Insecticidal Efficacy of Geranium Oil Nanoemulsion and Synergism with Sesame Oil and their Acetylcholinesterase Inhibition
Insecticidal Efficacy of Geranium Oil Nanoemulsion and Synergism with Sesame Oil and their Acetylcholinesterase Inhibition
Shawky M Aboelhadid1*, Abdel-Azeem S Abdel-Baki2, Khaled M Hassan3,
Samar M Ibrahium4, Saleh Al-Quraishy5, Ahmed O Hassan6 and Asmaa A Kamel1
1Parasitology Department, Faculty of Veterinary Medicine, Beni-Suef University, Beni-Suef 62511, Egypt
2Zoology Department, Faculty of Science, Beni-Suef University, Beni-Suef, Egypt
3Department of Parasitology, Beni-Suef Laboratory, Animal Health Research Institute, Agriculture Research center, Egypt.
4Department of Parasitology, Animal Health Research Institute, Fayum Branch, Fayum, Egypt
5Zoology Department, College of Science, King Saud University, Riyadh, Saudi Arabia
6Department of Medicine, Washington University School of Medicine, St. Louis, MO 63110, USA
ABSTRACT
Nowadays, many studies have been carried out to develop new eco-friendly alternatives consequently, the essential oils (EOs) have acquired more interest due to their insecticidal activities, low harmfulness, and rapid degradation in the environment. Following this approach, herein we tried to improve the insecticidal activities of Pelargonium graveolens (G) through using the geranium oil in nano-emulsion (GN) form and in combination with sesame seed oil (SO). Different concentrations ranged from 0.313 to 10% were prepared from GN and the combination of G with S and tested for their larvicidal and pupicidal activities against Musca domestica and Culex pipiens, while their adulticidal activities were tested against red flour beetle Tribolium castaneum. The LC50 of G alone was 4.29% against house fly larvae. The values of LC50 decreased to 1.50% for GN and 0.32% for the combination of G+S. GO and GN form induced (pupal toxicity) 100% inhibition rate (PIR) in the treated housefly pupae at the concentration of 10%. Meanwhile, GO+SO combination showed 100% PIR at the concentration of 2.5%. Furthermore, the LC50 for GO, GN and GO+SO was 0.22, 0.19 and 0.079 % respectively against larvae of C. pipiens, while it was 1.04 % for sesame oil. Regarding the contact and fumigant assays against adult T. castaneum, the combination of G and S achieved the best results when compared with GO alone or GN form. All treatments exhibited inhibition in the activity of acetylcholinesterase enzyme in the house fly larvae 24h post application. In addition, all the treatments induced significant increase in the malondialdehyde activity. In conclusion, nanoemulsion tech and mixing GO+SO increased their insecticidal potency against house fly, C. pipiens and T. castenum. This is a useful method in the integrated pest management.
Article Information
Received 18 April 2022
Revised 20 February 2023
Accepted 26 March 2023
Available online 19 June 2023
(early access)
Published 13 July 2024
Authors’ Contribution
Conceptualization: SMA, A-ASA-B. Investigation: KMH, AAK. Methodology: AAK, SMI, KMH. Validation: AOH, SA-Q, SMA. Visualization: AAK, SMI, KMH, AOH. Roles/Writing original draft: AAK, SMI, KMH. Writing review and editing: A-ASA-B, SMA.
Key words
Geranium oil, Sesame oil, Nanoemulsion, Synergism, Acetylcholinesterase
DOI: https://dx.doi.org/10.17582/journal.pjz/20220418100455
* Corresponding author: shawky.abohadid@vet.bsu.edu.eg
0030-9923/2024/0005-2067 $ 9.00/0
Copyright 2024 by the authors. Licensee Zoological Society of Pakistan.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Insects represent the principal percentage of the world’s organisms; they have over one million species and are still countinuing as pests and vectors (Gross, 2006). The housefly Musca domestica (Linnaeus, 1758) (Diptera: Muscidae) is an important mechanical vector of many diseases for domestic animals and humans (Sasaki et al., 2000; De Jesús et al., 2004; Singh et al., 2009; Sinthusiri and Soonwera, 2014). Also, mosquitoes (Diptera: Culicidae) are considered one of the most dangerous insects throughout the world as they transmit organisms that cause some of the fatal and debilitating diseases to both domestic animals and humans (Service, 2012). Of this disease malaria, West Nile fever, dengue fever, encephalitis, lymphatic filariasis, and yellow fever are able to induce millions of deaths each year (Bhatt et al., 2013; Stanaway et al., 2016; Lee et al., 2017). Because there are no vaccines for these diseases, prevention strategies are necessary. As well as, stored grain pests are the most damaging insects. Their control is very difficult due to their small size, feeding behavior, and to destroy grain prior to harvest (Raghavendra et al., 2017). Tribolium castaneum (Coleoptera: Tenebrionidae) is a major pest of stored products. If no prophylactic measures are taken, infestation of the storage with this pest can prompt losing of stored grains completely within 6 months (Abouelatta et al., 2020; Aboelhadid and Youssef, 2021).
Synthetic insecticides are the main choice to control these insect species, which has resulted in several serious problems, including the development of resistance (Khan et al., 2013), toxic effects to non-specific organisms, and environmental pollution (Shaalan et al., 2005). Consequently, extensive researches have been carried out to develop new eco-friendly alternatives (Carreño et al., 2014). Essential oils (EOs) have acquired considerable interest as insect control agents because of their insecticidal effectiveness with low impacts and rapid degradation in the environment (Chintalchere et al., 2020). These oils comprised mixtures of chemical compounds that cause toxicity in insects through a variety of mechanisms as enzymatic inhibition, membrane, and protein denaturation (Rey et al., 2001; Cavalca et al., 2010).
The main obstacle of field applying the plant EOs as insecticides is their chemical instability in the different environmental conditions, which may lead to rapid evaporation and destruction of active ingredients (Echeverría et al., 2019). In addition, many plant oils have low water solubility which limits their application as insecticides in the field (Aboelhadid et al., 2021). Therefore, several studies have been carried out to develop more effective formulations to avoid the problem associated with their Field application as insect control agents (Sharifian et al., 2011; Saranya et al., 2012; Hashem et al., 2018; Massoud et al., 2018). Among the recommended preparations, nanoemulsions may help in solving these problems (Saranya et al., 2012; Hashem et al., 2018; Massoud et al., 2018). Nanoemulsions are more stable and can increase the biological activity by decreasing droplet size (Donsi and Ferreri, 2016). Recently, nanocapsules and nanoemulsions containing cinnamon oil showed high efficacy against engorged female of Rhipicephalus (Boophilus) microplus (Dos Santos et al., 2017; Lazcano et al., 2019). Pelargonium graveolens L’Her (Geraniaceae) (Geranium) is a South African economic plant that contains essential oil (EO) known commercially as geranium oil (GO) (Bakkali et al., 2008). Egypt is one of the common countries in the production of GO (Abd El-Wahab et al., 2016) which can be extracted from leaves, flowers, and stalks by steam or hydro distillation (Boukhris et al., 2012). GO is non-toxic, non-irritant, and non-sensitizing with no side effects (Boukhatem et al., 2013). Additionally, GO is known to have antifungal, antibacterial, anti-inflammatory, spasmolytic, and hypoglycemic properties (Lis-Balchin et al., 1997; Maruyama et al., 2006; Lalli et al., 2010; Hassane et al., 2011). Citronellol and geraniol (trans-geraniol) are the two main components found in GO and they are known to have insecticidal activities (Babu and Kaul, 2005; Bouzenna and Krichen, 2013). GO showed a weak toxic effect on M. domestica (Pavela, 2008; Saraiva et al., 2020). Also, Norris et al. (2015) and Ríos et al. (2017) reported moderate toxicity for GO against Aedes aegypti and Anopheles gambiae only at higher concentrations.
Sesame (Sesamum indicum) is an ancient crop cultivated in tropical and sub-tropical regions of the world (Ram et al., 1990). Several literatures documented the synergistic effect of the sesame oil (SO) and also confirmed that sesamin and sesamolin were the SO main components. Therefore, several parts of the plant were tested for its insecticidal activities (Kato et al., 1998; Begum et al., 2000). SO showed synergist action when it was used with the botanical insecticides pyrethrin and rotenone against M. domestica (Eagleson, 1940). Kranthi (2005) postulated that synergistic effect augments the insecticide efficacy by inhibiting insecticide detoxifying enzymes.
The current investigation aimed to assess the improvement of the insecticidal activities of P. graveolens essential oil as nano-emulsion and in combination with SO against 3rd instar larvae and pupae of M. domestica and Culex pipiens larvae and one economically important adults of T. castinum.
Materials and Methods
Preparation of geranium (GO) and sesame seed oils (SO)
The oils were purchased from Trust Scientific for Natural Products, Cairo, Egypt. Six concentrations (10, 5, 2.5, 1.25, 0.625 and 0.312%; volume/volume) were prepared for both oils by dissolving in 70 % ethanol. The binary mixtures from GO and SO were prepared at a rate of (1:1) for all concentrations. The GO and SO were analysed using GC-MS and TRACE GC Ultra Gas Chromatographs at the Nawah Scientific Educational Research Center in Egypt (https://nawah-scientific.com/) (THERMO Scientific Corp., USA).
Preparation of geranium oil nanoemulsion (GN)
The nanoemulsion was prepared according to Nirmala et al. (2020). Briefly, the oil/water macroemulsion was prepared by combination of GO with a surfactant (Tween 80) (one oil to two T80, 1:2V:V ) then water to final volume was added and mixed using a magnetic stirrer (speed of 500 rpm for 10 min) to obtain concentration of 10% of G. An ultrasonicator was used to sonicate the prepared macroemulsion for 10 min (750 W, Branson Probe sonicator-Advanced model, 20 kHz). At 340 nm, a UV-visible spectrophotometer (UV-2600, Shimadz, Japan) was used to characterise the resulting nanoemulsion.
