Influence of Seed Priming on Growth, Yield and Fodder Quality of Pearl Millet Cultivars
Research Article
Influence of Seed Priming on Growth, Yield and Fodder Quality of Pearl Millet Cultivars
Muhammad Zeeshan Nadeem1, Muhammad Farrukh Saleem1*, Muhammad Ashfaq Wahid1 and Muhammad Anwar ul Haq2
1Department of Agronomy, University of Agriculture Faisalabad, Pakistan; 2Institute of Soil and Environmental Sciences, University of Agriculture Faisalabad, Pakistan.
Abstract | Less fodder yield with poor quality cannot fulfill the livestock fodder and nutrition requirements in semi-arid regions, particularly during lean period. To improve initial stand establishment and the fodder yield and quality in pearl millet cultivars through seed priming, a two years field study was conducted at research area of department of agronomy, University of Agriculture Faisalabad, Pakistan. Three seed priming treatments viz. hydro priming (48h), ascorbate (40 mg L-1) and calcium chloride (100 mg L-1) including one control (no-priming) were tested with four pearl millet cultivars (YBS-98, 18-BY, Ghana White and Sargodha Bajra 2011) during the experimentation. Treatments were arranged in randomized complete block design and replicated thrice. The results revealed that seed germination, plant growth, fodder yield and quality were improved by all seed priming agents over the control, but CaCl2 priming outclassed all other priming agents for all the attributes. For example, seed priming of CaCl2 increased plant height significantly during both years of study over the other priming agents. Similarly, maximum fodder yield during both growing seasons (67 t ha-1) was observed under CaCl2 seed priming. Likewise, fodder crude proteins, crude fiber contents, ash contents and ether extractable fat were increased prominently by CaCl2 over the other priming agents. Among the cultivars, Sargodha Bajra 2011 performed better than other cultivars it showed better adaptability for more pearl millet fodder yield and quality. Therefore, seed priming with CaCl2 can be used to increase pearl millet fodder yield and quality to overcome the fodder scarcity under semi-arid regions.
Received | January 03, 2022; Accepted | March 14, 2022; Published | March 30, 2022
*Correspondence | Muhammad Farrukh Saleem, Department of Agronomy, University of Agriculture Faisalabad, Pakistan; Email: mfsuaf@yahoo.com
Citation | Nadeem, M.Z., M.F. Saleem, M.A. Wahid and M.A. Haq. 2022. Influence of seed priming on growth, yield and fodder quality of pearl millet cultivars. Pakistan Journal of Agricultural Research, 35(1): 215-226.
DOI | https://dx.doi.org/10.17582/journal.pjar/2022/35.1.215.226
Keywords | Pennisetum varieties, Priming agents, Green fodder, Emergence
Copyright: 2022 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
In dry land areas of Pakistan, a vital sector of livelihood is livestock. On regular basis, people have 5 to 6 heads of sheep and around 2 to 3 cattle per family. Livestock plays pivotal role in the country’s economy; it contributes 60.56% and 11.69% to agriculture sector and national GDP, respectively (GoP, 2020). Fodder scarcity is a major stumbling block in livestock development in Pakistan. The researchers reported in Pakistan, fodder production is virtually 50% below than the original need of animals (Khan et al., 2007). Fodder shortage has increased because of depletion in cultivated region of fodder yields by around 2% during every past decade (Yadav et al., 2012). In order to cater for the escalating demand of livestock related products, emphasis needs to be enhanced on raising production of fodder which is the cheaper source of feed for animals (Sarwar et al., 2002).
Pearl millet is traditionally low water requiring crop and grown in rainfed areas of Pakistan; however, the crop can successfully be grown in irrigated areas of the country as well (Yusuf et al., 2012). Fodder of pearl millet contains around 50% digestible nutrients which comprises of 8% protein, 45% extract free of nitrogen and 2.5% fat (Jukanti et al., 2016). Varieties vary notably both in quality and yield attributes like crude fiber, ash and crude protein (Bukhari et al., 2011) and resistance to numerous diseases (Wilson et al., 2008). Dry matter production was higher in tall growing varieties mainly because of large leaf area duration compared with those of short statured varieties (Gong et al., 2020). Single type of cultivation cannot be produced in the entire country while having different types of environmental conditions (Delrot et al., 2020). Dry matter yield as well as quality of fodder are influenced by climatic factors therefore, genotypes need to be evaluated in various environments (Silva-Pérez et al., 2020). Nutrient composition of a feedstuff is mainly determined by genotype, but different soil, environmental and crop factors also influence the chemical composition of a feedstuff.
Interaction between genotype and the environment has always been problematic in crop growth while recommending or approving diversity for some agricultural areas in developing countries (De Ollas et al., 2019). Breeders have always worked on the collection and creation of genetic differences among crops for improvement of variations which are appropriate to diversified agro-climate regions (Khan et al., 2020). Seed priming is an attractive option as it is a cost effective, simple, low risk and short gun approach to overcome any stress conditions (Farooq et al., 2006). Seed priming boosts germination as well as seedling growth under normal and salt stress areas (Basra et al., 2005). Treating seeds before sowing with inorganic or organic substances with high or low temperatures improve plant growth and yield under abiotic stresses (Cantliffe, 2003). Seed priming with CaCl2 improves crop yield under field and green house conditions (Ghana and Schillinger, 2003). Similarly, seed priming with hormones, inorganic solute, or antioxidant composites improves water utilization efficiency under drought conditions (Coulibaly et al., 2019), enhances salt stress tolerance (Basra et al., 2005; Demir et al., 2006) and increases activity of catalase (CAT) and superoxide dismutase (SOD) which are responsible for protection of plants from oxidative stress (Salvi et al., 2020).
There is dire need to collect, categorize and evaluate the variable response manifested by the peal millet genotypes under both the promising and non-promising environmental conditions. Moreover, there is a knowledge gap related to the use of multiple pearl millet genotypes under varying levels of inorganic priming agents. It is therefore, hypothesized that priming (with some suitable agent) of best adopted pearl millet cultivar can help to improve its productivity in semi-arid environments. The purpose of this study was to appraise the effect of inorganic seed priming agents on the growth, yield and quality of selected (screened in a preliminary pot trial) pearl millet cultivars.
Materials and Methods
Study site, experimental design and treatments
Field experiments were performed to evaluate the influence of seed priming technique on growth and fodder yield of pearl millet cultivars at the Farm Area of Department of Agronomy, University of Agriculture Faisalabad Pakistan (latitude 31°-26′N, longitude 73°-06′E, and altitude 184.4 m). The experimental design was randomized complete block design under factorial arrangement having three repeats. The soil had loamy texture with EC = 2.05-1.93 dS m−1, pH = 8.0-7.6, organic matter = 0.60-0.58%, total nitrogen 0.047-0.045%, total available phosphorous 8.10-7.90 mg kg−1, and total available potassium 181-177 mg kg−1 during both years of study (ICARDA, 2013).
