Growth Performance and Morphology Traits Associated with Neuropeptide Y (NPY) Genes Expression in Native Chickens
Research Article
Growth Performance and Morphology Traits Associated with Neuropeptide Y (NPY) Genes Expression in Native Chickens
Jennarong Kammongkun1, Doungnapa Promket2*
1Bureau of Animal Husbandry and Genetic Improvement, Department of Livestock, Development, Pathum Thani, Thailand; 2Branch of Animal Science, Department of Agricultural Technology, Faculty of technology, Mahasarakham University, Mahasarakham, Thailand.
Abstract | This study investigates the association between neuropeptide Y (NPY) gene expression and growth performance and morphology traits in 281 PH chickens using PCR-RFLP genotyping. The analysis revealed significant associations between growth performance and morphology traits in PH chickens. The correlations between average daily gains (ADG4 to ADG16) and body weights (BW0 to BW16) ranged from 0.33 to 0.99, indicating a consistent upward trend over time. The high body weight (HBW) group exhibited superior growth performance and morphology traits compared to the low body weight (LBW) group (P<0.01), except for shank length (SL). Notably, NPY gene polymorphisms showed significant associations with growth performance and morphological traits (P<0.01). The Bb genotype was linked to higher body weight and average daily gain during weeks 4 to 16 compared to the bb genotype (P<0.01). This suggests an influence of the genotype on these traits. Additionally, NPY polymorphism affected body length (BL), with Bb genotype (21.10 cm) showed significantly higher BL values compared to the BB genotype (20.57 cm). These findings highlight the potential role of NPY gene variants in influencing growth performance and morphology traits in PH chickens, providing important genetic markers for selective breeding programs. Incorporating NPY polymorphisms into breeding strategies could boost productivity and efficiency in poultry farming. Future research should explore additional genetic factors influencing these traits.
Keywords | Growth performance, Morphology, Gene marker, Native chickens, Candidate gene, Economic trait
Received | June 20, 2024; Accepted | August 18, 2024; Published | October 07, 2024
*Correspondence | Doungnapa Promket, Branch of Animal Science, Department of Agricultural Technology, Faculty of technology, Mahasarakham University, Mahasarakham, Thailand; Email: Napakran@hotmail.com
Citation | Kammongkun J, Promket D (2024). Growth performance and morphology traits associated with neuropeptide y (npy) genes expression in native chickens. Adv. Anim. Vet. Sci. 12(11): 2263-2274.
DOI | https://dx.doi.org/10.17582/journal.aavs/2024/12.11.2263.2274
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright: 2024 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
INTRODUCTION
The demand for animal protein, particularly chicken meat, is expected to increase significantly by 2050 when the global population is projected to surpass 9.7 billion. Of all the meat produced worldwide, approximately 36% comes from poultry, with 89% of that production being chicken. Over 136.5 million tons of poultry meat were produced worldwide in 2020, a 2.4% rise from the previous year (Dorota and Joanna, 2021). Chickens, which have 39 pairs of chromosomes and a substantial number of genes. The haploid chicken genome, approximately 40% smaller than that of mammals, consists of 1.2 x 109 base pairs and an estimated 20,000 to 23,000 genes lower than in mammalian genomes (Furlong, 2005; Wang et al., 2024). Despite this, numerous genes have been mapped to chicken chromosomes, allowing comparative genomics to reveal insights into genetic functions and evolutionary relationships across species. Understanding the genetic architecture of chickens is crucial for optimizing breeding strategies aimed at enhancing growth performance, disease resistance, and meat quality, thus ensuring sustainable poultry production to meet the rising consumer demand. Compared to broiler chicken, indigenous chickens are less productive but are more suited to the harsh tropical climate and shifting nutritional requirements. However, the taste, leanness, firmness, high protein content, and appropriateness for certain meals may account for the preferences for native chicken raised on free range (Rashid et al., 2020).