Rearing of house fly colony
Adult house flies were collected from animal farms in different villages of Beni-Suef province, Egypt. The collected house flies were transported to the Parasitology Lab at Faculty of Veterinary Medicine, Beni-Suef University. The flies were kept at temperature of 28 ± 2 °C and 60–70% relative humidity (RH) in plastic jars (35 × 15 cm), covered with muslin cloth for several generations. A cotton swab soaked in milk (10% w/v) was introduced as food to the adult flies and also served as a substratum for oviposition. The eggs were transported to another set of jars containing animal feed or cotton swab soaked in milk for hatching and development of larvae. Similarly, pupae were collected and kept in a separate container until they emerged as adults. The bioassays were carried out on larvae and pupae (Jesikha, 2014; Kamel et al., 2019).
Rearing of Culex pipiens
Egg rafts of laboratory reared colony of C. pipiens were sieved into large plastic containers with water. The resulting larvae were then placed in enamel trays with 1 liter of dechlorinated water and 0.15 g of Brewer’s yeast (lactalbumin) (50:50). Water was replaced every other day and food was added on a daily basis. Adults were kept in 0.51 m3 aluminum screen cages and fed on 10% sucrose solution on cotton wicks. To blood-feed the insect female, restrained quail was used. A 400 ml plastic container was used to collect the deposited egg rafts. The colony was kept at 26 °C and a 75% RH with a 16 L: 8 D photoperiod. In the bioassays, the third and fourth larval instars were used (Sayed et al., 2018).
Rearing adults of Tribolium castaneum
Adult T. castaneum insects were raised in a dark incubator at a temperature of 28°C and RH of 70–80%. The culture for insect rearing was wheat flour mixed with active yeast (10:1, w/w). The adult insect was employed in subsequent applications after a week (Aboelhadid and Youssef, 2021).
Larvicidal and pupicidal bioassays against Musca domestica
The larvicidal and pupicidal bioassays were conducted according to the methods of Busvine (1971) and Palacios et al. (2009), respectively with few modifications. Briefly, 10 individuals of the 3rd instar larvae/ pupae in each test (5 replicates) were treated with different concentrations (10, 5, 2.5, 1.25, 0.625 and 0.312%) of the investigated materials (G, GN, S and G+S) to evaluate the toxicity and the values of LC50 and LC90. For the residual film method, 1 mL of the various concentrations was uniformly applied over filter paper and kept inside a glass Petri dish with a diameter of 9 cm. The treated Petri-dishes were first air-dried for few minutes to allow the solvent to evaporate before being incubated at 28 °C and 75% RH for 24 h. Acetone was used as a negative control, while deltamethrin at 2 ml/L was used in the positive control group. The treated larvae were observed for mortality for 24 h.

In the pupal bioassay, the treated pupae were observed for adult emergence for 6 days. The adult emergence rate was determined according to the method of Kumar et al. (2011). Percentage inhibition rate (PIR) was calculated as follows:
%PIR = Cn−Tn /Cn× 100
where Cn is the number of newly emerged houseflies in the control group and Tn is the number of newly emerged houseflies in the treated group.
Larvicidal bioassay against Culex pipiens
The standard method of World Health Organization was used in this bioassay (WHO, 2005). This was done in plastic cups (250 mL). The EOs were dissolved in ethyl alcohol at the tested concentrations 0.078 to 10 % then the working solution prepared by aliquot one mL of these dilutions were added to 99 mL distilled water. Thirty Culex pipiens third-instar larvae were placed in the prepared concentrations in the plastic cups (5 replicates for each concentration). In the negative control, larvae were exposed to one mL of the solvent dissolved in the water, however deltamethrin at 3.5µl/L was used as positive control group The dead larvae (motionless) were recorded after 24 h, and the average percentage mortality was estimated according to Abbott’s formula (Abbott, 1925).
Adulticidal bioassay against T. castaneum
Fumigant toxicity
The fumigant effect of the EO and the nanoemulsion against adults of T. castaneum were assessed according to Ko et al. (2009). Briefly, filter paper of 6 cm diameter soaked in the prepared dilutions (0.312, 0.625, 1.25, 2.5, 5, and 10%) of the EO and the nanoemulsion forms. This paper was connected to the undersurface of the screw cap of glass jars (170 cm3). Ten adults of T. castaneum were transported to glass jars covered with their screw caps that were attached with treated filter papers. Filter paper treated with acetone was used as negative control, while deltamethrin (1 ppm) was used in the positive control group (Arthur, 2019). Five replicates were done for each treatment and for each control. After 24 h exposure, the mortality was recorded.
Contact toxicity (Surface film bioassay)
This bioassay was done according to Broussalis et al. (1999). The tested compounds were prepared in acetone. To get a series of concentrations ranging from 0.312 to 10 %, one millilitre of each concentration was put to the bottom of a glass Petri dish (9 cm diameter). Before introducing the adults of T. castaneum, the Petri dishes were left open for 5 min to allow acetone to evaporate. Ten adults insect were transported to each Petri dish. Control group were treated with acetone, while deltamethrin (1 ppm) (Arthur, 2019) was used in the positive control group. Five replicates were used for each concentration and the control as well. The dead insects were counted after 24 h, and LC50 values were calculated by probit analysis (Finney, 1971).
Inhibition of acetylcholinesterase (AchE) activity in treated house fly larvae
The treated house fly larvae were suspended in lysis buffer and homogenized with a glass homogenizer on ice. The resulted homogenate was centrifuged at 10, 000 rpm for 10 min at 4 °C. The supernatant was taken and preserved to be use in the detection of AchE (Chintalchere et al., 2020). The AchE activity in treated larvae was estimated by the modified technique of Ellman et al. (1961) and using kits of QuantiChromTM Acetylcholinesterase Assay Kit (DACE-100) (www.bioassaysys.com). The larvae in the control group were treated with acetone. The AchE activity were detected using UV-spectrometer (at 412 nm). The inhibition of AchE in the treated insects was calculated according to Anderson and Coats (2012): AchE inhibition (%) = 100 - [(As / Ac) x 100], where: As = AchE activity for each concentration; Ac = Negative control.
Lipid peroxidation (Malondialdehyde assay) in treated house flies larvae
The lipid peroxidation (LPO) in the homogenate of house fly larvae was detected by the method of Bar-Or et al. (2001) and the reading of the test was done at 535 nm.
Statistical analysis
Three replicates were done for all the treatments and mean ± SE values were calculated. Larval mortality analysis was performed by using ANOVA and subsequent Duncan’s multiple range tests (p < 0.05). Probit analysis was applied to determine the LC50 and LC90values with their 95% confidence limits (Finney, 1952). All statistical analyses were achieved using SPSS for Windows (version 22.0). The synergistic factor (SF) was calculated by dividing the LC50 value of the individual test insecticide with the corresponding LC50 value of the test insecticide + synergist mixture (Chou, 2006).
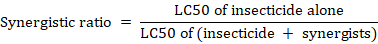
If the synergistic ratio is < 1 it means antagonistic effect; >1 means synergistic effect; and 1 means no effect.
Results
GC-MAS analysis of GOand SO
The analysis showed that the citronellol (14.44%) and geraniol (11.08%) were the most predominant constituents of the GO with other minor components (Table I), while linoleic acid (37.27 %), oleic acid (26.07 %) and palmitic acid (13.34 %) were the main constituents of the sesame oil (Table II).
Table I. GC-MAS of Pelargonium graveolens.
|
RT |
Compound name |
Area% |
|
3.30 |
β-Pinene |
0.75 |
|
3.89 |
Cis-Linalool oxide |
0.55 |
|
4.32 |
Linalool |
7.74 |
|
4.72 |
Rose oxide |
0.76 |
|
5.17 |
Trans-p-Menthone |
0.33 |
|
5.41 |
Isomethane |
4.35 |
|
5.73 |
Citronellal |
0.44 |
|
5.85 |
Isopulegol |
0.41 |
|
6.63 |
Citronellol |
14.44 |
|
6.94 |
β-Geranial |
0.40 |
|
7.31 |
Geraniol |
11.08 |
|
7.55 |
Citronellyl formate; Formic acid |
7.66 |
|
8.01 |
Geranyl formate |
3.91 |
|
8.69 |
Geranyl acetal |
0.63 |
|
8.95 |
Citronellyl acetate |
1.23 |
|
9.44 |
Copaene |
1.05 |
|
9.63 |
a-Bourbonene |
3.28 |
|
10.10 |
ι-Gurjunene |
0.26 |
|
10.32 |
Caryophyllene |
2.55 |
|
10.49 |
β-Copaene-4α-ol |
0.36 |
|
10.75 |
Aromadendrene |
1.73 |
|
Table continued on next page............... |
||
|
RT |
Compound name |
Area% |
|
10.88 |
ç-Muurolene |
0.96 |
|
10.99 |
Humulene |
0.83 |
|
11.13 |
Epi-β-Caryophyllene |
0.48 |
|
11.38 |
Geranyl propionate |
2.01 |
|
11.56 |
Germacrene D |
2.63 |
|
11.65 |
Epi-β-Selinene |
0.30 |
|
11.85 |
Elemene |
1.60 |
|
11.91 |
Epicubebol |
0.53 |
|
12.04 |
a-Farnesene |
0.30 |
|
12.19 |
Naphthalene |
0.89 |
|
12.39 |
σ-Cadinene |
3.27 |
|
12.64 |
α-Gurjunene |
0.30 |
|
12.82 |
γ-costol |
0.82 |
|
13.08 |
Geranyl butyrate |
2.36 |
|
13.48 |
Spathulenol |
1.13 |
|
13.60 |
phenylethyl tiglate |
2.71 |
|
13.76 |
(-)-Globulol |
0.29 |
|
13.91 |
Neryl 2-methylbutyrate |
0.41 |
|
14.03 |
Caryophylene oxide |
0.30 |
|
14.34 |
10-epi-y-eudesmol |
4.93 |
|
14.42 |
Cubenol |
0.17 |
|
14.57 |
Agarospirol |
0.40 |
|
14.72 |
Guaiene |
0.69 |
|
14.89 |
Elemol |
1.93 |
|
15.07 |
Citronellyl tiglate |
0.79 |
|
15.40 |
2, 6-octadiene, 2, 6-dimethyl |
0.47 |
|
15.75 |
Geranyl tiglate |
2.62 |
|
16.02 |
Geranyl palmitate |
0.84 |
|
16.66 |
Geranyl isobutyrate |
0.37 |
|
17.77 |
Citronellol heptanoate |
0.23 |
|
18.36 |
Geranyl heptanoate |
0.36 |
|
20.00 |
Geranyl caprylate |
0.20 |
|
Total |
100 |
|
Characterization of GN
The UV-visible spectrophotometer (UV-2600, Shimadz, Japan) measured the absorbance of GN at 340 nm. The zeta potential (-0.569), droplet size distribution (18.7 d, nm) (analysis by volume) and polydispersity index (PDI) (0.299 d. nm) of nanoemulsions were measured by a zeta sizer apparatus (dynamic light scattering technique) (Nano-ZS90, Malvern, UK). Zeta potential dimensions showed formation of GN (Supplementary Figs. 1, 2 and 3).