Experimental treatments were comprised of two factors; priming agents viz. no priming/control, hydropriming, ascorbate (40 mg L-1) and CaCl2 (100 mg L-1) and four pearl millet cultivars (Sargodha Bajra 2011, YBS-98, 18-BY and Ghana White).
The hydration of seeds in a column of aerated water to a level of moisture which is close to the one required for radical protrusion is termed as hydro priming or aerated hydration (Pawar and Laware, 2018). Sodium hypochlorite (5%) was used to sterilize the seeds against fungal infections. Thereafter the seeds were washed twice with distilled water. Seed was soaked for 12 h in aerated 5% (w/v) solution of all the salts. Seed was soaked in water for hydropriming; keeping seed and solution ratio of 1:5 (w/v). The seed was washed with distilled water for 3 times after removing from the respective solution and dried back to its original weight by enforced air at 27oC ± 2. These seeds were then packed in polythene bags and kept at 5oC until used (Ashraf and Foolad, 2005). Before start of experiments, wheat crop was harvested from the selected field during both study years. Cultivation of field was done before starting the experiments; leveled and watered the field for planting of crop. The plantation of crops was made on July 13, 2017 and July 17, 2018 as per treatments in plots having the gross size of 7.0 m × 2.4 m and replicated thrice. Treated seed, at the rate of 12 kg ha-1, was sown using hand drill. Seed was placed to a depth of about 50 mm, with spacing of rows of 0.45 m. The plant spacing was maintained by thinning when the seedlings have attained the height of about 0.15 m. Nitrogen (N), phosphorus (P) and potassium (K) were applied at the rate of 80 kg ha-1, 60 kg ha-1 and 30 kg ha-1, respectively in all plots. One third N and full P and K doses were given at the time of sowing. The remaining dose N was side dressed in two equal splits with first and second irrigation. In addition to the rainfall, crop received four irrigations throughout its growing period. Weather data, during both study years, were collected from the meteorological observatory of the Department of Agronomy, University of Agriculture Faisalabad and it was presented in Figure 1.
Germination attributes
According to AOSA (1990) seeds germination counts were made on daily basis until achieving a constant count. Time for starting the germination (TSG) was noted when the length of radical and first hypocotyls germinated seeds length was a little above 2 mm. Calculation of mean emergence time was done by using Ellis and Robert (1981) formula:

Coolbear et al. (1984) formula was used to compute completion of 50% emergence:

Where; N= Emerged seedlings number ni, nj= Accumulative of the emerged seedlings numbers at each adjacent count at time ti and tj, while, ni< N/2 < nj.
Growth and yild attributes
Crop was harvested on September 29, 2017 and October 1, 2018. The stand density or plant population was counted from each replication from specific area at the time of harvest. Ten randomly selected plants were tagged and used for measuring of all the growth, biomass and yield attributes from each replicate. Height of the plants was measured from the level of soil to the flag leaf tip and at the end average height was measured using meter rod. By using vernier callipers the girth of tagged plants was calculated from the middle, base and upper portions of the stem and then average was measured. Leaves of tagged plants from all plots were counted and average was taken after every 15 days. Leaves of three selected/tagged plants from each plot were detached and the area of leaves was determined using leaf area meter (model L1-3000) with 15 days gap. Three tagged plants in each plot were harvested from ground level and their fresh weight was recorded before detaching leaves for recording leaf area. Thereafter, average fresh weight per plant was calculated. After recording fresh weight and leaf area, subsamples (each of 500 g) were taken from the harvested above ground biomass of three plants (in each plot). These subsamples were kept in oven at 70oC for recording dried weight. This per plot fodder yield was converted to t/ha. After recording the weight of each dried plant, dry matter proportion was noted.
Quality attributes
Determination of crude protein content was carried out by following method No. 46-10 of AACC (2000). Two-gram sample of dried biomass along with 30 ml concentrated H2SO4 and five gram of digestion mixture was allowed to react in digestion flask till the transparent mixture was obtained. Diluted this digested mixture with distilled water up to 200 ml. Distillation of 10 ml of this mixture was done with forty percent sodium hydroxide in distillation equipment. As a result, NH3 gas was produced that was trapped in four percent boric acid solution having indicator. At the end titrated the distillated mixture with 0.1N H2SO4till end point of golden yellow.
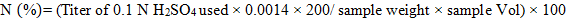
Nitrogen percentage was multiplied with factor 6.25 to obtain protein percentage.
The determination of fiber was performed by following the protocols of AACC (2000) method No. 32-10. After thirty minutes of sample boiling, added 200 ml of 1.25% sulfuric acid in a 500 ml beaker. After boiling, the residues were washed with hot water for the purpose of getting acid free sample. After this step these residues were transferred to another beaker having capacity of 500 ml and boiled for almost 30 minutes with the addition of 200 ml of 1.25% NaOH. After boiling, the residues were washed with hot water for the purpose of getting alkali free sample. After charring the sample to get flame free sample and then put into muffle furnace at temperature of 500oC until a grey colored material obtained. To calculate the amount of fiber in sample following formula was used:
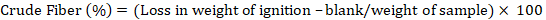
Each sample was run for ash determination by adopting the protocol outline as discussed in AACC (2000) technique number 08-01. Dried samples in already weighed crucibles were burnt on Bunson burner prior to putting in muffle furnace, where a temperature of 550°C was adjusted until grayish white residue appeared.
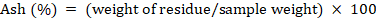
For the determination of fat soxhlet equipment was used. For sample preparation AACC (2000) method No. 30-25 was used. In this regard 2 g sample was taken and placed in thimbles. Petroleum ether was used as a solvent in the extraction of fat and temperature was adjusted according to protocols. The extraction process was carried out for 4 to 6 hours followed by the transfer of these residues into china dish. This pre weighed china dish was put into hot air oven for the removal of solvent for at least one to two hours and then for cooling purpose placed into desiccator and again weighed. To calculate the amount of fat in sample following formula was used:
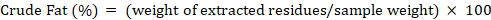
Statistical analysis
A two-way analysis of variance was used to assess the effect of seed priming × varieties (P ≤ 0.05) using the Fisher’s analysis of variance technique (Steel et al., 1997) under field conditions. Tukey’s Honestly Significant Difference (Tukey’s HSD) test was employed to compare the means at 5% probability level using STATISTIX 8.1 software (Gomez and Gomez, 1984). Both the factors were studied independently in two years but years were not taken as factor.
Results and Discussion
Findings showed that time to start emergence, time to 50% emergence and mean emergence time were significantly decreased due to priming, however, final emergence count showed non-significant results (Table 1). Priming with CaCl2 significantly decreased the time to start emergence (63.81 and 63.82%), time to 50% emergence (41.68 and 41.13%) and mean emergence time (53.43 and 53.34%) for 2017 and 2018, respectively followed by ascorbate priming and then by hydro priming while control treatment (no priming) showed the maximum values for these parameters. However, final emergence count remained similar i.e., it was not affected by any priming agent. Among the cultivars, minimum time to start emergence was noticed in Sargodha Bajra 2011 as compared to rest of pearl millet cultivars.
