Impact of Some Forage Species Derived from Egyptian Rangelands on Rumen Fluid Parameters and Methane Production: In Vitro
Impact of Some Forage Species Derived from Egyptian Rangelands on Rumen Fluid Parameters and Methane Production: In Vitro
Mohamed S. Abbas1*, Adel E.M. Mahmoud2, Hemat S. Mohamed3, Adam Cieślak4 and Małgorzata Szumacher-Strabel4
1Natural Resources Department, Faculty of African Postgraduate Studies, Cairo University, 12613, Cairo, Egypt
2Animal Production Department, Faculty of Agriculture, Cairo University, 12613, Cairo, Egypt
3Animal Nutrition Department, Animal Production Research Institute, Agriculture Research Center, Giza, Egypt
4Department of Animal Nutrition, Faculty of Veterinary Medicine and Animal Science, Poznan University of Life Sciences, Poland
ABSTRACT
This study was aimed to assess the effect of dietary forge plants (grasses, legumes and forbs) contents and concentration on ruminal fermentation, pH, ammonia (NH3), total gas production (TGP), methane and volatile fatty acids (VFA) concentration. Twenty-six wild palatable forage plants were identified and analyzed for pH, NH3, TGP, methane and VFA. The results indicated that in grasses the highest pH and NH3 concentration was found in Aeluropus lagopoides, TGP in Ammophila arenaria and methane in Phragmites australis. In legumes the highest concentration of pH was found in Lygos raetum, NH3 and methane in Vicia sativa and TGP in Trigonella maritime. Whereas, in forbs the highest concentration of pH was found in Periploca angustifolia, NH3 in Anacyclus nummularia and TGP and methane in Anacyclus alexandrinex. From among grasses: Aegilops kotschyi had the highest value of acetic, propionic, VFA, APB (acetic, propionic, butyric) and A/P (acetic to propionic ratio) and Aeluropus lagopoides had the highest value of isobutyric, butyric, isovaleric and valeric. In legumes: Lotus polyphyllos had the highest value of acetic, propionic, butyric, walerianowy, total VFA and OPB (oil from among, propionic acid, and butyric acid). However, Vicia monantha had the highest value of isobutyric, isowaleriaowy and A/P. Whereas, forbs Anacyclus alexandrinex had the highest value of acetic, propionic, isobutyric, butyric, isowalerianowy, walerianowy, total VFA and OPB.
Article Information
Received 08 June 2021
Revised 05 May 2022
Accepted 20 May 2022
Available online 28 October 2022
(early access)
Published 22 February 2023
Authors’ Contribution
MSA, AEMM, AC and MS-S suggested and plannedthe idea, performed field trials, analysed the data and prepared the manuscript. HSM revised thedata and helped in manuscript writing.
Key words
Grasses, Legumes, Forbs, Ammonia, Total gas produced, Volatile fatty acids
DOI: https://dx.doi.org/10.17582/journal.pjz/20210608200641
* Corresponding author: akkb2010@gmail.com
0030-9923/2023/0003-1051 $ 9.00/0
Copyright 2023 by the authors. Licensee Zoological Society of Pakistan.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Range productivity has been reduced by overgrazing. One of the factors that negatively affects livestock is the poor use of pastures and negatively affects livestock and the use of inappropriate grazing systems, whereas the livestock needs of fodder has exceeded the sustainable yield of rangelands and other forage resources in nearly all developing countries now (Taha and Khidr, 2011). The malicious and excessive use of rangelands has contributed to their degradation while reducing the availability of livestock feed resources further (Ahmadi-Beni et al., 2014). It is necessary to provide the animals with sufficient nutrition necessary to increase productive and reproductive performance (Taha and Khidr, 2011). The cattle tend to graze long weeds that sheep may reject, while sheep graze near livestock deposits that cattle avoid (Forbes and Hodgson, 1985). It is estimated that rangelands (i.e., grasslands, shrubs, forest lands and tundra) provide more than 70% of the feed consumed by livestock worldwide (Lund, 2007). Rangeland is also home to a variety of wildlife species (Krausman et al., 2009).
In the North Western Coast (NWC) of Egypt raising sheep considered to be the main economic activity of Bedouins inhabiting the area and constitutes the majority of their incomes. Rangeland is the main feedstuff for livestock population in NWC. However, rangeland has been deteriorated rapidly as a result of mismanagement and overgrazing and inadequate nutrition are the major constraints preventing the animals from exhibiting their genetic potential. Thus, sheep productivity has deteriorated as a result of lack of genetic and poor feeding. The range vegetation consists of sparse stands of shrubs and sub-shrubs and herbaceous perennials with a winter-spring covers of ephemerals of variable density depending upon soil depth and surface topography (NEPAD–CAADP Bankable Investment Project Profile “Improving Range-Livestock Productivity in the North–Western Desert of Egypt, 2005”). These species represent 50% of the total flora of Egypt (Heneidy, 2012). Heneidy (1992), discovered a list of plant species with different life-forms in El-Omayed Biosphere that consisted of 171 species. Subshrubs are the dominant plant type of rangeland in the north-western coastal zone of Egypt since animal husbandry depends to a large extent on this feed source (Van Duivenbooden, 1993). Mahmoud et al. (2017) have also concluded that Ammophila arenaria, Trigonella maritime, Vicia sp., Ononis vaginalis, Atriplex sp. and Lycium shawii have high protein, moderate fiber contents and high in vitro digestibility that make them good quality wild fodder plants for ruminants’ nutrition in Egyptian rangelands.
The area of rangeland around the world is more than 52.5 million km2, of which 13.8% of terrestrial rangelands is woody savanna, 12.7% are open and closed shrub lands, 8.3% are no weedy grasslands, and 5.7% are tundra Sandhage-Hofmann (2016). Most of rangelands which is distributed in dry land refer to areas with primary productivity limited by water. They cover about 40% of the land surface and contain about one fifth of the human population (Heneidy, 2012). The exploitation of the productivity of this vast area of rangelands depends on ruminant animals, given their ability on converting roughage to meat, milk, leather and wool (Beukes et al., 2002).