Toxicity of GO forms against larvae and pupae of Musca domestica
The GO showed no larvicidal effect against the 3rd instar larvae of M. domestica at concentrations of ≤ 0.312%. Meanwhile, at 10% concentration the larval mortality was 77.67 % with LC50 value (4.29%). However, the GN form revealed better larvicidal activity which is evidenced by the reduced value of the LC50 (1.50%) (Tables III, IV, Fig. 1). Conversely the sesame oil (S) also showed no larvicidal effect against the 3rd instar larvae of M. domestica. Interestingly, the combination of GO+S showed significant larvicidal activity at the all tested concentrations with LC50 attained at a concentration of 0.32%. The treated larvae died within 24 h with clear blackening of the cuticle. The synergistic factor was 49.13 (Tables III, IV, Fig. 1).
Regarding the pupal toxicity, GO showed a percentage inhibition rate (PIR) ranging from 6.67% to 100% at different concentrations after six days of application with LC50 at concentration of 1.48%. Meanwhile, the GN induced PIR ranged from 3.3 to 100% with LC50 achieved at concentration of 1.98%. The 100% of PIR for both GO and GN achieved only at the concentration of 10%. In contrary, SO showed no any pupicidal activity at the all-tested concentrations. The combination of GO+SO showed significant pupicidal activity at the all tested concentrations with LC50 reached at a concentration of 0.50% and 100% PIR was achieved at a concentration of 2.5% concentration. The synergism factor was 2.96 (Tables V, VI).
Table II. The phytochemical composition of sesame oil (SO) by GC-MS.
|
Peak |
R.t* |
Name |
Area % |
Molecular weight |
Molecular formula |
MF** |
|
1 |
17.63 |
Phenol, 2, 4-bis(1, 1-Dimethylethyl)- |
0.40 |
206 |
C14H22O |
932 |
|
2 |
22.24 |
Methyl tetradecanoate |
0.27 |
242 |
C15H30O2 |
933 |
|
3 |
26.39 |
Hexadecanoic acid, methyl ester (palmitic acid) |
13.34 |
270 |
C17H34O2 |
925 |
|
4 |
29.61 |
9,12-Octadecadienoic acid (Z, Z)-, methyl ester (Linoleic acid) |
37.27 |
294 |
C19H34O2 |
905 |
|
5 |
29.73 |
9-Octadecenoic acid (Z)-, methylester (oleic acid) |
26.07 |
296 |
C19H36O2 |
939 |
|
6 |
30.12 |
Methyl stearate |
10.22 |
298 |
C19H38O2 |
929 |
|
7 |
30.35 |
9,12-Octadecadienoic acid (Z, Z)- |
1.26 |
280 |
C18H32O2 |
898 |
|
8 |
30.45 |
Oleic Acid |
1.78 |
282 |
C18H34O2 |
930 |
|
9 |
30.61 |
Linoleic acid ethyl ester |
0.36 |
308 |
C20H36O2 |
912 |
|
10 |
30.71 |
Ethyl oleate |
0.32 |
310 |
C20H38O2 |
908 |
|
11 |
32.93 |
11-Eicosenoic acid, methyl ester |
0.47 |
324 |
C21H40O2 |
913 |
|
12 |
33.40 |
Eicosanoic acid, methyl ester |
2.14 |
326 |
C21H42O2 |
884 |
|
13 |
36.52 |
Docosanoic acid, methyl ester |
0.30 |
354 |
C23H46O2 |
880 |
|
14 |
38.27 |
9,12,15-Octadecatrienoic acid, 2, 2-dimethyl-1, 3-dioxolan-4- Ylmethyl ester, (Z, Z, Z)- |
0.82 |
392 |
C24H40O4 |
797 |
|
15 |
38.33 |
Oleic acid, (2, 2-dimethyl-1, 3-dioxolan-4-yl)methyl ester |
0.95 |
396 |
C24H44O4 |
892 |
|
16 |
38.94 |
9-Octadecenoic acid (Z)-, 2-hydroxy-1-(hydroxymethyl)ethyl ester |
4.03 |
356 |
C21H40O4 |
883 |
Table III. Mortality percentages (Mean±SEM) of P. graveolens (GO and GN) and the synergistic action with sesame oil (SO) against Musca domestica larvae.
|
Concentration (%) |
GO |
GN |
SO |
GO+SO |
|
0.312 |
0.00±0.00g |
8.67±1.20f |
0.00±0.00b |
53.33±3.33c |
|
0.625 |
5.00±1.15f |
25.77±2.33e |
0.00±0.00b |
76.67±5.77b |
|
1.25 |
16.00±2.08e |
40.00±1.76d |
0.00±0.00b |
100.00±0.00a |
|
2.50 |
33.67±2.03d |
62.00±1.53c |
0.00±0.00b |
100.00±0.00a |
|
5.00 |
53.33±2.40c |
83.33±2.40b |
0.00±0.00b |
100.00±0.00a |
|
10.00 |
77.67±1.45b |
100.00±0.00a |
0.00±0.00b |
100.00±0.00a |
|
Deltamethrin (2ml/L) (control positive) |
100.00±0.00a |
100.00±0.00a |
100.00±0.00a |
100.00±0.00a |
|
Acetone (negative control) |
0.00±0.00g |
0.00±0.00g |
0.00±0.00b |
0.00±0.00d |
Means within a column followed by the same letter are not significantly different (Duncan’s multiple range test: P > 0.05).
Table IV. Larvicidal activity of P. graveolens (GO and GN) and the synergistic action with sesame oil against Musca domestica larvae.
|
Treatment |
LC50 (%) |
95% CL |
LC90 |
95% CL |
X2 (df = 4) |
P* |
Synergism factor |
||
|
LCL |
UCL |
LCL |
UCL |
||||||
|
P. graveolens oil (GO) |
4.29 |
3.71 |
5.07 |
14.08 |
11.02 |
19.42 |
1.74 |
0.784 |
- |
|
P. graveolens nanoemulsion (GN) |
1.50 |
1.31 |
1.72 |
6.27 |
5.09 |
8.15 |
8.26 |
0.082 |
- |
|
Sesame oil (SO) |
- |
- |
- |
- |
- |
- |
- |
- |
- |
|
GO+SO |
0.32 |
0.26 |
0.36 |
0.77 |
0.66 |
0.95 |
6.92 |
0.140 |
13.41 |
LCL, lower confidential limit; UCL, upper confidential limit; X2, Chi-square; df, degree of freedom; LC50 and LC90 were lethal concentration at which 50% and 90% population dies, respectively. * p > 0.05 is non-significant
Table V. Percentage inhibition rate (PIR) (Mean±SEM) of P. graveolens (GO and GN) and the synergistic action with SO against housefly pupae.
|
Concentration (%) |
G |
GN |
SO |
GO+SO |
|
0.312 |
6.67±3.33f |
3.33±3.33f |
0.00±0.00b |
30.00±5.77d |
|
0.625 |
20.00±5.77e |
11.67±4.41e |
0.00±0.00b |
60.00±5.77c |
|
1.25 |
43.33±3.33d |
30.00±5.77d |
0.00±0.00b |
83.33±3.33b |
|
2.50 |
66.67±3.33c |
60.00±5.77c |
0.00±0.00b |
100.00±0.00a |
|
5.00 |
86.67±3.33b |
83.33±3.33b |
0.00±0.00b |
100.00±0.00a |
|
10.000 |
100.00±0.00a |
100.00±0.00a |
0.00±0.00b |
100.00±0.00a |
|
Deltamethrin (2ml/L) (control positive) |
100.00±0.00a |
100.00±0.00a |
100.00±0.00a |
100.00±0.00a |
|
Acetone (negative control) |
0.00±0.00f |
0.00±0.00f |
0.00±0.00b |
0.00±0.00e |
Means within a column followed by the same letter are not significantly different (Duncan’s multiple range test: P > 0.05).