In comparison with the control different osmopriming techniques showed positive effect in enhancing the number of leaves of pearl millet cultivars. All the priming agents including the hydropriming significantly increased the leaf number per plant after various days’ intervals. Priming with CaCl2 showed a significant increase in leaf number per plant when recorded at 15 days after sowing (DAS) (29.20 and 27.71%), 30 DAS (27.90 and 27.54%), 45 DAS (24.40 and 22.73%), 60 DAS (19.52 and 19.28%) and 75 DAS (14.73 and 14.76%) for 2017 and 2018, respectively followed by ascorbate priming and then by hydro priming while control (no priming) showed minimum values. Cultivars also showed significant differences for number of leaves recorded at various intervals except at 45 DAS. Maximum number of leaves were noticed in Sargodha Bajra 2011, followed by in YBS-98, while minimum number of leaves were noticed in Ghana White. The decreasing pattern in terms of number of leaves for pearl millet cultivars was Sargodha Bajra 2011> YBS-98> 18-BY > Ghana White (Table 2).
Table 1: Effect of priming agent on the emergence related attributes of various pearl millet cultivars.
|
Experimental treatments |
Time to start emergence (days) |
Time to 50% emergence (Days) |
Mean emergence time (Days) |
Final emergence count (%) |
||||
|
2017 |
2018 |
2017 |
2018 |
2017 |
2018 |
2017 |
2018 |
|
|
Pearl millet cultivars (V) |
||||||||
|
V1 = YBS-98 |
4.04 B |
4.09 B |
4.30 |
4.29 |
4.25 |
4.24 |
85.05 |
86.49 |
|
V2 = 18-BY |
4.37 A |
4.35 A |
4.19 |
4.17 |
4.75 |
4.78 |
84.58 |
85.53 |
|
V3 = Sargodha Bajra 2011 |
3.72 C |
3.67 C |
4.30 |
4.32 |
4.08 |
4.11 |
86.15 |
87.25 |
|
V4 = Ghana White |
4.58 A |
4.62 A |
4.12 |
4.15 |
4.83 |
4.89 |
85.03 |
85.00 |
|
HSD (V) (p ≤ 0.05) |
0.23 |
0.21 |
- |
- |
- |
- |
- |
- |
|
Priming Agents (P) |
||||||||
|
P1 = Control (No-Priming) |
5.72 A |
5.80 A |
5.11 A |
5.13 A |
6.70 A |
6.73 A |
83.3 |
84.02 |
|
P2 = Hydro-priming |
4.04 B |
4.01 B |
4.98 B |
4.99 B |
5.21 B |
5.26 B |
84.93 |
85.68 |
|
P3 = Ascorbate (40 mg L-1) |
3.42 C |
3.47 C |
4.34 C |
4.38 C |
4.53 C |
4.59 C |
84.05 |
85.03 |
|
P4 = CaCl2 (100 mg L-1) |
2.07 D |
2.11 D |
2.98 D |
3.02 D |
3.12 D |
3.14 D |
85.18 |
86.59 |
|
HSD (P) (p ≤ 0.05) |
0.23 |
0.21 |
0.11 |
0.11 |
1.48 |
|||
|
Significance Level (V) |
* |
* |
NS |
NS |
NS |
NS |
NS |
NS |
|
Significance Level (P) |
* |
* |
* |
* |
* |
* |
NS |
NS |
|
Significance Level (V × P) |
NS |
NS |
NS |
NS |
NS |
NS |
NS |
NS |
Means following different alphabets are statistically different from each other at probability (p) ≤ 0.05 according to honestly significant difference (HSD) test; n = 3; NS = non-significant; * = significant at p ≤ 0.05.
Table 2: Effect of priming agent on leaf number per plant of various pearl millet cultivars.
|
Experimental treatments |
15 DAS |
30 DAS |
45 DAS |
60 DAS |
75 DAS |
|||||||||
|
2017 |
2018 |
2017 |
2018 |
2017 |
2018 |
2017 |
2018 |
2017 |
2018 |
|||||
|
Pearl millet cultivars (V) |
||||||||||||||
|
V1 = YBS-98 |
2.51 B |
2.57 B |
5.01 B |
5.34 AB |
6.76 |
6.79 |
8.09 A |
8.13 B |
9.01 B |
9.11 A |
||||
|
V2 = 18-BY |
2.37 C |
2.41 B |
4.84 B |
4.91 AB |
6.42 |
6.50 |
7.76 B |
7.81 C |
8.26 C |
8.33 B |
||||
|
V3 = Sargodha Bajra 2011 |
2.67 A |
2.77 A |
5.34 A |
5.42 A |
7.09 |
7.15 |
8.59 A |
8.64 A |
9.51 A |
9.56 A |
||||
|
V4 = Ghana White |
2.04 D |
2.13 C |
4.51 D |
4.68 B |
6.09 |
6.11 |
7.01 C |
7.03 D |
7.92 D |
7.94 B |
||||
|
HSD (V) (p ≤ 0.05) |
0.14 |
0.16 |
0.15 |
0.31 |
- |
- |
0.54 |
0.48 |
0.31 |
0.45 |
||||
|
Priming agents (P) |
||||||||||||||
|
P1 = Control (No-Priming) |
2.26 C |
2.31 C |
5.34 D |
5.41 C |
7.09 D |
7.26 D |
8.81 D |
8.87 D |
9.37 D |
9.42 C |
||||
|
P2 = Hydro-priming |
2.56 B |
2.59 B |
6.02 C |
6.11 B |
7.98 C |
8.05 C |
9.33 C |
9.43 C |
9.89 C |
9.97 B |
||||
|
P3 = Ascorbate (40 mg L-1) |
2.78 A |
2.79 A |
6.45 B |
6.59 A |
8.57 B |
8.64 B |
9.98 B |
10.06 B |
10.13 B |
10.21 B |
||||
|
P4 = CaCl2 (100 mg L-1) |
2.92 A |
2.95 A |
6.83 A |
6.90 A |
8.82 A |
8.91 A |
10.53 A |
10.58 A |
10.75 A |
10.81 A |
||||
|
HSD (P) (p ≤ 0.05) |
0.14 |
0.16 |
0.15 |
0.31 |
0.24 |
0.25 |
0.54 |
0.48 |
0.31 |
0.45 |
||||
|
Significance Level (V) |
* |
* |
* |
* |
NS |
NS |
* |
* |
* |
* |
||||
|
Significance Level (P) |
* |
* |
* |
* |
* |
* |
* |
* |
* |
* |
||||
|
Significance Level (V × P) |
NS |
NS |
NS |
NS |
NS |
NS |
NS |
NS |
NS |
NS |
||||
Means following different alphabets are statistically different from each other at probability (p) ≤ 0.05 according to honestly significant difference (HSD) test; n: 3; NS: non-significant; *: significant at p ≤ 0.05
Analysis of variance showed that various priming agents and pearl millet cultivars showed significant differences in leaf area per plant recorded at various intervals, however interactive effect of V × P on this parameter was significant at initial growth stages (15 and 30 DAS) only, thereafter the differences became non-significant. Priming with CaCl2 significantly increased the leaf area per plant at 15 DAS (29.24 and 29.86%), 30 DAS (24.77 and 26.02%), 45 DAS (21.36 and 21.66%), 60 DAS (19.61 and 19.81%) and 75 DAS (12.53 and 13.33%) for 2017 and 2018, respectively. It was followed by ascorbic acid priming and then by hydro priming while no priming) resulted in minimum leaf area per plant. The maximum leaf area was noticed in Sargodha Bajra 2011, followed by in YBS-98, while minimum leaf area was noticed in Ghana White (Table 3). As regards interactive effect of V × P, maximum leaf area per plant (recorded at 15 DAS and 30 DAS) was observed in Sargodha Bajra 2011 when its seeds were primed with CaCl2 (Figure 2) during both study years. More leaf area per plant was observed during second year of study than first year.