Forage species with low concentrations of N, such as winter pastures can improve digestibility, because N fertilization can stimulate microbial activity in the rumen. Forage contain 30-90% of neutral detergent fiber (NDF) and bulky feeds are essential to stimulate rumination and maintain the health of the ruminant. Legumes may contain 15-23% crude protein (CP); grasses however, contain 8-18% CP (according to the level of nitrogen fertilization) and crop residues (straw or stubble) may have only 3-4% of CP. Forage can make up two-thirds of the dry matter in the diet of ruminants. However, good quality forages in good balanced diets, can provide much of the protein and energy needed for milk production (Roque, 2015). Herbaceous species play an important role in livestock feeding in arid and semi-arid regions (Arzani et al., 2006).
The malicious and excessive use of rangelands has contributed to their degradation while reducing the availability of livestock feed resources further (Ahmadi-Beni et al., 2014). A thoughtful understanding of the associative effects between forages could help to optimise feed use efficiency, resulting in greater productivity, a reduction of the environmental impact of animal emissions and more sustainable animal production (Niderkorn and Baumont, 2009).
Many studies have proved that there were no differences in pH of the rumen between different dietary groups during the treatment period (Abdullah et al., 2000). The rumen pH for cow fed the control diet or one of the four treatment diets during the treatment period was 6.62–6.67. Whereas, Schauf and Clark (1989) that indicating that feeding rumen protected fat at up to 7.2% of the ration DM did not adversely affect rumen pH. And, Grummer (1988) indicated that feeding rumen protected fat at up to 5% of the ration DM does not change rumen pH.
In recent years there has been an increasing interest of nutritionists in bioactive plant factors– phyto factors as natural feed additives that can modify the rumen fermentation processes, improve the protein metabolism and, at the same time, reduce ammonia production and emission (Szumacher-Strabel1 and Cieślak, 2010). Despite the fact that ruminants can benefit from almost all nitrogen sources, excessive dietary protein leads to ammonia formation (Place and Mitloehner, 2010). Szumacher-Strabel and Cieslak (2012) indicated that the process of ammonia composition in the rumen involves most of the protein-soluble bacteria, including the bacteria of excessive ammonia production (HAP), whereas, they observed that efficiency of ruminal metabolism was a significant factor affecting production and release of pollutants, i.e., methane and ammonia.
Zmora et al. (2012) observed that Mentha piperita supplementation affected the total gas production (TGP). TGP was increased by 18.5% in the group with the highest MP dose compared with control. In addition, MP supplementation caused an increase in TGP. Williams (2000) indicated that there has been much interest in in vitro gas production techniques because of their potential to simulate fermentation kinetics in the rumen (Getachew et al., 1998); BluÈmmel and Becker, 1997) and in vivo digestibility (Menke and Steingass,1988; BluÈmmel and Érskov, 1993). Whereas, Aiple et al. (1992) showed that the highest correlations of TGP were obtained from rumen liquor (TGPRL) or from faeces (TGPFA) was combined with chemical analysis (CP, ash, fat or CF). Furthermore, the determination between TGP at 48, 96 h or 21 h using RL or FA, improved when combined with ADF.
Cieslak et al. (2014) concluded that in rumen the highest CH4 decrease (21.9%) was with Vaccinium vitis idaea (VVI), and other diets containing Quercus cortex (QC) extract and mixture QC+ VVI decreased CH4 production by 18.0 and 18.6%, respectively. However, Zmora et al. (2012) indicated that the reduction of CH4 will increase the concentration of substrates (H2 and VFA) for methane synthesis and thus might increase TGP or total VFA. On the other hand, efficient and balanced ruminal fermentation reduces the emission of gases, particularly methane, to the atmosphere and the total amount of methane produced by ruminants can be reduced by a reduction in the size of the dairy population by an increase in their productivity (Szumacher-Strabel and Cieslak, 2012). Yulistiani et al. (2016) said that in rumen methane production tended (P<0.07) to decrease by complete rumen modifier supplementation in rice straw basal diet
Abdullah et al. (2000) indicated that no differences were noted in rumen VFA and A:P ratio between cows fed the control diet or one of the four treatment diets and between cows fed diets with different levels of forage or fat. On the other hand, Priolo et al. (2001) indicated that animals raised on different production systems produce different concentrations of VFA in the rumen. The metabolism of propionate is very different from that of acetate and from the VFA have origin some different compounds responsible for specific meat flavors. This study aimed to assess the rumen gases and the total VFA value of some wild forage plant species derived from Egyptian rangelands.
MATERIALS AND METHODS
The experiments were conducted at the Experimental Laboratories of the Natural Resources Department, Animal Production Department, Cairo University, Egypt and Department of Animal Nutrition, Poznan University of Life Sciences, Wolynska 33, 60-637 Poznan, Poland during the period from 2015 to 2018.
Plant materials
Twenty-four wild palatable forage plants were collected from Egyptian Rangelands in Mediterranean coast region of Egypt during spring season (Table I). Plant species were firstly identified at the field, collected and prepared as herbarium sheets after being pressed. All collected and preserved plants were taken to the Cairo University herbarium for accurate identification. Floristic identifications were performed according to Täckholm (1974) and the scientific names of species were updated by Boulos (1999, 2000, 2002, 2005).
Batch culture
The batch culture method was adopted from Szumacher-Strabel et al. (2004). Briefly, rumen fluid was mixed with the buffer solution (292 mg K2HPO4, 240 mg KH2PO4, 480 mg (NH4)2SO4, 480 mg NaCl, 100 mg MgSO4x7H2O, 64 mg CaCl2x2H2O, 4 mg Na2CO3 and 600 mg cysteine hydrochloride per 1 liter of double distilled water in the 1:4 (v/v) ratio. Incubations were done at 39°C under CO2 in 40 ml buffered rumen fluid added to pre-warmed 125 ml serum flasks. 400 g from each feed were used. The incubation flasks, sealed with rubber stoppers and Al caps, were placed in an incubator for 24 h and periodically mixed every few hours.
Estimation of pH and NH3concentration
Immediately after withdrawal, rumen contents were strained through four layers of cheesecloth to obtain rumen fluid in which the following determinations were made: pH using a CP-1-4 type pH meter, and ammonia nitrogen by the Nessler method by Szumacher-Strabel et al. (2002).
