.25a± 0.20 |
1.21b± 0.03 |
1.10c± 0.07 |
0.97d± 0.09 |
1.15b± 0.35 |
1.31a±0.08 |
1.12c±0.04 |
Mean values are presented as mean±SD. Different small letters in the same row indicated significantly different (P < 0.05).
CONCLUSIONS and Recommendations
The physicochemical attributes differed among muscle positions. Due to its reduced fat, greater protein content, superior texture qualities (Warner-Bratzler shear force value and Pressing loss), and lower values of cooking loss, the spent hen meat meets the preferences of consumers who are seeking chicken meat. Broiler meat had higher cooking loss value, more essential and non-essential amino acids, greater intramuscular fat, and lower values of protein and drip loss. The overall moisture content of Spent hen meat is significantly higher. From a healthy human lifestyle point of view, breast meat and drumstick seem to be superior to thigh and wing meat. In conclusion, spent hen meat was refereed to have better physicochemical attributes than broilers.
ACKNOWLEDGEMENT
This work was supported by the Ministry of Science and Technology (MOST), the Army Welfare Trust, and the UGC Merit Scholarship.
Novelty Statement
The manuscript is relevant and novel with respect to the existing literature including the author’s own published work. The novelty of this study is physicochemical attributes of spent hen meat was greater than broiler meat. I think this research lead to new information or add something new to the existing literature.
AUTHOR’S CONTRIBUTION
SAT: Laboratory analysis, statistics, data curation, and manuscript writing. SAB: Data analysis. MAB and MNU: Conceptualization, interpretation of the data, and revision of the manuscript. All authors have read and agreed to the published version of the manuscript.
Conflict of interest
The authors have declared no conflict of interest.
REFERENCES
AOAC International (2007). Official methods of analysis of association of official analysis chemists international. Method, 2007.04.18th edn. AOAC, Gaithersburg, MD.
Biesek J, Kuzniacka J, Banaszak M, Maiorano G, Grabowicz M, Adamski M (2020). The effect of various protein sources in goose diets on meat quality, fatty acid composition, and cholesterol and collagen content in breast muscles. Poult. Sci., 99(11): 6278-6286. https://doi.org/10.1016/j.psj.2020.08.074
Boni I, Huda H, Noryati I (2010). Comparison of meat quality characteristics between young and spent quails. Int. Food Res. J., 17: 661-666.
Chen Y, Qiao Y, Xiao Y, Chen H, Zhao L, Huang M, Zhou G (2016). Differences in physicochemical and nutritional properties of breast and thigh meat from crossbred chickens, commercial broilers, and spent hens. Asian-Australas. J. Anim. Sci., 29(6): 855. https://doi.org/10.5713/ajas.15.0840
Choe J, Kim HY (2020). Physicochemical characteristics of breast and thigh meats from old broiler breeder hen and old laying hen and their effects on quality properties of pressed ham. Poult. Sci., 99(4): 2230-2235. https://doi.org/10.1016/j.psj.2019.10.076
Franco D, Rois D, Vazquez JA, Purrinos L, Gonzalez R, Lorenzo JM (2012). Breed effect between Mos rooster (Galician indigenous breed) and Sasso T-44 line and finishing feed effect of commercial fodder or corn. Poult. Sci., 91(2): 487-498. https://doi.org/10.3382/ps.2011-01546
Geldenhuys G, Hoffman LC, Muller N (2015). The fatty acid, amino acid, and mineral composition of Egyptian goose meat as affected by season, gender, and portion. Poult. Sci., 94: 1075-1087. https://doi.org/10.3382/ps/pev083
https://en.wikipedia.org/wiki/Broiler
Huo W, Weng K, Gu T, Luo X, Zhang Y, Zhang Y, Chen G (2021). Effects of integrated rice-duck farming system on duck carcass traits, meat quality, amino acid, and fatty acid composition. Poult. Sci., 100(6): 101107. https://doi.org/10.1016/j.psj.2021.101107