Table VI. Pupicidal activity of P. graveolens (GO and GN) and the synergistic action with SO against Musca domestica pupae.
|
Treatment |
LC50 (%) |
95% CL |
LC90 |
95% CL |
X2 (df = 4) |
P* |
Synergism factor |
||
|
LCL |
UCL |
LCL |
UCL |
||||||
|
P. graveolens oil (GO) |
1.48 |
1.31 |
1.68 |
5.29 |
4.39 |
6.66 |
4.05 |
0.39 |
- |
|
P. graveolens nanoemulsion (GN) |
1.98 |
1.76 |
2.23 |
6.32 |
5.29 |
7.87 |
6.95 |
0.12 |
- |
|
Sesame oil (SO) |
- |
- |
- |
- |
- |
- |
- |
- |
- |
|
GO+SO |
0.50 |
0.44 |
0.57 |
1.38 |
1.18 |
1.70 |
4.38 |
0.36 |
2.96 |
For abbreviations and statistical details, see Table IV.
Table VII. Mortality percentages of P. graveolens (Mean± SE) (GO and GN) and the synergistic action with SO against culex pipiens larvae.
|
Concentration (%) |
GO |
GN |
Sesame oil (S) |
GO+SO |
|
0.078 |
8.67±1.33e |
11.67± 4.41d |
0.00±10.00e |
50.00±5.77c |
|
0.156 |
33.33±3.33d |
40.00± 5.77c |
1.67±1.67e |
86.67±3.33b |
|
0.312 |
70.00±5.77c |
70.00± 5.77 b |
10.00±2.89e |
100.00±0.00a |
|
0.625 |
90.00±5.77b |
100.00± 0.00a |
23.33±1.67d |
100.00±0.00a |
|
1.25 |
100.00±0.00a |
100.00± 0.00a |
50.00±5.77c |
100.00±0.00a |
|
2.50 |
100.00±0.00a |
100.00± 0.00a |
88.33±1.67b |
100.00±0.00a |
|
5.00 |
100.00±0.00a |
100.00± 0.00a |
100.00±0.00a |
100.00±0.00a |
|
10.000 |
100.00±0.00a |
100.00± 0.00a |
100.00±0.00a |
100.00±0.00a |
|
Deltamethrin (3.5µl/L) (control positive) |
100.00±0.00a |
100.00± 0.00a |
100.00±0.00a |
100.00±0.00a |
|
Ethyl alchol70% (negative control) |
1.67±1.67e |
1.67±1.67e |
1.67±1.67e |
1.67±1.67d |
Means within a column followed by the same letter are not significantly different (Duncan’s multiple range test: P > 0.05).
Larvicidal activity of GO forms against Culex pipiens
GO caused 100% mortality in Culex pipiens larvae at concentration of 1.25 % and LC50 was achieved at concentration of 0. 22 %. While, GN showed 100% larval mortality at concentration of 0.625 % with LC50 was reached at 0.19 % (Tables VII, VIII, Fig. 2). SO showed larval toxicity at of 5.00 and 10.00 % with LC50 attained at 1.04 %. Furthermore, the combination of G+S induced significant larvicidal activity at the all tested concentrations with LC50 reached at concentration of 0.079 %. The synergistic factor was 2.78 (Tables III and V, Fig. 2).
Table VIII. Larvicidal activity of P. graveolens (GO and GN) and the synergistic action with sesame oil against Culex pipiens.
|
Treatment |
LC50 (%) |
95% CL |
LC90 |
95% CL |
X2 (df = 6) |
P* |
Synergism factor |
||
|
LCL |
UCL |
LCL |
UCL |
||||||
|
P. graveolens oil (GO) |
0. 22 |
0. 19 |
0. 24 |
0. 56 |
0. 48 |
0. 67 |
1.81 |
0.936 |
- |
|
P. graveolens Nanoemulsion (GN) |
0. 19 |
0. 17 |
0. 21 |
0. 44 |
0. 38 |
0. 53 |
8.101 |
0.231 |
- |
|
Sesame oil (SO) |
1.04 |
0. 93 |
1.16 |
2.87 |
2.46 |
3.47 |
9.81 |
0.133 |
- |
|
GO+SO |
0.079 |
0.068 |
0.089 |
0.162 |
0.142 |
0.196 |
1.22 |
0.976 |
2.78 |
For abbreviations and statistical details, see Table IV.
Toxicity activities of GO forms against T. castaneum
Contact toxicity
In contact toxicity assay, the mortality percentage of T. castaneum was increased with increasing the exposure time. After 24 h of exposure, the concentration 10% achieved 100% mortality for each oil and their combination. The LC50 was attained at concentrations of 1.74%, 0.711%, 0.97% and 0.302% for GO, S, GN and GO+S, respectively. The synergistic factor was SF=5.26 (Tables IX, X).
Fumigant toxicity
At 10% concentration the GO and GN showed 80.00% and 94.67% mortality, respectively after 24h of exposure with LC50 value at 4.65% and 3.5%, respectively (Tables XI, XII, and Fig. 4). Oppositely, sesame oil showed no effect on T. castaneum. Furthermore, the combination of GO+S at 5 and 10% showed 90 and 100% mortality, respectively, with an LC50 reached at a concentration of 1.97%. The synergistic factor was 2.36 (Tables XI, XII; Fig. 4).
AchE inhibition and MDA activity in house fly larvae
As shown in Figure 5A, all treatments induced reduction in the activity of AchE enzyme of the house fly larvae after 24 h with non-significant difference between these treatments. Meanwhile, all treatments exhibited significant increase in the MDA production when compared with that of the control untreated larvae (Fig. 5B).
Discussion
Traditional insecticides are commonly used to control various insect species; however, many of them do not have the expected efficacy, due to the development of insecticidal resistance (Scott et al., 2000). In addition, some insecticides are known to pose severe threats to human health and the environment. Therefore, it is necessary to investigate natural products such as essential oils as alternative insecticides (Koul et al., 2008). Following this prospective, the present work was aimed to investigate the insecticidal activities of the geranium essential oil and to evaluate the improvement in its activity when it was in nano-emulsion form and when it was combined with sesame oil.
Generally, the larval stage of insect causes most of the damage, thus determining the larvicidal activity of essential oils is critical (Ebadollah, 2012). The GO showed moderate activity against 3rd instar larvae of Musca domestica with LC50 4.29%. Similarly, Pavela (2008) reported moderate toxicity for geranium oil against the adults of M. domestica. In addition, Saraiva et al. (2020) found no larvicidal activity for the GO at concentrations from 2.5% to 20% against M. domestica. Meanwhile, the GN showed 100% larval mortality at concentration of 10% with LC50 achieved at a concentration of 1.50%. Our findings are in agreement with Boito et al. (2018) as they reported higher insecticidal effect for the cinnamon nanoemulsion with respect to the cinnamon oil against M. domestica and Haematobia irritans flies. Also, SO did not show larvicidal activity against M. domestica. Similarly, Mesbah et al. (2006) reported a weak toxic effect for the SO on the cotton leaf-worm Spodoptera littoralis. In addition, Soe et al. (2019) found no insecticidal activity for the SO against the pulse beetle Callosobruchus maculatus.
Interestingly, combination of GO and SO showed strong larvicidal activities against M. domestica with LC50 reached at concentration of 0.32% that attributed to synergistic action between SO and GO as evidenced by the synergist factor of 49.13. Several authors found that the combination of EOs induced great improvement in the potency of their insecticidal effects due to the synergetic effect between the different oils constituents like terpenes and phenylpropanoids (Gallardo et al., 2012; Tong and Bloomquist, 2013; Faraone et al., 2015; Gallardo et al., 2015). Similarly, Mesbah et al. (2006) realized that the combination of clove and SOs greatly improved its larvicidal effect when compared with their use alone against the 4th larval instar of Spodoptera littoralis. Also, Soe et al. (2019) observed synergistic effect for SO after mixing with clove oil against the pulse beetle, Callosobruchus maculatus.
The pupicidal effect of the tested EOs was also important for the control of the housefly population (Chintalchere et al., 2020). In the present study, GO and GN induced 100% inhibition of pupal emergence at concentration of 10% which was similar to those reported by Pavela (2008), Boito et al. (2018) and Saraiva et al. (2020). Meanwhile, SO showed no pupicidal activity at
Table IX. Contact toxicity of P. graveolens (Mean±SE) (GO and GN) and the synergistic action with SO against adults of Tribolium castaneum after 24h.
|
Concentration (%) |
G |
GN |
SO |
GO+SO |
|
0.312 |
3.33±0.33f |
13.33±3.33c |
20.00±5.77c |
53.33±3.33b |
|
0.625 |
11.67±1.76e |
20.00±5.77c |
30.00±5.77c |
100.00±0.00a |
|
1.25 |
37.70±1.45d |
60.00±5.77b |
76.66±3.33b |
100.00±0.00a |
|
2.50 |
65.00±2.65c |
90.00±5.77a |
100.00±0.00a |
100.00±0.00a |
|
5.00 |
84.33±2.33b |
100.00±0.00a |
100.00±0.00a |
100.00±0.00a |
|
10.000 |
100.00±0.00a |
100.00±0.00a |
100.00±0.00a |
100.00±0.00a |
|
Deltamethrin (1 ppm) (control positive) |
100.00±0.00a |
100.00±0.00a |
100.00±0.00a |
100.00±0.00a |
|
Acetone (negative control) |
16.67±3.33e |
16.67±3.33c |
16.67±3.33d |
16.67±3.33c |
Means within a column followed by the same letter are not significantly different (Duncan’s multiple range test: P > 0.05).