Various priming agents and pearl millet cultivars significantly (p ≤ 0.01) affected the fresh weight per plant recoded at 15 days interval during both years of study (Table 4). Maximum increase in fresh weight per plant was noticed by priming with CaCl2 followed by ascorbate priming and then by hydro priming while control (no priming) showed minimum values. Among the pearl millet cultivars, highest fresh weight per plant was recorded in Sargodha Bajra 2011 followed by YBS-98, while minimum fresh weight per plant was in Ghana White for study period. Interactive effect of the factors under study remained non-significant most of the times except for 2017 at 15 DAS. In this case Sargodha Bajra 2011 produced the maximum fresh weight per plant when its seeds were sown after priming with CaCl2 (Figure 3).
The highly significant differences in dry weight per plant were observed by various osmopriming techniques upto 60 DAS (Table 5). Priming with CaCl2 increased the dry weight per plant by 23.28-25.02% at 15 DAS, 25.19-26.88% at 30 DAS, 24.97-25.22% at 45 DAS and by 18.55-19.29% at 60 DAS for 2017 and 2018, respectively followed by ascorbate priming and then by hydro priming while control (no priming) showed minimum values for study period. Interactive effect also remained non-significant in this regard.
All the growth and yield attributes were influenced significantly by priming agents (P) and pearl millet cultivars (V) during the study period except for stand density (Table 6). However, interactive effect of V × P showed statistically similar response except for plant height and fodder yield during 2017. Priming with CaCl2 significantly increased the plant height (14.42 and 14.49%), stem girth (30.86 and 18.68%), fodder yield (13.66 and 13.67%) and dry matter yield (42.96 and 38.71%) for 2017 and 2018, respectively followed by ascorbate priming and then by hydro priming while control (no priming) remained at the bottom. Maximum values for all the growth and yield attributes were noticed in Sargodha Bajra 2011, followed by in YBS-98, while Ghana White showed least values for all these attributes (Table 6). Significant interactive values depict that maximum plant height and fodder yield (Figure 3) (during 2017) was observed in Sargodha Bajra 2011when its seeds were primed with CaCl2 before sowing.
During 2017, all the four varieties differed significantly from each other for crude protein, crude fibre and total ash contents (Table 7). Maximum values for these parameters were recorded in Sargodha Bajra 2011 followed by YBS-98, 18-BY and Ghana White. However, the differences in varieties for these quality traits could not reach to significant level during 2018. Percentage of ether extractable fat remained statistically similar in all varieties during both years of study.
Table 3: Effect of priming agent on the leaf area per plant (cm2) of various pearl millet cultivars.
|
Experi-mental Treat-ments |
15 DAS |
30 DAS |
45 DAS |
60 DAS |
75 DAS |
|||||
|
2017 |
2018 |
2017 |
2018 |
2017 |
2018 |
2017 |
2018 |
2017 |
2018 |
|
|
Pearl millet cultivars (V) |
||||||||||
|
V1 = YBS-98 |
89.14A |
90.83B |
121.22 B |
124.61 B |
167.71 B |
169.25 B |
207.65 B |
209.69 B |
202.30AB |
203.03 A |
|
V2 = 18-BY |
81.40B |
85.09C |
113.15 C |
119.94 C |
163.32 C |
164.86 C |
200.87 C |
203.17 C |
199.02BC |
202.00 B |
|
V3 = Sargodha Bajra 2011 |
93.34A |
96.04A |
125.60 A |
129.89 A |
173.07A |
174.61 A |
213.91 A |
216.20 A |
204.89 A |
206.87 A |
|
V4 = Ghana white |
78.27B |
81.97D |
112.53 C |
113.82 D |
158.85D |
160.38D |
196.36D |
197.90D |
196.11 C |
199.09 B |
|
HSD (V) (p ≤ 0.05) |
5.00 |
3.05 |
3.49 |
3.94 |
3.46 |
2.34 |
4.05 |
4.10 |
4.28 |
3.90 |
|
Priming agents (P) |
||||||||||
|
P1 = Control (No-Priming) |
78.07D |
79.77D |
106.38 D |
107.42 D |
148.64D |
151.18D |
191.54D |
193.56D |
183.95 C |
185.93 D |
|
P2 = Hydro-priming |
85.34C |
87.23C |
110.33 C |
116.61 C |
158.06 C |
159.60 C |
204.65 C |
205.41 C |
194.36 B |
201.34 C |
|
P3 = Ascorbate (40 mg L-1) |
91.32B |
96.01B |
123.07 B |
127.86 B |
175.85 B |
176.40 B |
224.65 B |
227.69 B |
201.03 A |
205.01 B |
|
P4 = CaCl2 (100 mg L-1) |
100.90 A |
103.59 A |
132.73 A |
135.37 A |
180.39 A |
183.93 A |
229.11 A |
231.90 A |
206.99 A |
210.72 A |
|
HSD (P) (p ≤ 0.05) |
5.00 |
3.05 |
3.49 |
3.94 |
3.46 |
2.34 |
4.05 |
4.10 |
4.28 |
3.90 |
|
Significance level (V) |
* |
* |
* |
* |
* |
* |
* |
* |
* |
* |
|
Significance level (P) |
* |
* |
* |
* |
* |
* |
* |
* |
* |
* |
|
Significance level (V × P) |
* |
* |
* |
* |
NS |
NS |
NS |
NS |
NS |
NS |
Means following different alphabets are statistically different from each other at probability (p) ≤ 0.05 according to honestly significant difference (HSD) test; n: 3; NS: non-significant; *: significant at p ≤ 0.05.