Ismoyowati I, Sumarmono J (2019). Duck production for food security. IOP Conf. Ser. Earth Environ. Sci., 372: 012070. https://doi.org/10.1088/1755-1315/372/1/012070
Jaturasitha S, Srikanchai T, Kreuzer M, Wicke M (2008). Differences in carcass and meat characteristics between chicken indigenous to northern Thailand (Black-boned and Thai native) and imported extensive breeds (Bresse and Rhode Island red). Poult. Sci., 87(1): 160-169. https://doi.org/10.3382/ps.2006-00398
Jayasena DD, Jung S, Kim HJ, Bae YS, Yong HI, Lee JH, Jo CR (2013). Comparison of quality traits of meat from korean native chickens and broilers used in two different traditional korean cuisines. Asian-australas. J. Anim. Sci., 26(7): 1038-1046. https://doi.org/10.5713/ajas.2012.12684
Jeon HJ, Choe JH, Jung YK, Kruk ZA, Lim DG, Jo CR (2010). Comparison of the chemical composition, textural characteristics, and sensory properties of North and South Korean native chickens and commercial broilers. Food Sci. Anim. Resour., 30(2): 171-178. https://doi.org/10.5851/kosfa.2010.30.2.171
Jukna V, Klementaviciute J, Meskinyte-kausiliene E, Peciulaitiene N, Samborskyte M, Ambrasunas L (2012). Comparative evaluation of quality and composition of ostrich, Turkey and broiler meat. Biotechnol. Anim. Husb., 28(2): 385-392. https://doi.org/10.2298/BAH1202385J
Jung YK, Jeon HJ, Jung S, Choe JH, Lee JH, Heo KN, Jo CR. (2011). Comparison of Quality Traits of Thigh Meat from Korean Native Chickens and Broilers. Korean J. Food Sci. Anim. Resour., 31(5): 684-692. http://dx.do.org/10.5851/kosfa.2011.31.5.684
Kaic A, Janjecic Z, Golub K, Potocnik K (2023). Comparison between standardized and modified ez-driploss determination methods in chicken breast meat. Animals, 13(6): 1054. https://doi.org/10.3390/ani13061054
Kamboh AA, Zhu WY (2013). Effect of increasing levels of bioflavonoids in broiler feed on plasma anti-oxidative potential, lipid metabolites, and fatty acid composition of meat. Poult. Sci., 92(2): 454-461. https://doi.org/10.3382/ps.2012-02584
Kang GH, Kim SH, Kim JH, Kang HK, Kim DW, Na JC, Choi YH (2009). Effects of washing methods on gel properties of chicken surimi prepared from spent hen breast muscle. Poult. Sci., 88(7): 1438-1443. https://doi.org/10.3382/ps.2008-00212
Kim JA, Cho ES, Jeong YD, Choi YH, Kim YS, Choi JW, Kim JS, Jang A, Hong JK, Sa SJ (2020). The effects of breed and gender on meat quality of Duroc, Pietrain, and their crossbred. J. Anim. Sci. Technol., 62: 409–419. https://doi.org/10.5187/jast.2020.62.3.409
Kokoszynski D, Arpasova H, Hrncar C, Zochowska-Kujawska J, Kotowicz M, Sobczak M (2020). Carcass characteristics, chemical composition, physicochemical properties, texture, and microstructure of meat from spent Pekin ducks. Poult. Sci., 99(2): 1232-1240. https://doi.org/10.1016/j.psj.2019.09.003
Lee SH, Kim HY (2021). Comparison of quality and sensory characteristics of spent hen and broiler in South Korea. Animals, 11(9): 2565. https://doi.org/10.3390/ani11092565
Li C, Liu D, Zhou G, Xu X, Qi J, Shi P, Xia T (2012). Meat quality and cooking attributes of thawed pork with different low field NMR T 21. Meat Sci., 92: 79-83. https://doi.org/10.1016/j.meatsci.2011.11.015
Lorenzo JM, Maggiolino A, Gallego L, Pateiro M, Serrano MP, Dominguez R, De Palo P (2019). Effect of age on nutritional properties of Iberian wild red deer meat. J. Sci. Food Agric., 99(4): 1561-1567. https://doi.org/10.1002/jsfa.9334
Macelline SP, Chrystal PV, Liu SY, Selle PH (2021). The dynamic conversion of dietary protein and amino acids into chicken-meat protein. Animals, 11(8): 2288. https://doi.org/10.3390/ani11082288
Moula MM, Bary MA, Shaon MTW, Arefin N, Ali MZ, Bhuiyan ZA (2020). Evaluation of broiler health status through flock health monitoring program in Bangladesh. Poult. Sci. J., 8(1): 59-72.