Table X. Adulticidal activity of P. graveolens (GO and GN) and the synergistic action with SO against T. castaneum in contact toxicity assay after 24h.
|
Treatment |
LC50 (%) |
95% CL |
LC90 |
95% CL |
X2 (df = 4) |
P* |
Synergism factor |
||
|
LCL |
UCL |
LCL |
UCL |
||||||
|
P. graveolens oil (GO) |
1.74 |
1.54 |
1.95 |
5.43 |
4.57 |
6.71 |
4.47 |
0.346 |
- |
|
P. graveolens nanoemulsion (GN) |
0.97 |
0.73 |
1.27 |
2.59 |
1.86 |
4.64 |
11.72 |
0.02 |
- |
|
Sesame oil (SO) |
0.711 |
0.496 |
0.99 |
1.75 |
1.21 |
3.88 |
17.04 |
0.002 |
- |
|
GO+SO |
0.302 |
0.27 |
0.33 |
0.46 |
0.42 |
0.56 |
1.50 |
0.83 |
5.76 |
For abbreviations and statistical details, see Table IV.
Table XI. Fumigant toxicity of P. graveolens (Mean ± SE) (GO and GN) and the synergistic action with SO against adults of Tribolium castaneuma after 24h.
|
Concentration (%) |
GO |
GN |
SO |
GO+SO |
|
0.312 |
0.00±0.00e |
0.00±0.00f |
0.00±0.00 |
4.21± 0.53d |
|
0.625 |
0.00±0.00e |
0.00±0.00f |
0.00±0.00 |
6.33± 0.88d |
|
1.25 |
6.00±3.33d |
8.00±1.73e |
0.00±0.00 |
26.67± 3.33c |
|
2.50 |
26.6±3.33c |
35.33±1.45d |
0.00±0.00 |
60.00± 5.77b |
|
5.00 |
53.33±3.33b |
64.00±2.08c |
0.00±0.00 |
90.00± 5.77a |
|
10.000 |
80.00±5.77a |
94.67±2.60b |
0.00±0.00 |
100.00±0.00a |
|
Deltamethrin (1 ppm) (control positive) |
100.00±0.00a |
100.00± 0.00a |
100.00±0.00a |
100.00±0.00a |
|
Acetone control negative |
0.00±0.00e |
0.00±0.00f |
0.00±0.00 |
0.00±0.00d |
Means within a column followed by the same letter are not significantly different (Duncan’s multiple range test: P > 0.05).
Table XII. Adulticidal activity of P. graveolens (GO and GN) and the synergistic action with SO against T. castaneum in fumigant toxicity assay.
|
Treatment |
LC50 (%) |
95% CL |
LC90 |
95% CL |
X2 (df = 3) |
P* |
Synergism factor |
||
|
LCL |
UCL |
LCL |
UCL |
||||||
|
P. graveolens oil (GO) |
4.65 |
4.13 |
5.29 |
13.88 |
11.27 |
18.29 |
1.717 |
0.633 |
- |
|
P. graveolens Nanoemulsion (GN) |
3.50 |
3.16 |
3.89 |
8.64 |
7.39 |
10.52 |
3.187 |
0.527 |
- |
|
Sesame oil (SO) |
- |
- |
- |
- |
- |
- |
- |
- |
- |
|
GO+SO |
1.97 |
1.77 |
2.19 |
4.91 |
4.23 |
5.91 |
1.783 |
0.619 |
2.36 |
For abbreviations and statistical details, see Table IV.
the all tested concentrations which in agreement with the finding of Mesbah et al. (2006) and Soe et al. (2019). Furthermore, the combination of GO+SO induced strong pupicidal effect and reduced the LC50 concentration to 0.50% with SF of 2.96. Several previous studies reported similar synergistic effect for sesame oil when mixed with other EOs (Mesbah et al., 2006; Karso and Al-Mallah, 2015; Soe et al., 2019).
The larvicidal activity of GO against Culex pipiens reached to 100% mortality at concentration of 1.25 % with LC50 achieved at concentration of 0. 22 %. Our findings are similar to those of Norris et al. (2015) as they recorded moderate toxicity for the GO against Aedes aegypti and Anopheles gambiae. Also, Ríos et al. (2017) reported larvicidal effect of 80% for the GO against Aedes aegypti larvae especially at higher concentrations (130mg/L). However, the lower concentration of GN (0.625 %) induced 100% larval mortality with LC50 achieved at concentration of 0.19 %. Several literatures demonstrated the strong insecticidal activity for the nano-emulsified preparations of EOs (Sugumar et al., 2014; Ramar et al., 2017; Sundararajan et al., 2018). Also, Jesser et al. (2020) reported significant larvicidal effect for Geranium maculatum L. nanoemulsion form against Culex pipiens and decreasing the LC50 from 80.97 ppm for EO to 48.27 ppm. Interestingly, combination of GO+SO led to decrease in concentration of the LC50 to 0.079 % with SF of 2.78. Our results are constant with work of Salama et al. (2002) as they confirmed a synergistic action for the SO against a Baygon– resistant strain of C. pipiens. Also, Jan (2001) emphasized the synergistic and antioxidant effects of SO and attributed these effects to presence of sesamin, sesamolin, and sesamol. In addition, Karso and Al-Mallah (2015) reported synergistic effect for SO when mixed with acetamiprid against the larvae of Trogoderma granarium.
Regarding the adulticidal activity against Tribolium castaneum; G was found to exert high contact toxicity than the fumigant effect. In contact assay, 100% mortality was achieved at concentration of 5% for GN after 24 h of exposure, with LC50 was reached at concentration of 0.97%. While in fumigant assay, 10 % GO showed 80.00% mortality with LC50 was achieved at concentration of 4.65%. The variation in the results of the two techniques could be attributed to the high volatility of EOs while in the contact assay the insects were in direct contact with the tested substance (Kabera et al., 2011). Similar variation was also previously reported by Odeyemi et al. (2008) and Kabera et al. (2011). In contrary, Abouelatta et al. (2020) noticed no toxic effect for P. graveolens EO on T. castaneum in the contact assay, while it showed a fumigant effect. This contradiction might be attributed to the variation in components of the used essential oil. GN induced no significant mortality at the lower concentration while at 10% concentration; mortality of 100% and 94.6 was achieved in both contact and fumigant assays, respectively similar to those reported by Boito et al. (2018). SO has no effect against T. castaneum adult in fumigant assay but it induced 100 % mortality in the direct contact assay after 24h of exposure. The mixture of GO and SO exerted 100% adulticidal activity against T. castaneum either by contact or fumigant assays with LC50 was reached at concentration of 1.97% and SF of 5.76 in agreement with the results of Mesbah et al. (2006), Karso and Al-Mallah (2015) and Soe et al. (2019). Several studies reported the synergistic action for the sesame oil to various insecticides through the obstruction of the detoxification process (Gowda, 1996; Visetson et al., 2003; Vastrad et al., 2004).
The insecticidal action of P. graveolens EO is due to the fact that it contains large quantity of monoterpenes, mainly citronellol and trans-geraniol (Babu and Kaul, 2005; Bouzenna and Krichen, 2013). The insecticidal effect of the constituents found in essential oils is caused by the oil penetration into the insect’s tissues and altering the insect’s main physiological functions (Babu and Kaul, 2005). According to Pavela (2005), the effectiveness of essential oils may vary based on the method of application and the ability of each insect species for detoxification. Although the mode of action of EOs is unknown, it was found to have acute, sub-acute, and sub-lethal effects (Hummelbrunner and Isman, 2001).
Our results demonstrated that the predominant constituents in SO are linoleic acid (37.27 %), oleic acid (26.07 %) and palmitic acid (13.34 %). Similar findings were reported by Rounizi et al. (2021). The action of SO on insects may refer to that the oil disrupting gas exchange (respiration) and destruct the cell membrane function or structure of the tick. So, SO toxic action is more physical than chemical and is short-lived (Cranshaw and Baxendale, 2013).
In the present study, to improve the efficacy of GO; two forms were prepared; GN formulation and a binary mixture with SO. Anjali et al. (2012) proposed that the GN preparation as a new promising way to improve the properties and effectiveness of botanical insecticides for commercial use. Our results revealed that the lower concentrations of GN induced no significant mortality while the higher concentrations caused 100% mortality. Another approach to increase the efficiency of GO is the combination with the SO for its synergistic action. This combination increased the percentage of mortality even at the lower concentrations when compared to their use alone against insects.
In the current study, all treatments caused neurotoxicity as evidenced by inhibiting the AchE enzyme of the M. domestica larvae after 24h of treatment. Many secondary metabolites of aromatic plants such as EOs and monoterpenes were known to be able to inhibit the AchE activity of insects (Senthil-Nathan et al., 2007). Our findings go parallel with Chintalchere et al. (2020) as they reported inhibition in AchE activity of M. domestica larvae treated with bay EO. Similarly, Anderson and Coats (2012) and Rajashekar et al. (2014) found inhibition in AchE of M. domestica treated with carvacrol and coumaran, extracted from Lantana camara.
All treatments induced an increase in the MDA production in M. domestica larvae which reflect the role of oxidative stress in the insecticidal effects of GO. These results are in accordance with Rahimi et al. (2018) as they observed increase in the level of MDA in Helicoverpa armigera larvae treated with P. persicaria agglutinin. Rahimi et al. (2018) postulated that the plant-derived compounds were able to enhance high levels of lipid peroxidation, that induces cytotoxicity in insect midgut epithelial cells.
It is worth mentioning that the search for synergy among the essential oil constituents is a promising approach for increasing the insecticidal activity of natural compounds. Synergistic mixtures enable a defined level of effect to be achieved at lower dose of constituents than that of using them alone. Thus, in the present study, mixing the GO with the SO could be an effective strategy to improve insecticidal activity and decrease insect control costs due to the lower price of sesame than geranium oil. This synergy may be attributed to the different mode of action of both oils. The geranium effects on the nervous system of insect, meanwhile, sesame oil acts through physical and mechanical effect on the insect.