Table 4: Effect of priming agent on the fresh weight per plant (g) at 15 days interval of various pearl millet cultivars.
|
Experimental treatments |
15 DAS |
30 DAS |
45 DAS |
60 DAS |
75 DAS |
|||||
|
2017 |
2018 |
2017 |
2018 |
2017 |
2018 |
2017 |
2018 |
2017 |
2018 |
|
|
Pearl millet cultivars (V) |
||||||||||
|
V1 = YBS-98 |
93.32 |
91.90 B |
103.30 AB |
105.33 B |
125.08 B |
129.34 B |
154.87 B |
155.81 B |
158.21 B |
160.34 B |
|
V2 = 18-BY |
79.71 |
82.62 C |
98.98 BC |
101.01 C |
126.93 B |
128.52 B |
148.71 C |
151.66 C |
152.35 C |
153.49 C |
|
V3 = Sargodha Bajra 2011 |
88.29 |
97.20 A |
106.20 A |
109.23 A |
132.57 A |
136.91 A |
167.74 A |
169.67 A |
164.14 A |
166.19 A |
|
V4 = Ghana white |
76.83 |
79.74 D |
94.93 C |
95.95 D |
118.54 C |
120.13 C |
140.21D |
142.15D |
148.25 C |
149.36 D |
|
HSD (V) (p ≤ 0.05) |
- |
2.48 |
4.39 |
3.59 |
4.56 |
3.57 |
3.91 |
4.12 |
4.30 |
3.66 |
|
Priming agents (P) |
||||||||||
|
P1 = Control (No-Priming) |
71.23 |
71.78D |
83.42D |
84.81D |
112.35D |
114.60D |
139.89D |
140.20D |
147.35D |
149.47 D |
|
P2 = Hydro-priming |
81.38 |
84.28 C |
91.23 C |
94.89 C |
123.16 C |
125.75 C |
149.12 C |
151.06 C |
152.15 C |
155.30 C |
|
P3 = Ascorbate (40 mg L-1) |
84.58 |
87.49 B |
99.11 B |
102.00 B |
129.98 B |
131.32 B |
156.24 B |
157.19 B |
158.47 B |
160.54 B |
|
P4 = CaCl2 (100 mg L-1) |
87.41 |
91.32 A |
106.19 A |
107.22 A |
137.63 A |
142.21 A |
162.90 A |
167.84 A |
164.97 A |
165.08 A |
|
HSD (P) (p ≤ 0.05) |
- |
2.48 |
4.39 |
3.59 |
4.56 |
3.57 |
3.91 |
4.12 |
4.30 |
3.66 |
|
Significance level (V) |
NS |
* |
* |
* |
* |
* |
* |
* |
* |
* |
|
Significance level (P) |
NS |
* |
* |
* |
* |
* |
* |
* |
* |
* |
|
Significance level (V × P) |
NS |
* |
NS |
NS |
NS |
NS |
NS |
NS |
NS |
NS |
Means following different alphabets are statistically different from each other at probability (p) ≤ 0.05 according to honestly significant difference (HSD) test; n: 3; NS: non-significant; *: significant at p ≤ 0.05
Table 5: Effect of priming agent on the dry weight per plant (g) of various pearl millet cultivars.
|
Experimental treatments |
15 DAS |
30 DAS |
45 DAS |
60 DAS |
75 DAS |
||||||
|
2017 |
2018 |
2017 |
2018 |
2017 |
2018 |
2017 |
2018 |
2017 |
2018 |
||
|
Pearl millet cultivars (V) |
|||||||||||
|
V1 = YBS-98 |
16.37 |
17.49 |
20.47 |
21.66 |
25.20 |
28.10 |
30.26 |
32.18 |
34.85 |
36.92 |
|
|
V2 = 18-BY |
15.99 |
17.11 |
20.09 |
21.28 |
24.63 |
26.51 |
29.19 |
31.11 |
33.95 |
36.03 |
|
|
V3 = Sargodha Bajra 2011 |
16.88 |
18.00 |
20.93 |
22.12 |
25.94 |
27.82 |
30.89 |
32.81 |
35.73 |
37.82 |
|
|
V4 = Ghana white |
15.51 |
16.63 |
19.55 |
20.74 |
24.03 |
25.92 |
29.51 |
31.43 |
33.30 |
35.38 |
|
|
Priming agents (P) |
|||||||||||
|
P1 = Control (No-Priming) |
14.99 B |
16.11 B |
17.71 B |
18.90 B |
22.79 B |
23.99 B |
27.12 B |
28.56 B |
33.92 |
34.91 |
|
|
P2 = Hydro-priming |
15.10 B |
17.54 B |
18.96 B |
20.15 AB |
24.05 B |
24.17 B |
29.41 AB |
31.33 AB |
35.01 |
35.67 |
|
|
P3 = Ascorbate (40 mg L-1) |
17.03 AB |
18.15 AB |
21.89 A |
23.08 A |
27.77 A |
29.66 A |
31.17 A |
33.09 A |
37.47 |
38.85 |
|
|
P4 = CaCl2 (100 mg L-1) |
18.74 A |
19.86 A |
22.47 A |
23.66 A |
28.38 A |
30.04 A |
32.15 A |
34.07 A |
38.85 |
39.75 |
|
|
HSD (P) (p ≤ 0.05) |
2.90 |
2.72 |
2.84 |
3.74 |
3.21 |
3.61 |
3.90 |
3.92 |
- |
- |
|
|
Significance level (V) |
NS |
NS |
NS |
NS |
NS |
NS |
NS |
NS |
NS |
NS |
|
|
Significance level (P) |
* |
* |
* |
* |
* |
* |
* |
* |
NS |
NS |
|
|
Significance level (V × P) |
NS |
NS |
NS |
NS |
NS |
NS |
NS |
NS |
NS |
NS |
|
Means following different alphabets are statistically different from each other at probability (p) ≤ 0.05 according to honestly significant difference (HSD) test; n: 3; NS: non-significant; *: significant at p ≤ 0.05
Table 6: Effect of priming agent on the growth and yield related attributes of various pearl millet cultivars.