Estimation of total gas produced (TGP) and methane production
All inoculated fermentation bottles were flushed with CO2 before being sealed and incubated at 39°C for 24 h. The gas headspace pressure inside each of the fermentation bottles was recorded at the end of the 24 h incubation period using a detachable pressure transducer. The TGP in each bottle was estimated using the equation:
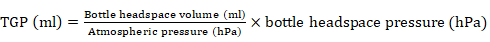
After 24 h of incubation, gas production was estimated by the displacement of syringe piston, which was connected to the serum flasks. The gas produced due to fermentation of substrate was calculated by subtracting gas produced in blank vessels (without substrate) from total gas produced in the vessels containing buffered rumen fluid and substrate
For methane determination 500 µl gas was sampled from the headspace of incubation vessels in a gastight syringe (GASTIGHT® Syringes, Hamilton Bonaduz AG, Switzerland) into SRI310 gas chromatograph equipped with a thermal conductivity detector (TCD) and Carboxen – 1000 column (mesh side 60/80, 15 FT x 1,8 INS.S, SUPELCO). Nitrogen was used as the carrier gas at a constant flow of 30.0 mL/min. The oven temperature was programmed as follows: Initially 180°C for 1.5 min, then increasing at 20°C/min to 220°C. 500 µL gas samples were injected. Observed peaks were identified by comparison of retention times with appropriate gases standards (mix gases 5.63% CO2, 5.56% CH4, 5.10% H2 and N2 remains, Multa S.C. Poland) using PeakSimple ver. 3.29.
Volatile fatty acids (VFA) estimation
At the end of incubation, 3.6 ml of fermented rumen fluid was stabilized with 0.4 ml of a 46 mM HgCl2 solution and frozen until analyses by HPLC. After thawing the mixture was centrifugated at 12000 rpm for 10 min, filtered through 0.22 µm and 10 µl of clear supernatant was injected to the High-Performance Liquid Chromatograph Waters 2690 equipped with Waters 2487 Dual λ detector and Aminex HPX-87H column (300 mm x 7.8 mm). As a mobile phases 0,004 M H2SO4 was used. A 10 µL sample volume was injected into the column. Quantitative and qualitative identification of individual peaks was made using the method based on external standard prepared by mixing of individual VFA purchased from Supelco and using the Millenium 2001 software (version 2.15).
Statistical analyses
Batch culture experiments (24 h incubation) were performed in four replication (4 incubation vessels) for each of each feeds (n = 4). Data were analyzed by ANOVA using the GLM procedures of SAS (Version 6.0; SAS Inst. Inc. Carry, NC, 1989) with treatment as a factor. Treatment means were calculated using the LSMEANS option of SAS.
RESULTS
The effect of feeds on forage plants in the rumen fluid
pH content in forbs was higher than legumes and grasses (Table I). Among forbs, cleared the lowest pH content was recorded 6.38% in rumen fluid feeds on Atriplex halimus, and the highest value (6.51%) was found in rumen fluid feeds on Periploca angustifolia. Among legumes, the pH ranged from 6.18% in rumen fluid feeds on Vicia monantha to 6.45% in rumen fluid feeds on Astragalus hamosus. Among grasses pH increased from 6.16% in rumen fluid feeds on Phalaris minor and to 6.43% in rumen fluid feeds on Aeluropus lagopoides.
The NH3 concentration of grasses varied between 276.62 mmol in rumen fluid feeds on Phalaris minor and 289.05 mmol in rumen fluid feeds on Aeluropus lagopoides. In contrast, among legumes, ammonia NH3 concentration varied between 9.54 mmol in rumen fluid feeds on Lygos raetam and 14.08 mmol in rumen fluid feeds on Vicia sativa. While, among forbs ammonia NH3 concentration of analyzed plants varied between 9.41 mmol in rumen fluid feeds on Deverra trotuosa and 14.02 mmol in rumen fluid feeds on Tamarix nilotica.
The highest value total gas produced (TGP)127.67 attained in rumen fluid feeds on Ammophila arenaria and 98.67 ml in rumen fluid feeds on Lygeum spartum, among grasses (Table I). In legumes, the TGP contents attained in 120.00 ml in rumen fluid feeds on Trigonella maritime and 100.00 ml in rumen fluid feeds on Lygos raetam. While, among forbs the highest values of TGP attained in Anacyclus alexandrines (91.67ml) and Atriplex halimus, Atriplex nummularia, Gymnocarpos decandrum, Lycium shawii, Periploca angustifolia and Tamarix nilotica (86.00ml).
The values of methane in rumen fluid feeds on legumes increased from 4.52mmol in Lygos raetam to 8.75 mmol in Vicia sativa and ranged from 5.16mmol in rumen fluid feeds on Panicum coloratum to 7.59 mmol in rumen fluid feeds on Phragmites australis as grasses. Among forbs, methane increased from 2.12mmol in rumen fluid feeds on Atriplex nummularia to 6.70 mmol in rumen fluid feeds on Anacyclus alexandrines.
Effect of grasses, legumes and forbs plants on fatty acids rumen fluid
Overall results showed significant differences among different groups (Table II). Acetic, propionic, isobutyric, butyric, total VFA and A/P showed a higher trend for the group of grasses compared to legumes and forbs.
The highest values of acetic (A) (mol/mol) were 43.84, 37.59, 34.96 and 34.93mol/mol in Aegilops kotschyi, Aeluropus lagopoides, Bromus rubens and Ammophila arenaria, respectively. In contrast, the lowest values of acetic were 13.57, 17.56, 17.59 and 18.32mol/mol in Tamarix Nilotica, Panicum coloratum, Periploca Angustifolia and Lycium shawii, respectively.
Propionic (P) values of analyzed rumen fluid varied between 5.58mol/mol for Panicum coloratum and 14.91mol/mol for Aegilops kotschyi among grasses. Among legumes propionic varied between 8.58mol/mol (Astragalus hamosus) and 10.58 mol/mol (Lotus polyphyllos) while, among forbs propionic of tested rumen fluid varied between 9.23mol/mol (Anacyclus alexandrines) and 5.30mol/mol (Tamarix nilotica).
Isobutyric (mol/mol) among forbs fluctuated between 0.50 mol/mol (Anacyclus alexandrines) to 0.26mol/mol (Tamarix nilotica). Among grasses, it varied from 0.89mol/mol (Aeluropus lagopoides) to 0.26mol/mol (Panicum coloratum). Likewise, among legumes, isobutyric acid of tested legumes ranged from 0.41mol/mol in Lygos raetam to 0.54mol/mol in Vicia monantha.