Pradu Hangdum (PH) chicken, it is one of the native chicken breeds of Thailand, which is well-liked by farmers. From 2002 until now, the Department of Livestock Development (DLD) has been gathering PH chickens from all across the country to be employed in selective breeding and herd development. The selection of qualitative characteristics is the main goal of the first phase, focusing on choosing phenotype traits that align with the characteristics of the PH species as described in the Biodiversity research project of the DLD. The result is a flock of PH hens that are at least 85% uniform in appearance and have characteristics consistent with the breed. As a result, the DLD has registered the breed of chicken. dated December 9, 2010, under the name “Pradu Hangdum Chiang Mai chicken,” registration number CND 01/2010. In later stages of breeding, PH chickens with higher growth were chosen based on quantitative characteristic information. Resulting in a body weight that was, at 12 and 16 weeks of age, 33% and 36% more than other native chickens, respectively. The benefits of PH are well known for their delicious meat, low fat content, and illness resistance. Their phenotypic features included a red face, tiny pea-sized comb, pale yellow skin, and black feathers. Thai native chickens are quite popular with customers (Mookprom et al., 2017). Because its flesh is of higher quality and has less fat, it also sells for more money than industrial chicken. Consumers preference for PH chicken depends on its good meat quality (Rueangwittayanusorn et al., 2022).
Growth performance traits are among the most significant economic characteristics in chicken production, as they directly impact profitability and efficiency (Chomchuen et al., 2022). These traits are regulated by multiple genes and involve complex neuroendocrine pathways, making them quantitative in nature and challenging to enhance through conventional selection methods. Traditional breeding techniques often lead to slow and incremental genetic improvements due to the intricate interplay of numerous genetic factors influencing growth (Padwar and Thakur, 2021). With the increasing understanding of the chicken genome, it is now possible to identify causal genes and develop new molecular markers that are selective for desired traits, facilitating more precise and rapid genetic improvements. Molecular breeding approaches, which leverage genomic information, are essential for overcoming the limitations of conventional selection, enabling the efficient enhancement of growth traits and ensuring sustainable advancements in poultry production (Xu et al., 2020; Gheyas et al., 2021).
Morphological traits in chickens, such as body size, feather pattern, and skeletal structure, are not only vital for breed identification and aesthetic value but also have significant implications for growth performance and overall productivity. These traits are often correlated with key economic factors, including feed efficiency, meat yield, and health status, making them critical indicators in poultry breeding programs (Sam et al., 2019). The relationship between morphology and growth traits is complex, as both are influenced by genetic and environmental factors that determine the physical and physiological development of chickens (Vilakazi et al., 2020; Bila and Tyasi. 2022). Understanding this relationship can help in developing more effective selection strategies to enhance growth performance, thereby improving the efficiency and sustainability of poultry production. A comparative analysis of the morphological characteristics of PH chickens with other indigenous breeds reveals several unique and advantageous traits. PH chickens are known for their robust body structure, which includes a well-developed breast and substantial body weight, distinguishing them from other native breeds such as Chee chickens. While Chee for its resilience in harsh environments, PH chickens excel in growth performance and meat quality. This genetic advantage not only enhances their productivity but also positions PH chickens as a valuable resource in selective breeding programs aimed at optimizing growth performance and morphological traits.
One option that may have long lasting impacts on the poultry industry is the genetic selection method of growth performance using molecular approaches. To quickly enhance growth performance and meet the significant market demand for increased production, it is imperative to study native chicken breeds, such as PH chickens, utilizing molecular marker techniques. Promising approaches for genetic enhancement in breeding programs are provided by molecular techniques, such as genetic marker approaches and molecular technologies (Promket et al., 2023; Promket et al., 2024). The utilization of marker assisted selection (MAS) to modify the genetic composition of native chickens and enhance their developmental potential is made possible by the identification of polymorphisms and DNA markers associated with growth performance attributes (Piorkowska et al., 2020; Mastrangelo et al., 2023). By incorporating these molecular approaches, breeders can achieve more precise and rapid genetic improvements, ensuring sustainable advancements in poultry production to fulfill rising consumer demands.