Park SY, Kim HY (2021). Effects of Marketing ages on the physicochemical properties and sensory aspects of cured broiler chicken breast meat. Foods, 10(9): 2152. https://doi.org/10.3390/foods10092152
Petek M, Cavusoglu E (2021). Carcass characteristics and physical meat quality properties of spent broiler breeder hens and commercial spent layer hens. Harran Univ. Vet. Fakultesi Dergisi, 10(2): 172-177. https://doi.org/10.31196/huvfd.996375
Polizer Rocha YJ, Lorenzo JM, Barros JC, Baldin JC, Trindade MA (2019). Effect of chicken meat replacement by spent laying hen meat on physicochemical properties and sensorial characteristics of fresh sausage. Br. Poult. Sci., 60(2): 139-145. https://doi.org/10.1080/00071668.2019.1568392
Qiao Y, Huang J, Chen Y, Chen H, Zhao L, Huang M, Zhou G (2017). Meat quality, fatty acid composition and sensory evaluation of Cherry Valley, spent layer and crossbred ducks. Anim. Sci. J., 88(1): 156–165. https://doi.org/10.1111/asj.12588
Reddy GVB, Mallika EN, Reddy BO, Azad SAK, Reddy DM (2016). Comparison on meat quality characteristics of spent breeder, layer and broiler birds. Int. J. Sci. Environ. Technol., 5: 2590-2595.
Sarker MSH, Habib M, Bhuiyan MSA, Hashem MA, Ali MS (2022). Meat yield characteristics and physicochemical properties of different duck genotypes. Bangladesh Meat Sci. Assoc., 2(5). https://doi.org/10.55002/mr.2.6.39
Talpur MZ, Wang K, Ahmed I, Li Z, Liu L, Li Q, Wang S (2018). Analysis of differentially expressed genes related to intramuscular fat and chemical composition in different breeds of chicken. Pak. J. Agric. Sci., 55(3): 615-623. https://doi.org/10.21162/PAKJAS/18.6537
Vargas-Ramella M, Pateiro M, Rois D, Arias A, Justo JR, Lopez-Pedrouso M, Franco D (2022). Effect of breed and diet on carcass parameters and meat quality of spent hens. Ann. Anim. Sci., 22(1): 477-500. https://doi.org/10.2478/aoas-2021-0036
WHO (World Health Organization) (2007). Protein and amino acid requirements in human nutrition: World Health Organization Report, Geneva, Switzerland.
Xie L, Qin J, Rao L, Tang X, Cui D, Chen L, Huang L (2021). Accurate prediction and genome-wide association analysis of digital intramuscular fat content in longissimus muscle of pigs. Anim. Genet., 52(5): 633-644. https://doi.org/10.1111/age.13121
Zhao GP, Cui HX, Liu RR, Zheng MQ, Chen JL, Wen J (2011). Comparison of breast muscle meat quality in 2 broiler breeds. Poult. Sci., 90(10): 2355-2359. https://doi.org/10.3382/ps.2011-01432
To share on other social networks, click on any share button. What are these?