The butyric acid (B) (mol/mol) contents of rumen fluid in Table II. Among grasses, the highest and lowest values of butyric contents were 11.78mol/mol (Aeluropus lagopoides) and 6.17mol/mol (Panicum coloratum). In legumes, the highest and lowest values of butyric acid contents were 10.95mol/mol and 8.76mol/mol in Lotus polyphyllos and Ononis variegate, respectively. In forbs, the highest and lowest values of butyric acid contents were 9.17mol/mol (Anacyclus alexandrines) and 5.64mol/mol (Tamarix nilotica).
Table I. Effect of grasses, legumes, and forbs feeds on pH, NH3, total gas production (TGP) and methane in the rumen fluid.
|
Plant species |
pH |
NH3 (mmol) |
TGP (mL) |
Methane (mmol) |
|
Grasses |
||||
|
Poaceae (Gramineae) |
||||
|
Aegilops kotschyi (A) |
6.21±0.11ab |
279.17±4.91ab |
120.00±3.06 b |
6.62 ±0.91 |
|
Aeluropus lagopoides (P) |
6.43±0.04a |
289.05±2.02a |
106.33±1.33ab |
6.06±1.44 |
|
Ammophila arenaria (P) |
6.24±0.01b |
280.21±0.40ab |
127.67±1.67 a |
6.71±1.20 |
|
Bromus rubens (A) |
6.31±0.00ab |
283.66±0.15 b |
109.67±2.03ab |
6.62±0.77 |
|
Hordeum marinum (A) |
6.32±0.01ab |
283.96±0.26ab |
115.33±1.20 b |
6.64±0.87 |
|
Lolium rigidum (A) |
6.25±0.02ab |
280.81±0.69ab |
114.67±5.49ab |
6.23±0.30 |
|
Lophochloa cristata (A) |
6.33±0.01ab |
284.41±0.45ab |
112.67±2.91ab |
7.39±0.49 |
|
Lygeum spartum (P) |
6.41±0.03a |
287.85±1.28a |
98.67±2.40 b |
5.74±0.91 |
|
Panicum coloratum (A) |
6.31±0.01ab |
283.36±0.60 b |
109.00±0.58ab |
5.16±0.16 |
|
Phalaris minor (A) |
6.16±0.04b |
276.62±1.59 b |
116.33±1.76ab |
7.32±0,84 |
|
Phragmites australis (P) |
6.28±0.02ab |
282.01±0.75ab |
107.67±1.45ab |
7.59±0.31 |
|
SEM total |
0.02 |
12.73 |
1.65 |
0.27 |
|
P-value |
0.007 |
0.002 |
0.061 |
0.003 |
|
Legumes |
||||
|
Fabaceae (Leguminose) |
||||
|
Astragalus hamosus (A) |
6.45± 0.01a |
11.52±0.33 |
107.00± 2.08ab |
6.68± 0.37ab |
|
Lotus polyphyllos (P) |
6.35± 0.03ab |
10.12± 0.75 |
106.67± 1.20ab |
6.17± 0.74ab |
|
Lygos raetam (P) |
7.35± 1.20ab |
9.54± 0.67 |
100.00± 1.00b |
4.52± 0.22b |
|
Ononis variegate (P) |
6.34± 0.02ab |
11.58± 1.59 |
102.00± 2.08ab |
6.05± 0.23ab |
|
Trigonella maritime (A) |
6.27± 0.03ab |
10.68± 1.48 |
120.00± 6.51ab |
7.35± 0.59ab |
|
Vicia monantha (A) |
6.18± 0.05b |
12.93± 0.04 |
115.67 ± 0.33a |
7.10± 0.82ab |
|
Vicia sativa (A) |
6.20± 0.03ab |
14.08± 0.95 |
110.00± 0.58ab |
8.75 ± 0.18a |
|
SEM total |
0.15 |
0.45 |
1.72 |
0.32 |
|
P-value |
0.02 |
0.07 |
0.02 |
0.03 |
|
Forbs |
||||
|
Asteraceae |
||||
|
Anacyclus alexandrines (P) Chenopodiaceae |
6.39± 0.01bc |
13.40± 1.01a |
91.67± 0.67 |
6.70± 0.49a |
|
Atriplex halimus (P) |
6.38± 0.02 c |
12.69± 0.49abc |
86± 0.00 |
3.38± 0.62b |
|
Atriplex nummularia (P) Apiaceae |
6.44± 0.01 ab |
13.73± 0.4a |
86± 0.00 |
2.12± 0.53b |
|
Deverra trotuosa (P) Caryophiyllaceae |
6.41± 0.02bc |
9.41± 0.27 c |
89± 0.00 |
2.89± 0.37b |
|
Gymnocarpos decandrum (P) Solanaceae |
6.48± 0.02a |
10.06± 0.67bc |
86± 0.00 |
3.10± 0.41b |
|
Lycium shawii (P) Apocynaceae |
6.47± 0.02 ab |
11.29± 1.16ab |
86± 0.00 |
3.98± 0.04b |
|
Periploca angustifolia (P) Tamariceae |
6.51± 0.02a |
13.25± 0.45ab |
86± 0.00 |
2.57± 0.14b |
|
Tamarix nilotica (P) |
6.39± 0.01bc |
14.02± 0.41a |
86± 0.00 |
2.66± 0.77b |
|
SEM total |
0.0109 |
0.396 |
0.421 |
0.314 |
|
P-value |
<0.001 |
<0.001 |
>0.05 |
<0.001 |
A, annual; P, peremmial; NH3, ammonia; TGP, total gas produced.