The neuropeptide Y (NPY) gene is one of the significant genetic markers for growth performance in chickens. It plays a crucial role in regulating gonadal activity, feeding behavior, and insulin secretion. Studies have shown that NPY injections can alter plasma levels of prolactin, luteinizing hormone, growth hormone, vasopressin, and thyrotropin. Moreover, NPY influences food intake and sexual development, affecting ovulation and linking to the age at first egg, potentially increasing the rate of egg production in hens (Sartsoongnoen et al., 2021). Given these extensive physiological functions, the NPY gene holds promise for improving growth and reproductive traits through genetic selection. If gene markers for economically important traits are identified, information on marker genotypes can be utilized to selectively breed chickens with desirable characteristics (Zubaidi et al., 2023). Therefore, understanding the genetic basis of growth traits is vital for advancing breeding programs and enhancing productivity in the livestock industry. The genetic underpinnings of growth traits and morphological characteristics in poultry are critical for understanding and improving breeding programs. NPY gene polymorphisms, in particular, play a significant role in regulating growth performance and morphological traits. Genetic variations in the NPY gene can influence feeding behavior, metabolic processes, and overall growth efficiency, making it a relevant candidate for genetic studies.
The NPY gene has been shown in previous studies to be a strong contender for regulating growth performance traits in hens. However, it remains unknown whether nucleotide polymorphisms in the NPY gene are associated with variables affecting the growth performance of PH chickens. This gap in knowledge underscores the need for further investigation to elucidate the genetic underpinnings of growth traits specific to this breed. Additionally, the relationship between NPY polymorphisms and morphological traits in PH chickens has not been thoroughly explored. The study of NPY polymorphism in PH chickens is crucial because it can provide insights into the genetic factors that influence growth performance and morphological traits, which are essential for selective breeding. Understanding these genetic variations can help optimize breeding strategies, leading to improved productivity and sustainability in poultry farming. Furthermore, investigating NPY gene polymorphisms in PH chickens can contribute to the broader field of avian genetics and enhance our knowledge of genetic determinants in indigenous chicken breeds. Therefore, the aim of this study is to identify polymorphisms in the NPY gene and examine their relationship to both growth performance and morphological traits in PH chickens. By addressing this gap, the research aims to improve our comprehension of the genetic characteristics that influence growth and morphology, which will help guide selective breeding strategies to enhance production efficiency and effectiveness.
MATERIALS AND METHODS
Animals Management and Data
The Chiangmai Livestock Research and Breeding Center, located in Thailand, Chiangmai district, provided the data from 281 PH. Every chicken utilized in this research was a female breeder chicken. Growth performance data were collected from birth until 16 weeks of age. The area experiences have hot and humid weather during the summer season, with temperatures ranging from 21.3°C to 32.5°C and humidity averaging around 52%. PH chickens resided in an open-air living arrangement. Individual chicken was identified by their wing bands. After a 21-day incubation period, the chicks will be raised in captivity at a density of 8 birds per square meter until they are 18 weeks old. After that, move them to separate battery cages. The PH chickens were kept under controlled lighting conditions, with 16 hours of light and 8 hours of darkness every day, in order to promote photo responsiveness. Each open pen (8 m wide x 8 m deep x 16 m high) contained one raised chicken, a room temperature atmosphere, and tools that make exact care and monitoring possible which met or exceeded Good Agricultural Practices for chicken farms recommendations. The chickens were given a commercial meal (pelleted) containing 19% crude protein (CP) or 3,200 kcal from birth to five weeks of age; from six to twelve weeks, they were given 15% CP (3,200 kcal ME/kg); and from thirteen to sixteen weeks, they were given 13% CP (3,200 kcal ME/kg). Water and food were available to the chickens at all times during the experiment (ad libitum). Following the widely used chicken vaccination methods, the farm veterinarian conducted the immunization regimen. Standard operating procedures of the Thai Agricultural Standard were followed in the provision of the management and feeding program. All procedures involving animals in this study were conducted in accordance with ethical standards and were approved by The Institutional Animal Care and Use Committee (IACUC) of Mahasarakham University in Thailand (IACUC-MSU-9/2023).