|
Experimental treatments |
Stand density (m-2) |
Plant height (cm) |
Stem girth (cm) |
Fodder yield (t ha-1) |
Dry matter yield (t ha-1) |
|||||
|
2017 |
2018 |
2017 |
2018 |
2017 |
2018 |
2017 |
2018 |
2017 |
2018 |
|
|
Pearl millet cultivars (V) |
||||||||||
|
V1 = YBS-98 |
26.05 |
27.81 |
196.75 B |
195.39 B |
0.86 A |
0.90 |
61.00 B |
64.11 B |
13.77 B |
14.78 |
|
V2 = 18-BY |
25.58 |
27.09 |
194.00 C |
192.48 C |
0.82 B |
0.91 |
59.15 B |
62.61 B |
13.23 C |
14.24 |
|
V3 = Sargodha Bajra 2011 |
27.67 |
28.07 |
199.25 A |
197.85 A |
0.89 A |
0.94 |
65.15 A |
68.51 A |
14.28 A |
15.31 |
|
V4 = Ghana White |
25.11 |
26.83 |
191.25 D |
189.83 D |
0.78 C |
0.85 |
58.13 B |
60.30 C |
12.67 D |
13.69 |
|
HSD (V) (p ≤ 0.05) |
- |
- |
1.33 |
1.29 |
0.03 |
- |
2.45 |
2.30 |
0.08 |
- |
|
Priming agents (P) |
||||||||||
|
P1 = Control (No-Priming) |
28.00 |
28.33 |
183.75 D |
182.35 D |
0.89 D |
0.91 D |
53.65 C |
54.06 D |
9.94 D |
10.36 D |
|
P2 = Hydro-priming |
29.00 |
29.30 |
190.00 C |
188.64 C |
0.93 C |
0.97 C |
55.30 C |
56.62 C |
12.74 C |
13.44 C |
|
P3 = Ascorbate (40 mg L-1) |
30.00 |
30.33 |
197.25 B |
195.81 B |
0.97 B |
1.03 B |
58.45 B |
59.12 B |
13.78 B |
13.97 B |
|
P4 = CaCl2 (100 mg L-1) |
31.33 |
31.00 |
210.25 A |
208.77 A |
1.06 A |
1.08 A |
60.98 A |
61.45 A |
14.21 A |
14.37 A |
|
HSD (P) (p ≤ 0.05) |
- |
- |
1.33 |
1.29 |
0.03 |
0.02 |
2.45 |
2.30 |
0.08 |
0.12 |
|
Significance level (V) |
NS |
NS |
* |
* |
* |
NS |
* |
* |
* |
NS |
|
Significance level (P) |
NS |
NS |
* |
* |
* |
* |
* |
* |
* |
* |
|
Significance level (V × P) |
NS |
NS |
* |
NS |
NS |
NS |
* |
NS |
NS |
NS |
Means following different alphabets are statistically different from each other at probability (p) ≤ 0.05 according to honestly significant difference (HSD) test; n; 3; NS: non-significant; *: significant at p ≤ 0.05
While all priming agents differed significantly among each other in affecting quality attributes of pearl millet fodder except ether extractable fats. So, crude protein contents (CPC), crude fiber contents (CFC) and total ash contents (TAC) were highest in crop that was sown with seeds primed with CaCl2 followed by ascorbate primed seeds and then by hydro primed seeds. Control recorded minimum values.
Seed priming enhanced plant growth, yield and quality attributes especially seed priming with calcium chloride (CaCl2) increased all attributes significantly over the other priming agents. Seed priming with CaCl2 prominently improved the seed emergence
Table 7: Effect of priming agent on the quality related attributes of various pearl millet cultivars.
|
Experimental treatments |
Crude protein contents (%) |
Crude fiber contents (%) |
Total ash contents (%) |
Ether extractable fat (%) |
|||||
|
2017 |
2018 |
2017 |
2018 |
2017 |
2018 |
2017 |
2018 |
||
|
Pearl millet cultivars (V) |
|||||||||
|
V1 = YBS-98 |
7.31 B |
7.41 |
38.43 B |
38.59 |
8.69 B |
8.72 |
1.99 |
197 |
|
|
V2 = 18-BY |
7.02 C |
7.20 |
37.81 C |
38.01 |
8.42 C |
8.51 |
2.00 |
2.01 |
|
|
V3 = Sargodha Bajra 2011 |
7.59 A |
7.78 |
38.86 A |
38.94 |
8.95 A |
9.06 |
2.01 |
2.02 |
|
|
V4 = Ghana White |
6.79 D |
6.97 |
37.30 D |
37.41 |
8.69 B |
8.20 |
1.97 |
1.99 |
|
|
HSD (V) (p ≤ 0.05) |
0.10 |
- |
0.29 |
- |
0.22 |
- |
- |
- |
|
|
Priming agents (P) |
|||||||||
|
P1 = Control (No-Priming) |
6.01 D |
6.13 D |
34.25 D |
34.30 D |
6.71 D |
6.85 D |
1.92 |
1.89 |
|
|
P2 = Hydro-priming |
6.52 C |
6.63 C |
37.89 C |
37.97 C |
7.88 C |
7.94 C |
1.93 |
1.90 |
|
|
P3 = Ascorbate (40 mg L-1) |
6.89 B |
7.01 B |
39.38 B |
39.45 B |
9.21 B |
9.28 B |
1.91 |
1.89 |
|
|
P4 = CaCl2 (100 mg L-1) |
7.28 A |
7.36 A |
41.11 A |
41.26 A |
10.29 A |
10.38 A |
1.90 |
1.91 |
|
|
HSD (P) (p ≤ 0.05) |
0.10 |
0.20 |
0.29 |
0.35 |
0.22 |
0.37 |
- |
- |
|
|
Significance level (V) |
* |
NS |
* |
NS |
* |
NS |
NS |
NS |
|
|
Significance level (P) |
* |
* |
* |
* |
* |
* |
NS |
NS |
|
|
Significance level (V × P) |
NS |
NS |
NS |
NS |
NS |
NS |
NS |
NS |
|
Means following different alphabets are statistically different from each other at probability (p) ≤ 0.05 according to honestly significant difference (HSD) test; n: 3; NS: non-significant; *: significant at p ≤ 0.05
over control and other priming agents. Interestingly, the increase in seed emergence would be due to the stimulation of seed coating enzymes, which increase the breakdown of reserve food. These results lead to a theory that seed priming with CaCl2 may increase the solute potential of seed and that potential increases the water absorption capacity and the vigor of pearl millet seeds. The results of Rehman et al. (2014) and Rai-Kalal and Jajoo (2021) and Farooq et al. (2020) are in accordance with our findings who reported that seed priming is a viable technique that has great potential to improve germination and early seedling establishment. This priming technique can be used in those areas where there is a shortage of water and for those seeds which have germination problem.
Plant height and leaf area are the important attributes of plant growth while plant fresh/dry weight and stem diameter are the significant outcomes of net assimilation rate. Averaged across during both years of study, the plant height; leaf area, plant fresh/dry weights and stem diameter (averaged across of 15, 30, 45, 60 and 75 days after sowing) were increased prominently under all priming agents over the control but CaCl2 priming outclassed all other agents for the improvement of all attributes. This might have happened due to high photosynthetic rate and high photosynthetic efficiency caused by CaCl2 priming. The activation of plant defensive system by CaCl2 priming would also be a possible reason for more accumulation of dry matter in pearl millet under field conditions. The findings of our results are in consistent with those of Panda and Mondal (2020) and Goa and Yan (2020). The significance of our findings could be applied in other fodder crops for better fodder yield to overcome the deficiency of fodder under normal and stressful conditions.