Table II. Level of volatile fatty acid (mol/mol) in the rumen fluid that feed on grasses feeds.
|
Plant species |
Acetic (A) |
Pro-pionic (P) |
Isobu-tyric acid |
Butyric acid (B) |
Isova- leric |
Valeric acid |
Izowa-leria-nowy |
Wale-ria-nowy |
Total VFA (mmol/l) |
APB |
A/P |
OPB |
|
Grasses |
||||||||||||
|
Aegilops kotschyi |
43.84± 1.01a |
14.91± 0.19a |
0.57± 0.03ab |
11.75± 0.27a |
0.91± 0.05ab |
1.20± 0.05ab |
ND |
ND |
73.08± 1.32a |
70.50± 1.25a |
2.94± 0.07ab |
--- |
|
Aeluro-pus lago-poides |
37.59± 1.96b |
13.12± 0.97ab |
0.89± 0.13a |
11.78± 1.00a |
1.06± 0.08a |
1.51± 0.19a |
ND |
ND |
65.95± 4.28a |
62.50± 3.90a |
2.87± 0.07ab |
--- |
|
Ammo-phila arenaria |
34.93± 1.63b |
12.49± 0.80ab |
0.67± 0.06a |
11.42± 0.63ab |
0.81± 0.06b |
1.29± 0.10a |
ND |
ND |
61.61± 3.21ab |
58.83± 3.03ab |
2.80± 0.07ab |
--- |
|
Bromus rubens |
34.96± 1.28b |
12.65± 0.58ab |
0.52± 0.05ab |
11.05± 0.28ab |
0.79± 0.01b |
1.05± 0.04ab |
ND |
ND |
61.03± 2.17ab |
58.67± 2.12ab |
2.77± 0.03ab |
--- |
|
Hordeum marinum |
24.20± 0.43c |
8.34± 0.19ab |
0.37± 0.03ab |
8.69± 0.21ab |
0.62± 0.01cd |
0.86± 0.03ab |
ND |
ND |
43.08± 0.80ab |
41.23± 0.76ab |
2.90± 0.05ab |
--- |
|
Lolium rigidum |
25.43± 0.86c |
9.01± 0.20ab |
0.43± 0.05ab |
9.20± 0.20ab |
0.64± 0.02cd |
0.96± 0.02ab |
ND |
ND |
45.67± 1.28ab |
43.64± 1.26ab |
2.82± 0.03ab |
--- |
|
Lopho-chloa cristata |
24.37± 0.48c |
9.05± 0.26ab |
0.40± 0.03ab |
9.01± 0.26ab |
0.68± 0.04c |
0.73± 0.00ab |
ND |
ND |
44.25± 0.99b |
42.43± 0,95ab |
2.69± 0.03b |
--- |
|
Lygeum spartum |
20.11± 0.21cd |
6.95± 0.10ab |
0.33± 0.02ab |
7.11± 0.03ab |
0.54± 0.01cd |
0.63± 0.02ab |
ND |
ND |
35.66± 0.28ab |
34.17± 0.28ab |
2.89± 0.00ab |
--- |
|
Panicum coloratum |
17.56± 0.03 d |
5.58± 0.30b |
0.26± 0.02b |
6.17± 0.30b |
0.48± 0.03d |
0.53± 0.03b |
ND |
ND |
30.58± 0.40ab |
29.31± 0.38b |
3.17± 0.18a |
--- |
|
Phalaris minor |
18.98± 0.40d |
6.79± 0.26b |
0.30± 0.02ab |
7.56± 0.28ab |
0.55± 0.03cd |
0.59± 0.02ab |
ND |
ND |
34.76± 1.01ab |
33.33± 0.94b |
2.80± 0.05ab |
--- |
|
Phrag-mites australis |
21.25± 0.20 c |
7.67± 0.04ab |
0.39± 0.01ab |
8.25± 0.22ab |
0.60± 0.00cd |
0.67± 0.03ab |
ND |
ND |
38.83± 0.50ab |
37.17± 0.45ab |
2.77± 0.01ab |
--- |
|
P-value |
<0,001 |
<0.001 |
0.001 |
<0.001 |
<0.001 |
<0.001 |
ND |
ND |
<0.001 |
<0.001 |
0.02 |
--- |
|
Legumes |
||||||||||||
|
Astra-galus hamosus |
23.18 ± 1.15 |
8.58± 0.47b |
0.47 ± 0.03 |
9.01± 0.56b |
ND |
ND |
0.65± 0.05b |
0.71± 0.06b |
42.59 ± 2.30 |
--- |
2.70± 0.02ab |
40.77 ±2.18 |
|
Lotus poly-phyllos |
26.98 ± 0.04 |
10.58± .10a |
0.48 ± 0.02 |
10.95± 0.15a |
ND |
ND |
0.79± 0.03b |
0.99± 0.03a |
50.77 ± 0.27 |
--- |
2.55± 0.03b |
48.51 ±0.20 |
|
Lygos raetam |
25.41 ± 1.23 |
9.85± 0.74ab |
0.41 ± 0.06 |
9.43± 0.54ab |
ND |
ND |
0.77± 0.01b |
0.88± 0.06ab |
46.75 ± 2.65 |
--- |
2.59± 0.06ab |
44.68 ±2.50 |
|
Ononis variegate |
23.89 ± 0.52 |
8.70± 0.12ab |
0.47 ± 0.01 |
8.76± 0.11b |
ND |
ND |
0.74± 0.03b |
0.82± 0.03ab |
43.38 ±0.53 |
--- |
2.75± 0.08ab |
41.35 ±0.50 |
|
Trigonella maritime |
25.68 ± 0.30 |
9.22± 0.29ab |
0.52 ± 0.01 |
9.63± 0.37ab |
ND |
ND |
1.04± 0.05a |
0.88± 0.02ab |
46.97 ± 0.99 |
--- |
2.79± 0.05a |
44.53 ±0.94 |
|
Vicia monantha |
24.34 ± 0.12 |
9.43± 0.10ab |
0.54 ± 0.04 |
9.97± 0.07ab |
ND |
ND |
1.16± 0.00a |
0.88± 0.02ab |
46.31 ± 0.25 |
--- |
2.58± 0.03ab |
43.73 ±0.23 |
|
Vicia sativa |
24.96 ± 1.07 |
9.49± 0.47ab |
0.52 ± 0.05 |
9.70± 0.58ab |
ND |
ND |
1.09± 0.05a |
0.89ab ± 0.07 |
46.65 ± 2.25 |
--- |
2.63± 0.02ab |
44.15 ±2.11 |
|
P-value |
0.071 |
0.043 |
0.27 |
0.034 |
ND |
ND |
<0.001 |
0.02 |
0.06 |
--- |
0.02 |
0.059 |
|
Forbs |
||||||||||||
|
Ana-cyclus alex-andrines |
23.74± 0.69a |
9.23± 0.19a |
0.50± 0.04a |
9.17± 0.35a |
ND |
ND |
1.05± 0.03a |
0.87± 0.03a |
44.56± 1.29a |
--- |
2.57± 0.02b |
42.14 ± 1.23 |
|
Atriplex halimus |
21.59± 1.41ab |
8.40± 0.70ab |
0.42± 0.07ab |
7.72± 0.69ab |
ND |
ND |
0.85± 0.09ab |
0.77± 0.09ab |
39.75± 3.03ab |
--- |
2.58± 0.05b |
37.70 ± 2.79 |
|
Table continues on next page..........................? |
||||||||||||
|
Plant species |
Acetic (A) |
Pro-pionic (P) |
Iso-butyric acid |
Butyric acid (B) |
Isova- leric |
Valeric acid |
Izo-waleria-nowy |
Wale-ria-nowy |
Total VFA (mmol/l) |
APB |
A/P |
OPB |
|
Atriplex mmularia |
20.52± 0.47abc |
7.68± 0.10ab |
0.36± 0.02ab |
6.36± 0.09bc |