Conclusions
Mixing the GO with SO increased the insecticidal activity with higher percentage of mortality even at lower concentrations when compared with the use of each one alone. The SO is of low price; thus, it’s use in combination with GO (expensive) decreasing its dose and saving money. This form of natural products is safe for environment, animals and human consequently it could be utilized in the integrated pest management.
Acknowledgments
The authors appreciate the help of veterinarians and technicians for conducting this work.
Funding
This work was supported by Researcher supporting Project (RSP-2023/3), King Saud University.
IRB approval
This work was approved by the Institutional Review Board of Beni-Suef University.
Ethics approval and consent to participate
Not applicable.
Availability of data and materials
All the study data are in this article.
Consent to Publish
Not applicable.
There is supplementary material associated with this article. Access the material online at: https://dx.doi.org/10.17582/journal.pjz/20220418100455
Statement of conflict of interest
The authors have declared no conflict of interest.
References
Abbott, W.S., 1925. A method of computing the effectiveness of an insecticide. J. Econ. Entomol., 18: 265–267, https://doi.org/10.1093/jee/18.2.265a
Abd El-Wahab, M.A., Toaima, W.I.M. and Hamed, E.S., 2016. Effect of different planting locations in Egypt on volatile oil of geranium (Pelargonium graveolens L.) plant. J. Basic appl. Res., 2: 522-533.
Aboelhadid, S.M. and Youssef, I.M.I., 2021. Control of red flour beetle (Tribolium castaneum) in feeds and commercial poultry diets via using a blend of clove and lemon grass extracts. Environ. Sci. Pollut. Res. Int., Epub ahead of print. PMID: 33582963. https://doi.org/10.1007/s11356-021-12426-7
Aboelhadid, S.M., Arafa, W.M., Abdel-Baki, A.S., Sokmen, A., Al-Quraishy, S., Hassan, A.O., Kamel, A.A., 2021 Acaricidal activity of Foeniculum vulgare against Rhipicephalus annulatus is mainly dependent on its constituent from trans-anethone. PLoS One. 2: e0260172. https://doi.org/10.1371/journal.pone.0260172
Abouelatta, A.M., Keratum, A.Y., Ahmed, S.I. and El-Zun, H.M., 2020. Repellent, contact and fumigant activities of geranium (Pelargonium graveolens L.’Hér) essential oils against Tribolium castaneum (Herbst) and Rhyzopertha dominica (F.). Int. J. trop. Insect Sci., 40: 1021–1030. https://doi.org/10.1007/s42690-020-00161-4
Anderson, J.A. and Coats, J.R., 2012. Acetylcholinesterase inhibition by nootkatone and carvacrol in arthropods. Pestic. Biochem. Physiol., 102: 124-128. https://doi.org/10.1016/j.pestbp.2011.12.002
Anjali, C.H., Sharma, Y., Mukherjee, A. and Chandrasekaran, N., 2012. Neem oil (Azadirachta indica) nanoemulsion. A potent larvicidal agent against Culex quinquefasciatus. Pest Manage. Sci., 68: 158–163. https://doi.org/10.1002/ps.2233
Arthur, F.H., 2019. Efficacy of combinations of methoprene and deltamethrin as long-term commodity protectants. Insects, 10: 50. https://doi.org/10.3390/insects10020050
Babu, K.G.D. and Kaul, V.K., 2005. Variation in essential oil composition of rose-scented geranium (Pelargonium sp.) distilled by different distillation techniques. Flavour Fragance J., 20: 222–231. https://doi.org/10.1002/ffj.1414
Bakkali, F., Averbeck, S., Averbeck, D. and Idaomar, M., 2008. Biological effects of essential oils. A review. Fd. Chem. Toxicol., 46: 446–475. https://doi.org/10.1016/j.fct.2007.09.106
Bar-Or, D., Rael, L.T., Lau, E.P., Rao, N.K., Thomas, G.W., Winkler, J.V., Yukl, R.L., Kingston, R.G. and Curtis, C.G., 2001. An analog of the human albumin N-terminus (Asp-Ala-His-Lys) prevents formation of copper induced reactive oxygen species. Biochem. biophys. Res. Commun., 284: 856–862. https://doi.org/10.1006/bbrc.2001.5042
Begum, S., Furumoto, T. and Fukui, H., 2000. A new chlorinated red naphthoquinone from roots of Sesamum indicum. Biosci. Biotech. Biochem., 64: 873–874. https://doi.org/10.1271/bbb.64.873
Bhatt, S., Gething, P.W., Brady, O.J., Messina, J.P., Farlow, A.W., Moyes, C.L., Drake, J.M., Brownstein, J.S., Hoen, A.G., Myers, M.F., George, D.B., Jaenisch, T., William, G.R., Simmons, C.P., Scott, T.W., Farrar, J. and Hay, S.I., 2013. The global distribution and burden of dengue. Nature, 496: 504–507. https://doi.org/10.1038/nature12060
Boito, J.P., Da Silva, A.S., dos Reis, J.H., Santos, D.S., Gebert, R.R., Biazus, A.H., Santos Roberto, C.V., Quatrin, P.M., Ourique, A.F., Boligon, A.A., Baretta, D., Baldissera, M.D., Stefani, L.M. and Machado, G., 2018. Insecticidal and repellent effect of cinnamon oil on flies associated with livestock. Rev. MVZ Córdoba, 23: 6628-6635. https://doi.org/10.21897/rmvz.1337
Boukhatem, M.N., Kameli, A., Ferhat, M.A., Saidi, F. and Mekarnia, M., 2013. Rose geranium essential oil as a source of new and safe anti-inflammatory drugs. Libyan J. Med., 8: 22520. https://doi.org/10.3402/ljm.v8i0.22520
Bouzenna, H. and Krichen, L., 2013. Pelargonium graveolens L’Her. And Artemisia arborescens L. essential oils: chemical composition, antifungal activity against Rhizoctonia solani and insecticidal activity against Rhysopertha dominica. Nat. Prod. Res., 27: 841–846. https://doi.org/10.1080/14786419.2012.711325
Boukhris, M., Bouaziz, M., Feki, I., Jemai, H., El-Feki, A. and Sayadi, S., 2012. Hypoglycemic and antioxidant effects of leaf essential oil of Pelargonium graveolens L’Hér. in alloxan induced diabetic rats. Lipids Hlth. Dis., 11: 81-81. https://doi.org/10.1186/1476-511X-11-81
Broussalis, A.M., Ferraro, G.E., Martino, V.S., Pinzon, R., Coussio, J.C. and Alvarez, J.C., 1999. Argentine plants as potential source of insecticidal compounds. J. Ethnopharmacol., 67: 219–223. https://doi.org/10.1016/S0378-8741(98)00216-5
Busvine, J.R., 1971. A critical review of the techniques for testing insecticides. CABI, London. p. 345.
Carre˜no, L.O., Vargas, M.L.Y., Duque, J.E.L. and Kouznetsov, V.V., 2014. Design, synthesis, acetylcholinesterase inhibition and larvicidal activity of girgensoh-nine analogs on Aedes aegypti, vector of dengue fever. Eur. J. med. Chem., 78: 392–400. https://doi.org/10.1016/j.ejmech.2014.03.067
Cavalca, P.A.M., de Assumpção Lolis, M.I.G., Reis, B. and Bonato, C.M., 2010. Homeopathic and larvicide effect of Eucalyptus cinerea essential oil against Aedes aegypti. Braz. Arch. Biol. Technol., 53: 835-843. https://doi.org/10.1590/S1516-89132010000400012
Chintalchere, J.M., Dar, M.A. and Pandit, R.S., 2020. Biocontrol efficacy of bay essential oil against housefly, Musca domestica (Diptera:Muscidae). J. Basic Appl. Zool., 81: 1-12. https://doi.org/10.1186/s41936-020-0138-7
Chou, T.C., 2006. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev., 58: 621-681. https://doi.org/10.1124/pr.58.3.10
Cranshaw, W.S. and Baxendale, B., 2013. Insect Control: Horticultural Oils. www.ext.colostate.edu.
De Jesús, A.J., Olsen, A.R., Bryce, J.R. and Whiting, R.C., 2004. Quantitative contamination and transfer of Escherichia coli from foods by houseflies, Musca domestica L. (Diptera:Muscidae). Int. J. Fd. Microbiol., 93: 259–262. https://doi.org/10.1016/j.ijfoodmicro.2003.12.003
Donsi, F. and Ferrari, G., 2016. Essential oil nanoemulsions as antimicrobial agents in food. J. Biotechnol., 233: 106-120.
Dos Santos, D.S., Boito, J.P., Santos, R.C.V., Quatrin, P.M., Ourique, A.F., dos Reis, J.H., Gebert, R.R., Glombowsky, P., Klauck, V., Boligon, A.A., Baldissera, M.D. and Da Silva, A.S., 2017. Nanostructured cinnamon oil has the potential to control Rhipicephalus microplus ticks on cattle. Exp. appl. Acarol., 73: 129–138. https://doi.org/10.1007/s10493-017-0171-5
Eagleson, C., 1940. Oil synergist for insecticides. 2, 202, 145, issued May 28.