Leaf area, plant height, plant fresh, dry weight and stem diameter due to seed priming with CaCl2 significantly increased the fodder fresh and dry matter yield. The results of our study suggest that seed priming with CaCl2 activates the genes of growth and plant defensive system, which promote the water and nutrients uptake and the fodder yield in pearl millet. Ramamurthy et al. (2005) and Asif et al. (2021) documented that seed priming in pearl millet and sorghum increased vegetative growth and seed yield, as it was found in this study. Before sowing of fodder crops under water deficit and fodder scarce areas, seed priming with CaCl2 has potential to overcome the deficiency of fodder scarcity particularly in lean periods. Better fodder quality is essential for better nutrition and palatability of pearl millet. Fodder crude protein, crude fiber, ash contents and fiber ether extractable fat contents were increased under all seed priming agents but the best quality was observed under seed priming with CaCl2. The improved fiber quality due to CaCl2 priming in our results would be due to expression of various genes for more root proliferation for better uptake of nutrients from soil. The malnutrition and low digestibility problems in animals may be overcome by priming seeds of fiber crops with CaCl2. The higher grain quality in sunflower and maize due to seed priming has already been reported by other researchers as well (Hussain et al., 2006; Houshmandfar and Asli, 2011).
The pearl millet cultivars behaved differently. Among the cultivars, Sargodha Bajra 2011 showed significantly higher germination, seedling establishment, leaf area, plant fresh weight, plant height, stem diameter, fresh fodder yield and dry weight over the other cultivars. Similarly, fodder crude protein, crude fiber, ash contents and fiber ether extractable fat contents were found higher in Sargodha Bajra 2011. The increase in all parameters in Sargodha Bajra 2011 was due to its early stand establishment, mobilization of reserved food, good balance between ROS and antioxidants, and the high rate of physiological activities. The results of Ayub et al. (2010), Goa and Yan (2020) and Singh et al. (2020) on the performance of various cultivars are in accordance with our findings. Our results suggest that pearl millet cultivar Sargodha Bajra 2011 could be grown in various areas for obtaining more fodder yield. The better fodder quality of this cultivar may overcome the low nutrition and low digestibility problems in animals.
Conclusions and Recommendations
In general, for improving the emergence, growth, yield and quality attributes of pearl millet, cultivars’ selection and priming are beneficial, as our study herein. Our findings revealed that priming agent especially CaCl2 significantly improved emergence, growth, yield and quality parameters. Therefore, sowing of the millet cultivar Sargodha Bajra 2011 primed with CaCl2 (100 mg L-1) is recommended for getting higher yield of better-quality fodder under the semiarid environment in Pakistan and similar semiarid regions.
Acknowledgements
Authors highly acknowledge the support provided by Analytical Laboratory of the Department of Agronomy, for determining quality related attributes of the fodder.
Novelty Statement
Role of seed priming with inorganic salts, in improving yield and quality of pearl millet fodder has yet not been studied.
Auhor’s Contribution
Muhammad Zeeshan Nadeem: Conducted research and collected data.
Muhammad Farrukh Saleem: Conceived the idea and wrote the paper.
Muhammad Ashfaq Wahid: Did statistical analysis.
Muhammad Anwar ul Haq: Provided technical input at every step.
Conflict of interest
The authors have declared no conflict of interest.
References
AACC, 2000. Approved methods of the AACC, 10th ed. Methods 08-12, 10-10B, 32-40, 44-15A, 54-21, 55-31, 56-81B and 66-20. St. Paul, MN: American Association of Cereal Chemists.
AOSA, 1990. Association of official seed analysts. 1990. Rules for testing seeds. J. Seed Technol., 12: 1-112.
Ashraf, M., and M.R. Foolad. 2005. Pre-sowing seed treatment a shotgun approach to improve germination, plant growth and crop yield under saline and non-saline conditions. Adv. Agron., 88: 223-271. https://doi.org/10.1016/S0065-2113(05)88006-X
Asif, M., H.A.U. Rahman, A. Aziz, M. Adnan, A. Ali and M.E. Safdar. 2021. Seed Priming improves Growth, yield and seed oil contents of Soybean (Glycine max L.) cultivars under semi-arid conditions of Sargodha, Pakistan. Plant Cell Biotechnol. Mol. Biol., 22 (5 and 6): 27-37.
Ayub, M., M.A. Nadeem, M. Tahir, A. Ghafoor, Z. Ahmed and M. Naeem. 2010. Comparative studies on the growth forage yield and quality of sorghum (Sorghum bicolor L.) varieties under irrigated conditions of Faisalabad. Pak. J. Life Soc. Sci., 8: 94-97.
Basra, S.M.A., M. Farooq and R. Tabassum. 2005. Physiological and biochemical aspects of seed vigor enhancement treatments in fine rice (Oryza sativa L.). Seed Sci. Technol., 33: 623-628. https://doi.org/10.15258/sst.2005.33.3.09
Bukhari, M.A., M. Ayub, R. Ahmad, K. Mubeen and R. Waqas. 2011. Impact of different harvesting intervals on growth, forage yield and quality of three pearl millet (Pennisetum americanum L.) cultivars. Int. J. Agro Vet. Med. Sci., 5(3): 307-315. https://doi.org/10.5455/ijavms.20110619114620
Cantliffe, D.J., 2003. Seed enhancements. Acta Hortic., 607: 53-62. https://doi.org/10.17660/ActaHortic.2003.607.8
Coolbear, P., A. ancis and D. Grierson. 1984. The effect of low temperature pre sowing treatment under the germination performance and membrane integrity of artificially aged tomato seeds. J. Exp. Bot., 35: 1609-1617. https://doi.org/10.1093/jxb/35.11.1609
Coulibaly, A., K. Woumou and J.B. Aune. 2019. Sustainable intensification of sorghum and pearl millet production by seed priming, seed treatment and fertilizer microdosing under different rainfall regimes in Mali. Agron., 9(10): 664. https://doi.org/10.3390/agronomy9100664
De Ollas, C., R. Morillón, V. Fotopoulos, J. Puértolas, P. Ollitrault, A. Gómez-Cadenas, and V. Arbona. 2019. Facing climate change: Biotechnology of iconic Mediterranean woody crops. Front. Plant Sci., 10: 427. https://doi.org/10.3389/fpls.2019.00427
Delrot, S., J. Grimplet, P. Carbonell-Bejerano, A. Schwandner, P.F. Bert, L. Bavaresco and S. Vezzulli. 2020. Genetic and genomic approaches for adaptation of grapevine to climate change. In: genomic designing of climate-smart fruit crops. Springer, Cham., pp. 157-270. https://doi.org/10.1007/978-3-319-97946-5_7
Demir, A.O., A.T. Goksoy, H. Buyukcangaz, Z.M. Turan and E.S. Koksal. 2006. Deficit irrigation of sunflower (Helianthus annuus L.) in a sub-humid climate. Irrig. Sci., 24: 279-289. https://doi.org/10.1007/s00271-006-0028-x
Ellis, R.A. and E.H. Roberts. 1981. The quantification of aging and survival in orthodox seeds. Seed Sci. Tech., 9: 373-409.