ND |
ND |
0.85± 0.00ab |
0.60± 0.04bc |
36.37± 0.58bc |
--- |
2.67± 0.03ab |
34.55 ±0.61 |
|
Deverra trotuosa |
20.01± 0.21bc |
7.66± 0.13ab |
0.43± 0.03a |
7.18± 0.09bc |
ND |
ND |
0.88± 0.09ab |
0.59± 0.03bc |
36.75± 0.45bc |
--- |
2.61± 0.03ab |
34.85 ± 0.41 |
|
Gymno-carpos decan-drum |
20.45± 0.36abc |
7.46± 0.10ab |
0.42± 0.02ab |
7.29± 0.15b |
ND |
ND |
0.86± 0.02ab |
0.65± 0.04b |
37.13± 0.63bc |
--- |
2.74± 0.01a |
35.20 ± 0.59 |
|
Lycium shawii |
18.32± 0.54bc |
6.69± 0.21ab |
0.38± 0.03ab |
6.38± 0.33bc |
ND |
ND |
0.79± 0.03bc |
0.56± 0.03bc |
33.12± 1.09c |
--- |
2.74± 0.01a |
31.39 ± 1.07 |
|
Periploca angusti-folia |
17.59± 0.73c |
6.60± 0.23ab |
0.34± 0.01ab |
6.47± 0.22bc |
ND |
ND |
0.76± 0.05bc |
0.58± 0.03bc |
32.35± 1.28c |
--- |
2.66± 0.02ab |
30.67 ± 1.18 |
|
Tamarix nilotica |
13.57± 0.87d |
5.30± 0.30b |
0.26± 0.02b |
5.64± 0.25c |
ND |
ND |
0.64± 0.04c |
0.42± 0.04c |
25.82± 1.52d |
--- |
2.56± 0.02b |
24.50 ± 1.43 |
|
P-value |
<0.001 |
<0.007 |
<0.007 |
<0.001 |
ND |
ND |
<0.001 |
<0.001 |
<0.001 |
--- |
<0.001 |
<0.001 |
VFA, volatile fatty acid; APB, acetic acid, propionic acid, butyric acid; A/P, acetic acid to propionic acid; OPB, oil propionic butyric acid; ND, not detected.
Isovaleric (mol/mol) and valeric (mol/mol) acids contents were found in grasses only. They varied from 1.06mol/mol and1.51mol/mol (Aeluropus lagopoides) to 0.48mol/mol and 0.53mol/mol (Panicum coloratum), respectively. In contrast, Izowalerianowy (mol/mol) and Walerianowy (mol/mol) were found among legumes and forbs only. Among legumes values of Izowalerianowy varied between 0.65mol/mol for Astraglus homosus and 1.16mol/mol for Vicia monantha. When the highest and the lowest values of Walerianowy content were 0.99mol/mol (Lotus polyphyllos) and 0.71mol/mol (Astragalus hamosus). While, among forbs Izowalerianowy content of analyzed rumen fluid varied between 0.64mol/mol for Tamarix nilotica and 1.05mol/mol for Anacyclus alexandrines. And, the highest and lowest values of Walerianowy contents were 0.87mol/mol (Anacyclus alexandrines) and 0.42mol/mol (Tamarix nilotica).
Among the tested of rumen fluid of animals were feeding of grasses, legumes and forbs, the highest total VFA contents (73.08, 50.77 and 44.56mol/mol) were recorded in Aegilops kotschyi, Lotus polyphyllos and Anacyclus alexandrines, respectively. In contrast, the lowest values of VFA contents (30.58, 42.59 and 25.82 mol/mol) were recorded in Panicum coloratum, Astragalus hamosus and Tamarix Nilotica, respectively.
APB content was found in grasses only, they varied from 70.50 (Aegilops kotschyi) to 29.31 (Panicum coloratum). OPB were found among legumes and forbs only. Among legumes the highest and lowest values of OPB contents were 48.51 (Lotus polyphyllos) and 40.77 (Astragalus hamosus). Among forbs values of OPB varied between 24.50 for Tamarix nilotica and 42.14 for Anacyclus alexandrines.
The highest percentage of A/P were found in grasses compared to legume and forbs (3.17, 2.79 and 2.58) in Panicum coloratum, Trigonella maritime and Atriplex halimus, respectively, while the lowest percent were record in legumes compared to forbs and grasses (2.55. 2.56 and 2.69) in Lotus polyphyllos, Tamarix nilotica and Lophochloa cristata.
DISCUSSION
In present study, the rumen pH fluid in forage plant species (grasses, legumes and forbs) regimen ranged from 6.16 to 6.51 and by incubation fluid pH the results indicated that have significant differences. These results were not in agreement with Abdullah et al. (2000) who indicated that rumen pH for cows fed on the control diet or one of the four treatment diets during treatment period was 6.62 and 6.67, respectively. There were no differences in pH of the rumen between different dietary groups during the treatment period. This was in disagreement with Cieslak et al. (2014) who indicated that the rumen pH during treatment period was 6.86 and 6.95 in tannin supplementation from Quercus cortex treatment and control treatment. When Sun et al. (2019) concluded that the rumen pH was not significantly different between the first and the second groups (6.50 and 6.53, respectively). However, the rumen pH in the third and the fourth groups was 7.11 and 7.13, respectively, and these values were significantly different from those of the first and second groups.