Ebadollahi, A., Nouri-Ganbalani, G., Hoseini, S.A. and Sadeghi, G.R., 2012. Insecticidal activity of essential oils of five aromatic plants against Callosobruchus maculatus F. (Coleoptera: Bruchidae) under laboratory conditions. J. Essent. Oil Bear Pl., 15: 256-262. https://doi.org/10.1080/0972060X.2012.10644044
Echeverría, J., and Duarte Galhardo de Albuquerque, R.D., 2019. Nanoemulsions of essential oils: New tool for control of vector-borne diseases and in vitro effects on some parasitic agents. Medicines, 6: 1-11. https://doi.org/10.3390/medicines6020042
Ellman, G.L., Courtney, K.D., Andres, V. Jr, and Featherstone, R.M., 1961. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol., 7: 88–95. https://doi.org/10.1016/0006-2952(61)90145-9
Faraone, N., Hillier, N.K. and Cutler, G.C., 2015. Plant essential oils synergize and antagonize toxicity of different conventional insecticides against Myzus persicae (Hemiptera: Aphididae). PLoS One, 10: e0127774. https://doi.org/10.1371/journal.pone.0127774
Finney, D.J., 1952. Probit analysis: A statistical treatment of the sigmoid response curve (2nd ed.). Cambridge University Press.
Finney, D.J., 1971. Probit analysis, 3rd edn. Cambridge University Press, London.
Gross, L., 2006. Gut bacteria cospeciating with Insects. PLoS Biol., 4: e357. https://doi.org/10.1371/journal.pbio.0040357
Gallardo, A., Picollo, M.I., Gonzlez-Audino, P. and Mougabure-Cueto, G., 2012. Insecticidal activity of individual and mixed monoterpenoids of geranium essential oil against Pediculus humanus capitis (Phthiraptera:Pediculidae). J. med. Ent., 49: 332–335. https://doi.org/10.1603/ME11142
Gallardo, A., Inés Picollo, M. and Mougabure-Cueto, G., 2015. Lethal activity of individual and mixed monoterpenoids of geranium essential oil on Musca domestica. Parasitol. Res., 114: 1229–1232. https://doi.org/10.1007/s00436-015-4315-4
Gowda, G., 1996. Studies on synergism with reference to the insecticide resistance management (IRM) of Helicoverpa armigera (Hubner). Ph.D. thesis., Tamil Nadu Agricultural University, Coimbatore.
Hassane, S.O.S., Ghanmi, M., Satrani, B., Mansouri, N., Mohamed, H., El-Hajaji, H. and Chaouch, A., 2011. Composition chimique et activités antibactériennes, antifongiques et antioxydante de l’huile essentielle de Pelargonium asperum Ehrh. ex Wilde des Comores. Acta Bot. Gall., 158: 225–237. https://doi.org/10.1080/12538078.2011.10516269
Hashem, A.S., Awadalla, S.S., Zayed, G.M., Maggi, F. and Benelli, G., 2018. Pimpinella anisum essential oil nanoemulsions against Tribolium castaneum-insecticidal activity and mode of action. Environ. Sci. Pollut. Res. Int., 25: 18802–18812. https://doi.org/10.1007/s11356-018-2068-1
Hummelbrunner, L.A. and Isman, M.B., 2001. Acute, sublethal, antifeedant, and synergistic effects of monoterpenoid essential oil compounds on the tobacco cutworm, Spodoptera litura (Lep., Noctuidae). J. Agric. Fd. Chem., 49: 715-720. https://doi.org/10.1021/jf000749t
Jan, K., 2001. Study on the antioxidative activities of extracts from sesame meal with different solvents. Dissertation, National Taiwan University, Taipei.
Jesser, E., Lorenzetti, A.S., Yeguerman, C., Murray, A.P., Dominic, C. and Werdin-Gonzalez, J.O., 2020. Ultrasound assisted formation of essential oil nanoemulsions: Emerging alternative for Culex pipiens pipiens Say (Diptera: Culicidae) and Plodia interpunctella Hubner (Lepidoptera: Pyralidae) management. Ultrason. Sonochem., 61: 104832. https://doi.org/10.1016/j.ultsonch.2019.104832
Jesikha, M., 2014. Control of Musca domestica using wastes from Citrus sinensis peel and Mangifera indica seed. Scrut. Int. Res. J. biol. environ. Sci., 1: 1.
Kabera, J., Gasogo, A., Uwamariya, A., Viateur, U.V. and Nyetera, P., 2011. Insecticidal effects of essential oils of Pelargonium graveolens and Cymbopogon citratus on Sitophilus zeamais (Motsch.). Afr. J. Fd. Sci., 5: 366-375.
Kamel, A.A., Mohamed, M.B. and El-Dakhly, K.M., 2019. Larvicidal activity and bio-efficacy of some products against larvae of the housefly, Musca domestica (L) (Diptera: Muscidae). J. appl. Sci., 19: 427-433. https://doi.org/10.3923/jas.2019.427.433
Karso, B.A. and Al-Mallah, N.M., 2015. Effectiveness of some vegetable oils and insecticide mixtures, against larvae of the khapra beetle Trogoderma granarium Everts (Coleoptera: Dermestidae). Egypt. J. Biol. Pest Contr., 25: 139-143.
Kato, M.J., Chu, A., Davin, L.B. and Lewis, N.G., 1998. Biosynthesis of antioxidant lignans in Sesamum indicum seeds. Phytochemistry, 47: 583–591. https://doi.org/10.1016/S0031-9422(97)00727-9
Khan, H., Akram, W., Shad, S.A., Razaq, M., Naeem-Ullah, U. and Zia, K., 2013. A cross sectional survey of knowledge, attitude and practices related to house flies among dairy farmers in Punjab, Pakistan. J. Ethnobiol. Ethnomed., 9: 18. https://doi.org/10.1186/1746-4269-9-18
Ko, K., Juntarajumnong, W. and Chandrapatya, A., 2009. Repellency, fumigant and contact toxicities of Melaleuca cajuputi Powell against Sitophilus zeamais Motschulsky and Tribolium castaneum Herbst. Thai J. agric. Sci., 42: 27–33.
Koul, O., Walia, S. and Dhaliwal, G.S., 2008. Essential oils as green pesticides: Potential and constraints. Biopestic. Int., 4: 63-84.
Kranthi, K.R., 2005. Insecticide resistance monitoring, mechanisms and management manual. CICR, Nagpur, and ICAC, Washington. pp. 46-47.
Kumar, P., Mishra, S., Malik, A. and Satya, S., 2011. Repellent, larvicidal and pupicidal properties of essential oils and their formulations against the housefly, Musca domestica. Med. Vet. Ent., 25: 302–310. https://doi.org/10.1111/j.1365-2915.2011.00945.x
Lalli, J.Y., Viljoen, A.M., and Van Vuuren, S.F., 2010. Potential interaction between the volatile and non-volatile fractions on the in vitro antimicrobial activity of three south African Pelargonium (Geraniaceae) species. Nat. Prod. Commun., 5: 1395–1400. https://doi.org/10.1177/1934578X1000500911
Lazcano, D.E., Padilla, C.E., Castillo, H.G.A., Estarrón, E.M., Espinosa, A.H., Paniagua, B.N.A., Gutiérrez, O.A. and Martínez, V.M., 2019. Development of essential oil-based phyto-formulations to control the cattle tick Rhipicephalus microplus using a mixture design approach. Exp. Parasitol., 201: 26-33.
Lee, B.Y., Alfaro-Murillo, J.A., Parpia, A.S., Asti, L., Wedlock, P.T., Hotez, P.J. and Galvani, A.P., 2017. The potential economic burden of Zika in the continental United States. PLoS Negl. Trop. Dis., 11: e0005531. https://doi.org/10.1016/j.exppara.2019.04.008
Lis-Balchin, M., Hart, S. and Roth, G., 1997. The spasmolytic activity of the essential oils of scented Pelargoniums (Geraniaceae). Phyther. Res. Int. J. Devoted Med. Sci. Res. Pl. Pl. Prod., 11: 583–584. https://doi.org/10.1002/(SICI)1099-1573(199712)11:8<583::AID-PTR148>3.0.CO;2-6
Maruyama, N., Ishibashi, H., Hu, W., Morofuji, S., Inouye, S., Yamaguchi, H. and Abe, S., 2006. Suppression of carrageenan-and collagen II-induced inflammation in mice by geranium oil. Mediat. Inflamm., 31: 625–637. https://doi.org/10.1155/MI/2006/62537
Massoud, M.A., Adel, M.M., Zaghloul, O.A., Mohamed, M.I.E. and Abdel-Rheim, K.H., 2018. Eco-friendly nano-emulsion formulation of Mentha piperita against stored product pest Sitophilus oryzae. Adv. Crop Sci. Tech., 6: 1–6. https://doi.org/10.4172/2329-8863.1000404
Mesbah, H.A., Mourad, A.K. and Rokaia, A.Z.M., 2006. Efficacy of some plant oils alone and/or combined with different insecticides on the cotton leaf-worm Spodoptera littoralis (Boisd.) (Lepidoptera: Noctuidae) in Egypt. Commun. Agric. Appl. Biol. Sci., 71: 305-328.