The growth performance of the PH chickens was evaluated according to standardized procedures by weighing each chicken (n = 281) using a calibrated scale by recording birth weight (BW0) and body weight at 4, 8, 12, and 16 weeks of age (BW4, BW8, BW12, and BW16, respectively) to ensure consistency and reliability in performance evaluation. These measurements were recorded in individual records kept for performance analysis. To assess the average daily gain (ADG) at different stages of growth, the following formula was used:
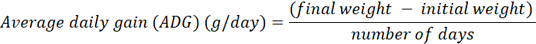
Based on these measurements, ADG at different intervals was calculated: ADG at 0-4 weeks (ADG4), ADG at 4-8 weeks (ADG8), ADG at 8-12 weeks (ADG12), and ADG at 12-16 weeks (ADG16). These values were used to analyze the growth performance of the chickens over specific time periods.
At one year of age, each chicken underwent six morphological evaluations, including breast width (BrW), breast length (BrL), shank length (SL), body length (BL), height toe to back (HB), and height toe to comb (HC). A Vernier caliper was used to measure six morphological characteristics. The measurements were conducted using standardized techniques to ensure accuracy and consistency following FAO’s descriptor for the characterization of chicken genetic resources (FAO, 2012) (Figure 1).
At year weight, the PH chickens were divided into two groups: low body weight (LBW) and high body weight (HBW). PH chickens from the 25 percent (Q1) were classified as belonging to the low body weight group (LBW), whereas those from the 95 percent were classified as belonging to the high body weight group (HBW). To compare the body weight and genotype distribution of the LBW and HBW populations.
Sample Collection and DNA Extraction
Extraction of DNA from the wing veins, 1.5 mL microtubes containing 100 μL of 0.5 M ethylenediaminetetraacetic acid (EDTA) were filled with each 1 mL of blood sample in order to prevent blood clotting. Using the guanidine hydrochloride method, genomic DNA was isolated from whole blood samples, as reported by Goodwin et al. (2011). The blood samples were treated with cell lysis buffer and protein precipitation. After that, they were centrifuged for five minutes at 10,000 rpm and 4°C, which separated the supernatant. After carefully transferring the supernatant to new 1.5 mL microtubes, 100% isopropanol was added to help in the precipitation of DNA. The DNA was then precipitated for five minutes at 10,000 rpm and 4°C. Using 75% ethanol, two rounds of DNA pellet washing were carried out. A Thermo Scientific (USA) Nanodrop 2000c Spectrophotometer was used to measure the genomic DNA volume and purity. After that, the isolated DNA was kept at -20°C.
Markers and Genotyping by PCR-RFLP Procedure
The NPY polymorphisms of PH, which were genotyped using PCR-RFLP, were used as markers to assess the effects on growth performance and morphological traits. A total reaction volume of 10 μL was used for each genotyping experiment using the polymerase chain reaction (PCR). The following elements made up the reaction mixture: 1 μL of 10X PCR buffer, 1 μL of 1 mM dNTPs, 1 μL of each 5 mM primer, 0.8 μL of 50 mM MgCl2, 0.1 μL of Taq DNA polymerase from Promega (San Diego, CA), 4.1 μL of nuclease-free water, and 1 μL of genomic DNA at a concentration of 50 ng/mL were used. The gene specific primer
(Forward primer- 5-TCTCAGAGCTCCAACGTATGA-3; Reverse primer: 5- ATATTTCTGTGCCTGAACAACA -3) was used to amplify the region of the NPY gene. The NPY gene is located on chromosome 2 (gene ID 396464) and type of polymorphism 4 bp deletion at position 494-499.
A thermal cycler (iCycler thermal cycler, BioLab, USA; Corbett Research, Australia 2003) was used to amplify PCR. A pre-denaturation stage at 94 °C was followed by a 5-minute incubation period. Following this, thirty seconds of denaturation at 94 °C, forty seconds of annealing at 60 °C, thirty seconds of extension at 72 °C, and five minutes of final extension at 72 °C were all completed in 35 cycles. The results of the PCR were kept cold (4°C) for additional examination.