The proportion of ammonia in rumen fluid in our study was high in forage plant species and ranged between 276 and 289 mmol but in legumes and forbs was ranged between 9.41 and 14.08 mmol but these results were in disagreement with Cieslak et al. (2014) who indicated that the proportion of ammonia ranged between 9.72 and 12.6 mmol. This was also not in agreement with Szumacher-Strabel et al. (2002) who indicated that the proportion of ammonia ranged between 7.03 and 29.07. On the other hand, Sun et al. (2019) observed that the concentration of rumen NH3–N was significantly higher in the third and the forest groups than in the first and the second groups.
In this study, the concentration of TGP in rumen fluid in forage plant species (grasses, legumes and forbs) ranged between 86 and 127 mL but Zmora et al. (2012) reported that concentration of TGP ranged between 42 and 51.33 mL. Aiple et al. (1992) reported that the TGP at 48, 96 h or at 21 h using RL or FA, were improved when combined with ADF.
Our study showed that the concentration of methane ranged between 2.12 and 7.59 mmol which was in agreement with Zmora et al. (2012) who indicated that methane concentration ranged between 3.62 and 6.26 mmol, although Cieslak et al. (2014) indicated that methane concentration ranged between (2.43 and 3.11 mmol).
The values of VFA in rumen fluid that feeding on rangeland in our study was higher than that reported by Zmora et al. (2012). The ratio of A/P was reported higher by Zmora et al. (2012) than our study. Szumacher-Strabel et al. (2002) indicated that the value of isobutyric acid ranged between 13.75 and 25.29 mmol and these results were in disagreement with our results because the value of isobutyric acid in our study ranged between 0.26 and 0.89 mmol. On the other hand, values of isovalyric acid ranged between 0.48 and 1.06 mmol but these results were in disagreement with Cieslak et al. (2014) results who reported these values between 4.77 and 8.11 mmol. Liu et al. (2019) observed that there was a decreasing tendency for total VFA, acetate and isovalerate concentration, but no significant differences were detected among the groups. The increases in dietary concentration level significantly decreased A:P ratio (P < 0.05).
Statement of conflict of interest
The authors have declared no conflict of interest.
REFERENCES
Abdullah, M., Young, J.W., Tyler, H.D., and Mohiuddin, G., 2000. Effect of feeding high forage diets with supplemental fat on blood metabolites, rumen fermentation and dry matter digestibility in dairy cows. Asian-Australas. J. Anim. Sci., 13: 451-456. https://doi.org/10.5713/ajas.2000.451
Ahmadi-Beni, M., Gharmakher, H.N., Azimi, M.S. and Maramaei, M.G., 2014. Investigation of forage quality of vetiveria zizanioides in semisteppe region of maravehtappeh, golestan province. Iran. J. Range. Sci., 4: 287-296.
Aiple, K.P., Steingass, H. and Menke, K.H., 1992. Suitability of a buffered faecal suspension as the inoculum in the Hohenheim gas test. J. Anim. Physiol. Nutr., 67: 57-66. https://doi.org/10.1111/j.1439-0396.1992.tb00583.x
Arzani, H., Basiri, M., Khatibi, F. and Ghorbani, G., 2006. Nutritive value of some zagros mountain rangeland species. Small Rumin. Res., 65: 128–135. https://doi.org/10.1016/j.smallrumres.2005.05.033
Beukes, P.C., Cowling, R.M. and Higgins, S.I., 2002. An ecological economic simulation model of a nonselective grazing system in the Nama Karoo, South Africa. Ecol. Econ., 42: 221-242. https://doi.org/10.1016/S0921-8009(02)00055-1
BluÈmmel, M., and Èrskov, E.R., 1993. Comparison of in vitro gas production and nylon bag degradability of roughage in predicting feed intake in cattle. Anim. Feed Sci. Technol., 40: 109-119. https://doi.org/10.1016/0377-8401(93)90150-I
BluÈmmel, M., and Becker, K., 1997. The degradability characteristics of fifty-four roughages and roughage neutral-detergent fibres as described by in vitro gas production and their relationship to voluntary feed intake. Br. J. Nutr., 77: 757-768. https://doi.org/10.1079/BJN19970073
Boulos, L., 1999. Flora of Egypt (Azollaceae- Oxalidaceae). 1, Al-Hadara Publishing, Cairo, Egypt, pp. 419. https://doi.org/10.1111/j.1756-1051.1999.tb01119.x
Boulos, L., 2000. Flora of Egypt (Geraiaceae- Boraginaceae). 2, Al-Hadara Publishing, Cairo, Egypt, pp. 352.
Boulos, L., 2002. Flora of Egypt (Verbenaceae- Compositae). 3, Al-Hadara Publishing, Cairo, Egypt, pp. 373. https://doi.org/10.1111/j.1756-1051.2002.tb01389.x
Boulos, L., 2005. Flora of Egypt (Monocotyledons). 4, Al-Hadara Publishing, Cairo, Egypt, pp. 617.
Cieslak, A., Zmora, P., Pers-Kamczyc, E., Stochmal, A., Sadowinska, A., Salem, A.Z.M., Kowalczyk, D., Zbonik, P. and SzumacherStrabel, M., 2014. Effects of two sources of tannins (Quercus L. and Vaccinium vitis-idaea L.) on rumen microbial fermentation: An in vitro study. Ital. J. Anim. Sci., 13: 290-294. https://doi.org/10.4081/ijas.2014.3133
Forbes, T.D.A. and Hodgson, J., 1985. The reaction of grazing sheep and cattle to the presence of dung from the same or the other species. In: Grass and forage science, June, pp. 177-182. https://doi.org/10.1111/j.1365-2494.1985.tb01735.x
Getachew, G., BluÈmmel, M., Makkar, H.P.S. and Becker, K., 1998. In vitro gas measuring techniques for assessment of nutritional quality of feeds: A review. Anim. Feed Sci. Technol., 72: 261-281. https://doi.org/10.1016/S0377-8401(97)00189-2
Grummer, R.R., 1988. Influence of prilled fat and calcium salts of palm oil fatty acids on ruminal fermentation and nutrient digestibility. J. Dairy Sci. 71: 117-123. https://doi.org/10.3168/jds.S0022-0302(88)79532-6
Heneidy, S.Z., 2012. Rangelands in arid ecosystem. J. Divers. Ecosyst., 7: 127-166.
Heneidy, S.Z., 1992. An ecological study of the grazing systems of marist, Egypt. UNESCO, Paris, pp. 51.