Farooq, M., L. Romdhane, A. Rehman, A.K. Al-Alawi, W.M. Al-Busaidi, S.A. Asad and D.J. Lee, 2020. Integration of seed priming and biochar application improves drought tolerance in cowpea. J. Plant Growth Regul., pp. 1-9. https://doi.org/10.1016/j.scienta.2020.109507
Farooq, M., S.M.A. Basra and A. Wahid. 2006. Priming of field-sown rice seed enhances germination, seedling establishment, allometry and yield. Plant Growth Regul., 49: 285-294. https://doi.org/10.1007/s10725-006-9138-y
Gao, L. and M. Yan. 2020. Calcium chloride priming increases chilling tolerance in Salvia miltiorrhiza Bunge. Chil. J. Agric. Res., 80(2): 219-226. https://doi.org/10.4067/S0718-58392020000200219
Ghana, S.G. and W.F. Schillinger. 2003. Seed priming winter wheat for germination, emergence and yield. Crop Sci., 43: 2135-2141. https://doi.org/10.2135/cropsci2003.2135
Gomez, K.A. and A.A. Gomez. 1984. Statistical procedures for agricultural research. 2nd Edition. John Wiley and Sons, New York. pp. 680.
Gong, X., U. Ferdinand, K. Dang, J. Li, G. Chen, Y. Luo and B. Feng. 2020. Boosting proso millet yield by altering canopy light distribution in proso millet/mung bean intercropping systems. Crop J., 8(2): 365-377. https://doi.org/10.1016/j.cj.2019.09.009
Government of Pakistan. 2020. Economic Survey of Pakistan 2019-20. Finance division, Economic Advisor’s Wing, Islamabad, Pakistan.
Houshmandfar, A. and D.E. Asli. 2011. Response of corn to seed pyridoxine-priming and different levels of nitrogen. Adv. Environ. Biol., 5: 53-57.
Hussain, M., M. Farooq, S.M.A. Basra and N. Ahmad. 2006. Influence of seed priming techniques on the seedling establishment, yield and quality of hybrid sunflower. Int. J. Agric. Biol., 8: 14-18.
ICARDA (International Center for Agricultural Research in the Dry Areas), 2013. Methods of soil, plant and water analysis: A manual for West Asia and North Africa region. In: (eds. G. Estefan, R. Sommer, and J. Ryan). International center for agricultural research in the dry areas.
Jukanti, A.K., C.L. Gowda, K.N. Rai, V.K. Manga and R.K. Bhatt. 2016. Crops that feed the world 11. Pearl Millet (Pennisetum glaucum L.): an important source of food security, nutrition and health in the arid and semi-arid tropics. Food Secur., 8(2): 307-329. https://doi.org/10.1007/s12571-016-0557-y
Khan, I., M.A. Raza, S.A. Awan, G.A. Shah, M. Rizwan, B. Ali and L. Huang. 2020. Amelioration of salt induced toxicity in pearl millet by seed priming with silver nanoparticles (AgNPs): The oxidative damage, antioxidant enzymes and ions uptake are major determinants of salt tolerant capacity. Plant Physiol. Biochem., 156: 221-232. https://doi.org/10.1016/j.plaphy.2020.09.018
Khan, S., A. Hussain, A. Bakhsh and M. Imran. 2007. Status paper on production of pearl millet in Pakistan. J. Sci. Dev., 26: 28-36.
Panda, D. and S. Mondal. 2020. Seed enhancement for sustainable agriculture: An overview of recent trends. Plant Arch., 20(1): 2320-2332.
Pawar, V.A. and S.L. Laware. 2018. Seed priming a critical review. Int. J. Sci. Res. Biol. Sci., 5: 94-101. https://doi.org/10.26438/ijsrbs/v5i5.94101
Rai-Kalal, P., and A. Jajoo., 2021. Priming with zinc oxide nanoparticles improves germination and photosynthetic performance in wheat. Plant Physiol. Biochem., 160: 341-351. https://doi.org/10.1016/j.plaphy.2021.01.032
Ramamurthy, V., K.S. Gajbhiye, M.V. Venugopalan and V.N. Parhad. 2005. On-farm evaluation of seed priming technology in sorghum (Sorghum bicolor L.). Agric. Trop. Subtrop., 38: 34-41.
Rehman, H., M.Q. Nawaz, S.M.A. Basra, I. Afzal, A. Yasmeen and F.U. Hassan. 2014. Seed priming influences on early crop growth, phenological development and yield performance of linola (Linum usitatissimum L.). J. Integr. Agric., 13: 990-996. https://doi.org/10.1016/S2095-3119(13)60521-3
Salvi, P., N.U. Kamble and M. Majee. 2020. Ectopic over-expression of ABA-responsive Chickpea galactinol synthase (CaGolS) gene results in improved tolerance to dehydration stress by modulating ROS scavenging. Environ. Exp. Bot., 171: 1039-1057. https://doi.org/10.1016/j.envexpbot.2019.103957
Sarwar, M., M.A. Khan and Z. Iqbal. 2002. Feed resources for livestock in Pakistan. Int. J. Agric. Biol., 4: 186-192.
Silva-Pérez, V., J. De Faveri, G. Molero, D.M. Deery, A.G. Condon, M.P. Reynolds and R.T. Furbank. 2020. Genetic variation for photosynthetic capacity and efficiency in spring wheat. J. Exp. Bot., 71(7): 2299-2311. https://doi.org/10.1093/jxb/erz439
Singh, N.B., A. Singh, S. Khare, V. Yadav, C. Bano and R.K. Yadav. 2020. Mitigating strategies of gibberellins in various environmental cues and their crosstalk with other hormonal pathways in plants. A review. Plant Mol. Biol. Rep., pp. 1-16.
Steel, R., J. Torrie and D. Dickey. 1997. Principles and procedures of statistics: A biometrical approach. 3rd Ed. McGraw Hill Book Co. Inc; New York. pp. 172-177.
Wilson, J.P., M.D. Sanogo, S.K. Nutsugah, I. Angarawal, A. Fofana, H. Traore, I. Ahmadou and F.P. Mukka. 2008. Evaluation of pearl millet for yield and downy mildew resistance across seven countries in sub-Saharan Africa. Afr. J. Agric. Res., 3: 371-378.
Yadav, O., K. Rai and S. Gupta. 2012. Pearl millet: genetic improvement in tolerance to abiotic stresses. Improving crop productivity in sustainable agriculture. Wiley Blackwell, pp. 261-288. https://doi.org/10.1002/9783527665334.ch12
Yusuf, M.J., G. Nabi, A. Basit, S.K. Husnain and L.H. Akhtar. 2012. Development of high yielding millet variety “Sargodha Bajra-2011” released for general cultivation in Punjab province of Pakistan. Pak. J. Agric. Sci., 49: 275-282.
To share on other social networks, click on any share button. What are these?