The PCR product of NPY gene was evaluated by electrophoresis on a 2% agarose gel. Following forty minutes of electrophoresis at 100 V, the gel was dyed for ten minutes using GELSTARTM (Gelstar Inc., NY). Following that, the PCR products were treated to an overnight digestion using the DraI enzyme at 37 °C. After that, restriction patterns were seen using 2.5% agarose gel electrophoresis at 100 V 45 min, GELSTARTM staining (Gelstar Inc., NY), and Gel Documentation equipment (Lab Focus, Inc.). This technique made it possible to accurately identify genotypes.
Statistical Analysis
With SAS (Statistical Analysis System, Version 9.4, 2019), all growth performance and morphological trait variables in all PH chickens (n=281) were examined using Proc means. The mean, standard deviation (SD), minimum (Min), maximum (Max), and coefficient of variation (CV) of the data were provided. In addition, Pearson’s correlation coefficients among growth performance and morphological parameters were also calculated using Proc corr (SAS, 2019).
Based on the YW trait, the chickens were split into two groups (LBW and HBW), and the means were analyzed using t-tests to determine the significance of the differences between the LBW and HBW groups. A statistical difference was considered significant if its P-value was less than 0.05. In SAS software version 9.4.
Analysis of Marker and Traits Associations
Gene genotype and allele frequencies were found at each locus. Hardy-Weinberg Equilibrium (HWE) and polymorphism information content (PIC) were tested using chi-square (χ2) (Falconer and Mackay, 1996).
The connections between genotype and growth performance and morphological characteristics were examined using the least-squares approach (GLM Procedure, Statistical Analysis System, Version 9.4, 2019). GLM assumptions include that the response variable has an exponential family distribution, the link function accurately connects the response mean to the linear predictor, and the observations are independent. Regarding the model that was applied to the data analysis, the following assumptions were made:
Yij= µ + Gi + eij
Where;
Yij: the PH chicken characteristics.
µ: population mean values for the characteristics.
Gi: fixed effects related to the genotype of NPY.
eij: residual error.
RESULTS AND DISCUSSION
Growth Performance and Morphology Traits
Table 1 shows the growth performance summary (BW0, BW4, BW8, BW12, BW16, ADG4, ADG8, ADG12, and ADG16) as well as the morphological features summary (BrW, BrL, SL, BL, HB, and HC). The dataset presents the mean values, standard deviations (SD), minimum (Min), maximum (Max), and coefficient of variation (CV) for these traits. For body weights from BW0 to BW16, the mean and standard deviations range from 29.54 ± 3.39 g to 1,485.70 ± 127.10 g, reflecting the various stages of growth. The average daily gains (ADG) show mean values of 9.38 g/day (ADG4), 13.72 g/day (ADG8), 13.99 g/day (ADG12), and 13.00 g/day (ADG16), indicating the rate of weight increase over time. These data points provide a clear understanding of the growth patterns and physical development of the PH chickens throughout the study period.
The means of breast width (BrW), breast length (BrL), shank length (SL), and body length (BL) were 7.45 cm, 12.99 cm, 7.70 cm, and 20.80 cm, respectively, according to descriptive statistics of morphological parameters. When the average was divided by the standard deviation to get the CV, the results showed that BrW had a 11.46 % CV and BrL had 7.41 % CV.
Table 1: Descriptive statistics of growth performance and morphology traits for PH chickens.