Krausman, P.R., Naugle, D.E., Frisina, M.R., Northrup, R., Bleich, V.C., Block, W.M. and Wright, J.D., 2009. Livestock grazing, wildlife habitat and rangeland values. Rangelands, 31: 15–19. https://doi.org/10.2111/1551-501X-31.5.15
Liu, H., Xu, T., Xu, S., Ma, L., Han, X., Wang, X., Zhang, X., Hu, L., Zhao, N., Chen, Y., Pi, L. and Zhao, X., 2019. Effect of dietary concentrate to forage ratio on growth performance, rumen fermentation and bacterial diversity of Tibetan sheep under barn feeding on the Qinghai-Tibetan plateau. Tibetan plateau. PeerJ., 7: e7462. https://doi.org/10.7717/peerj.7462
Lund, G.H., 2007. Accounting for the worlds rangelands. Rangelands, 29: 3-10. https://doi.org/10.2111/1551-501X(2007)29[3:AFTWR]2.0.CO;2
Menke, K.H. and Steingass, H., 1988. Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Anim. Res. Dev., 28: 7-12.
Mahmoud, A.E.M., Abbas, M.S., Cieslak A., and Szumacher-Strabel, M., 2017. Evaluation of chemical composition and in vitro dry and organic matter digestibility of some forage plant species derived from Egyptian rangelands. J. Anim. Pl. Sci., 27: 1573-1581.
Niderkorn, V. and Baumont, R., 2009. Associative effects between forages on feed intake and digestion in ruminants. Animal, 3: 951-960. https://doi.org/10.1017/S1751731109004261
Place, S.E. and Mitloehner, F.M., 2010. Invited review: Contemporary environmental issues: A review of the dairy industry’s role in climate change and air quality and the potential of mitigation through improved production efficiency. J. Dairy Sci., 93: 3407-3416. https://doi.org/10.3168/jds.2009-2719
Priolo, A., Micol, D. and Agabriel, J., 2001. Effects of grass feeding systems on ruminant meat colour and flavour. A review. Anim. Res., 50: 185–200. https://doi.org/10.1051/animres:2001125
Purcell, P.J., Boland, T.M., Brien, M.O. and Kiely, P.O., 2012. In vitro rumen methane output of forb species sampled in spring and summer. Agric. Fd. Sci., 21: 83-90. https://doi.org/10.23986/afsci.4811
Roque, R.L., 2015. Nutrition of grass carbohydrates. In: Grass nutrition (ed. R.R. Lozano). Palibrio Press, Bloomington, Indiana USA. ISBN: 9781506508986. pp. 29-56.
Sandhage-Hofmann, A., 2016. Rangeland management. Elsevier Reference Collection in Earth Systems and Environmental Sciences. https://doi.org/10.1016/B978-0-12-409548-9.10455-5
Schauf, D.J., and Clark, J.H., 1989. Effects of periled fatty acids and calcium salts of fatty acids on rumen fermentation, nutrient digestibilities, milk production, and milk composition. J. Dairy Sci., 72: 917-927. https://doi.org/10.3168/jds.S0022-0302(89)79185-2
Sun, S., Hou, Z., Dai, Q., and Wu, D., 2019. Effects of the forage type and chop length of ramie silage on the composition of ruminal microbiota in black goats. J. Anim., 9: 177-187. https://doi.org/10.3390/ani9040177
Szumacher-Strabel, M., Potkański, A., Kowalczyk, J., Cieślak, A., Czauderna, M., Gubała, A., and Jędroszkowiak, P., 2002. The influence of supplemental fat on rumen volatile fatty acid profile, ammonia and pH levels in sheep fed a standard diet. J. Anim. Feed Sci., 11: 577-587. https://doi.org/10.22358/jafs/67912/2002
Szumacher-Strabel, M., and Cielak, A., 2012. Dietary possibilities to mitigate rumen methane and ammonia production. In: Greenhouse gases capturing, utilization and reduction (ed., G Li)u. Intech Open, Poland. https://doi.org/10.5772/32105
Szumacher-Strabel, M. and Cieslak, A., 2010. Potential of phytofactors to mitigate rumen ammonia and methane production. J. Anim. Feed Sci., 19: 319-337. https://doi.org/10.22358/jafs/66296/2010
Szumacher-Strabel, M., Martin, S.A., Potkański, A., Cielak, A. and Kowalczyk, J., 2004. Changes in fermentation processes as the effect of vegetable oil supplementation in in vitro studies. J. Anim. Feed Sci., 13: 215-218. https://doi.org/10.22358/jafs/73843/2004
Täckholm, V., 1974. Student’s flora of Egypt. Cairo Univ. Pub., Cairo, Egypt, pp. 888.
Taha, E.A. and Khidr, R.E., 2011. Rangeland management and animal production sustainability under arid and semiarid conditions: Egypt overview. In: Economic, social and environmental sustainability in sheep and goat production systems (eds. A. Bernués, J.P. Boutonnet, I. Casasús, M. Chentouf, D. Gabiña, M. Joy, A. López-Francos, P. Morand-Fehr, F. Pacheco). pp. 317-322.
Van Duivenbooden, N., 1993. Grazing as a tool for rangeland management in semiarid regions: A case study in the north-western coastal zone of Egypt. J. Agric. Ecol. Environ., 43: 309-324. https://doi.org/10.1016/0167-8809(93)90094-6
Williams, B.A., 2000. Cumulative gas production techniques for forage evaluation. In: Forage evaluation in ruminant nutrition (eds. D.I. Givens, E. Owen, H.M. Omed and R.F.E. Axford). CAB International, Wallingford, pp. 189-213. https://doi.org/10.1079/9780851993447.0189
Yulistiani, D., Puastuti, W. and Widiawati, Y., 2016. In vitro digestibility and rumen fermentation of grass or rice straw basal diet with or without complete rumen modifier supplementation. Proc. Int. Sem. Livest. Prod. Vet. Technol., pp. 310-317. https://doi.org/10.14334/Proc.Intsem.LPVT-2016-p.310-317
Zmora, P., Cieslak, A., Pers-Kamczyc, E., Nowak, A., Szczechowiak, J. and Szumacher-Strabel, M., 2012. Effect of Mentha piperita L. on in vitro rumen methanogens and fermentation. Acta Agric. Scand. Sect., 62: 46-52. https://doi.org/10.1080/09064702.2012.703228
To share on other social networks, click on any share button. What are these?