Nirmala, M.J., Durai, L., Gopakumar, V. and Nagarajan, R., 2020. Preparation of celery essential oil-based nanoemulsion by ultrasonication and evaluation of its potential anticancer and antibacterial activity. Int. J. Nanomed., 8: 7651-7666. https://doi.org/10.2147/IJN.S252640
Norris, E.J., Gross, A.D., Dunphy, B.M., Bessette, S., Bartholomay, L. and Coats, J.R., 2015. Comparison of the insecticidal characteristics of commercially available plant essential oils against Aedes aegypti and Anopheles gambiae (Diptera: Culicidae). J. med. Ent., 52: 993-1002. https://doi.org/10.1093/jme/tjv090
Odeyemi, O., Masika, P.J. and Afolayan, A.J., 2008. Insecticidal activities of essential oil from the leaves of Mentha longifolia L. subsp. capensis against Sitophilus zeamais (Motschulsky) (Coleoptera: Curculionidae). Afr. Entomol., 16: 220-225. https://doi.org/10.4001/1021-3589-16.2.220
Palacios, S.M., Bertoni, A., Rossi, Y., Santander, R. and Urzua, A., 2009. Efficacy of essential oils from edible plants as insecticides against the house fly, Musca domestica L. Molecules. (Basel, Switzerland). 14: 1938-1947. https://doi.org/10.3390/molecules14051938
Pavela, R., 2005. Insecticidal activity of some essential oils against larvae of Spodoptera littoralis. Fitoterapia, 76: 691–696. https://doi.org/10.1016/j.fitote.2005.06.001
Pavela, R., 2008. Larvicidal effects of various Euro-Asiatic plants against Culex quinquefasciatus Say larvae (Diptera: Culicidae). Parasitol. Res., 102: 555–559. https://doi.org/10.1007/s00436-007-0821-3
Raghavendra, D., Manoharan, T. and Preeta, G., 2017. Evaluation of plant oils as synergists in suppression of malathion resistance in Sitophilus oryzae (L.) and Tribolium castaneum (Herbst.). Int. J. Curr. Microbiol. appl. Sci., 6: 909-917. https://doi.org/10.20546/ijcmas.2017.607.112
Rahimi, V., Hajizadeh, J., Zibaee, A. and sendi, J.J., 2018. Effect of Polygonum persicaria (Polygonales: Polygonaceae) extracted agglutinin on life table and antioxidant responses in Helicoverpa armigera (Lepidoptera: Noctuidae) larvae. J. econ. Ent., 111: 10.1093/jee/toy006. https://doi.org/10.1093/jee/toy006
Rajashekar, Y., Raghavendra, A. and Bakthavatsalam, N., 2014. Acetylcholinesterase inhibition by biofumigant (Coumaran) from leaves of Lantana camarain in stored grain and household insect pests. BioMed. Res. Int., 6: 187019. https://doi.org/10.1155/2014/187019
Ram, R., Catlin, D., Romero, J. and Cowley, C., 1990. Sesame: New approaches for crop improvement. In: Advances in new crops (eds. J. Janick, J.E. Simon). Timber Press, Portland. pp. 225-228.
Ramar, M., Manonmani, P., Arumugam, P., Kannam, S.K., Erusan, R.R., Baskaran, N. and Murugan, K., 2017. Nano-insecticidal formulations from essential oil (Ocimum sanctum) and fabricated in filter paper on adult of Aedes aegypti and Culex quinquefasciatus. J. Entl. Zool. Stud., 5: 1769–1774.
Rey, D., David, J.P., Besnard, G., Jullien, J.L., Lagnean, C. and Meyran, J.C., 2001. Comparative sensitivity of larval mosquitoes to vegetable polyphenols versus conventional insecticides. Ent. Exp. Appl., 8: 361-367. https://doi.org/10.1046/j.1570-7458.2001.00793.x
Ríos, N., Stashenko, E.E. and Duque, J.E., 2017. Evaluation of the insecticidal activity of essential oils and their mixtures against Aedes aegypti (Diptera: Culicidae). Rev. Bras. Ent., 61: 307–311. https://doi.org/10.1016/j.rbe.2017.08.005
Rounizi, S.K., Mohajeri, F.A., Broujeni, H.M., Pourramezani, F., Jambarsang, S., Kiani, H. and Sadrabad, E.K., 2021. The chemical composition and heavy metal content of sesame oil produced by different methods: A risk assessment study. Fd. Sci. Nutr., 9: 2886–2893. https://doi.org/10.1002/fsn3.2245
Salama, E.M., Hamed, M.S. and El-Hosary, S.M., 2002. Synergism and antagonism of Baygon with some additives against Baygon–resistant strain of Culex pipiens larvae. Egypt. J. Biol., 4: 127-132.
Saraiva, L.C., Magalhães de Matos, A.F.I., Cossetin, L.F., Couto, J.C.M., Petry, L.d.S. and Monteiro, S.G., 2020. Insecticidal and repellent activity of geranium essential oil against Musca domestica and Lucilia cuprina. Int. J. Trop. Insect Sci., 40: 1093–1098. https://doi.org/10.1007/s42690-020-00137-4
Sasaki, T., Kobayashi, M. and Agui, N., 2000. Epidemiological potential of excretion and regurgitation by Musca domestica (Diptera:Muscidae) in the dissemination of Escherichia coli O157: H7 to food. J. med. Ent., 3 945–949. https://doi.org/10.1603/0022-2585-37.6.945
Saranya, S., Chandrasekaran, N. and Mukherjee, A., 2012. Antibacterial activity of eucalyptus oil nanoemulsion against Proteus mirabilis. Int. J. Pharm. Pharm. Sci., 4: 668-671. f811/e54f0653236420785ecab5ec329c1638fa4e.pdf.
Sayed, R.M., Abdalla, R.S., Rizk, S.A. and El-Sayed, T.S., 2018. Control of Culex pipiens (Diptera: Culicidae), the vector of lymphatic filariasis, using irradiated and nonirradiated entomopathogenic nematode, Steinernema scapterisci (Rhabditida: Silver nanoparticles synthesis and its larvicidal potential against dengue, malaria Steinerne matidae). Egypt. J. Biol. Pest Contr., 28: 67. https://doi.org/10.1186/s41938-018-0070-z
Scott, J.G., Alefantis, T.G., Kaufmann, P.E. and Rutz, D.A., 2000. Insecticide resistance in house flies from caged-layer poultry facilities. Pest Manage. Sci., 56: 147–153. https://doi.org/10.1002/(SICI)1526-4998(200002)56:2<147::AID-PS106>3.0.CO;2-7
Service, M., 2012. Introduction to mosquitoes (Culicidae). In: Medical entomology for students, 5th ed Cambridge University Press, New York. pp. 2–34.
Senthil-Nathan, S., Choi, M.Y., Paik, C.H., Seo, H.Y., Kim, J.D. and Kang, S.M., 2007. The toxic effects of neem extract and azadirachtin on the brown planthopper, Nilaparvata lugens (Stål) (BPH) (Homoptera: Delphacidae). Chemosphere, 67: 80-88. https://doi.org/10.1016/j.chemosphere.2006.09.045
Shaalan, E.A.S., Canyon, D., Younes, M.W.F., Abdel-Wahab, H. and Mansour, A.H., 2005. A review of botanical phytochemicals with mosquitocidal potential. Environ. Int., 31: 1149–1166. https://doi.org/10.1016/j.envint.2005.03.003
Sharifian, I., Safaralizade, M. and Najafi-moghaddam, P., 2011. Investigation on the insecticidal efficacy of novel pellet formulation against stored products beetles. Mun. Ent. Zool., 6: 204–209.
Singh, V., Mishra, N., Awasthi, G., Dash, A.P. and Das, A., 2009. Why is it important to study malaria epidemiology in India? Trends Parasitol., 25: 452–457. https://doi.org/10.1016/j.pt.2009.06.004
Sinthusiri, J. and Soonwera, M., 2014. Oviposition deterrent and ovicidal activities of seven herbal essential oils against female adults of housefly, Musca domestica L. Parasitol. Res., 113: 3015–3022. https://doi.org/10.1007/s00436-014-3964-z
Soe, T.N., Ngampongsai, A. and Sittichay, W., 2019. Synergistic effect of sesame oil and clove oil on toxicity against the pulse beetle, Callosobruchus maculatus (Fabricius) (Coleoptera: Chysomelidae). Khon Kaen Agric. J., 47: 351-356.
Sugumar, S., Clarke, S.K., Nirmala, M.J., Tyagi, B.K., Mukherjee, A. and handrasekaran, N., 2014. Nanoemulsion of Eucalyptus oil and its larvicidal activity against Culex quinquefasciatus. Bull. entomol. Res., 104: 393–402. https://doi.org/10.1017/S0007485313000710
Sundararajan, B., Moola, A.K., Vivek, K. and Kumari, B.D.R., 2018. Formulation of nanoemulsion from leaves essential oil of Ocimum basilicum L. and its antibacterial, antioxidant and larvicidal activities (Culex quinquefasciatus). Microb. Pathog., 125: 475–485. https://doi.org/10.1016/j.micpath.2018.10.017
Stanaway, J.D., Shepard, D.S., Undurraga, E.A., Halasa, Y.A., Coffeng, L.E., Brady, O.J., Hay, S.I., Bedi, N., Bensenor, I.M., Casta˜neda-Orjuela, C.A., Chuang, T.W., Gibney, K.B., Memish, Z.A., Rafay, A., Ukwaja, K.N., Yonemoto, N. and Murray, C.J.L., 2016. Theglobal burden of dengue: An analysis from the global burden of disease study 2013. Lancet Infect. Dis., 16: 712–723. https://doi.org/10.1016/S1473-3099(16)00026-8
Tong, F. and Bloomquist, J.R., 2013. Plant essential oils affect the toxicities of carbaryl and permethrin against Aedes aegypti (Diptera: Culicidae). J. med. Ent., 50: 826–832. https://doi.org/10.1603/ME13002
Vastrad, A.S., Lingappa, S. and Basavan, G.K., 2004. Ovicides for managing resistant populations of diamondback moth (Plutella xylostella L.). Resistant Pest Manage., 14: 5.
Visetson, S., Milne, J., Milne, M. and Kanasutar, P., 2003. Synergistic effects of sesame oil with cypermethrin on the survival and detoxification enzyme activity of Plutella xylostella L. larvae Kasetsart J. (Nat. Sci.) 37: 52–59.
WHO, 2005. Guidelines for laboratory and field testing of mosquito larvicides. Bulletin WHO, pp. 1-41.
To share on other social networks, click on any share button. What are these?