|
Traits |
N |
Mean |
SD |
Min |
Max |
CV (%) |
|
Growth performance |
||||||
|
BW0, g |
281 |
29.54 |
3.39 |
21.00 |
39.20 |
11.47 |
|
BW4, g |
281 |
292.40 |
40.78 |
160.00 |
406.00 |
13.94 |
|
BW8, g |
281 |
797.90 |
77.60 |
579.00 |
1,004.00 |
9.72 |
|
BW12, g |
281 |
1,205.20 |
112.50 |
895.00 |
1,464.00 |
9.25 |
|
BW16, g |
281 |
1,485.70 |
127.10 |
1,100.00 |
1,800.00 |
8.55 |
|
ADG4, g/d |
281 |
9.38 |
1.41 |
4.68 |
13.29 |
15.02 |
|
ADG8, g/d |
281 |
13.72 |
1.36 |
9.85 |
17.38 |
9.94 |
|
ADG12, g/d |
281 |
13.99 |
1.31 |
10.33 |
17.11 |
9.39 |
|
ADG16, g/d |
281 |
13.00 |
1.12 |
9.56 |
15.80 |
8.64 |
|
Morphology |
||||||
|
BrW, cm |
279 |
7.45 |
0.85 |
5.00 |
9.90 |
11.46 |
|
BrL, cm |
279 |
12.99 |
0.96 |
10.30 |
15.30 |
7.41 |
|
SL, cm |
279 |
7.70 |
0.76 |
5.70 |
11.50 |
9.92 |
|
BL, cm |
279 |
20.80 |
1.31 |
18.00 |
25.00 |
6.31 |
|
HB, cm |
279 |
31.43 |
1.57 |
25.00 |
35.00 |
5.00 |
|
HC, cm |
279 |
49.68 |
2.37 |
40.00 |
55.00 |
4.77 |
BW0 is birth weight, BW4 is body weight at 4 weeks; BW8 is body weight at 8 weeks; BW12 is body weight at 12 weeks; BW16 is body weight at 16 weeks; ADG4 is ADG at 0-4 weeks; ADG8 is ADG at 4-8 weeks; ADG12 is ADG at 8-12 weeks; ADG16 is ADG at 12-16 weeks; BrW is breast width; BrL is breast length; SL is shank length; BL is body length; HB is height toe to back; HC is height toe to comb.
Based on their weight at one year of age, the chickens were divided into two groups: LBW (n=73) and HBW (n=73). The development performance and morphological features of the PH chickens in the LBW and HBW groups are displayed in Table 2. A comparison of the morphology and growth performance between the two groups revealed that the HBW group had significantly higher BW0–BW16 values than the LBW group (P < 0.01). Similarly, there was a highly significant difference (P < 0.01) in ADG4–ADG16 levels between the HBW and LBW groups.
Furthermore, the morphology traits (BrW, BrL, BL, HB, and HC) in the HBW group were higher than in the LBW group (P < 0.01), with the exception of the SL trait, which did not differ between the two comparison groups (P > 0.05). The means ± SE of BrW, BrL, BL, HB, and HC for the PH chickens in the HBW group were 7.88±0.08 cm, 13.31±0.09 cm, 21.53±0.15 cm, 32.17±0.16 cm, and 50.70±0.24 cm, respectively. Furthermore, Table 2 shows that the means ± SE of BrW, BrL, BL, HB, and HC for the chickens in the LBW group were 7.11±0.09 cm, 12.49±0.11 cm, 20.27±0.13 cm, 30.67±0.16 cm, and 48.54±0.28 cm, respectively.
Table 2: Growth performance and morphology traits of PH for LBW and HBW groups.
|
Traits |
LBW |
HBW |
P-Value |
||
|
Means |
SE |
Means |
SE |
||
|
Growth performance |
|||||
|
BW0, g |
28.89 |
0.36 |
29.99 |
0.37 |
0.03 |
|
BW4, g |
282.83 |
4.43 |
303.49 |
4.92 |
0.002 |
|
BW8, g |
765.36 |
9.18 |
827.69 |
9.35 |
0.001 |
|
BW12, g |
1,154.19 |
13.42 |
1,256.91 |
13.74 |
0.001 |
|
BW16, g |
1,421.53 |
16.03 |
1,548.43 |
15.26 |
0.001 |
|
ADG4, g/d |
9.06 |
0.15 |
9.76 |
0.17 |
0.003 |
|
ADG8, g/d |
13.15 |
0.16 |
14.24 |
0.16 |
0.001 |
|
ADG12, g/d |
13.39 |
0.15 |
14.60 |
0.16 |
0.001 |
|
ADG16, g/d |
12.43 |
0.14 |
13.55 |
0.13 |
0.001 |
|
Morphology |
|||||
|
Brw, cm |
7.11 |
0.09 |
7.88 |
0.08 |
0.001 |
|
BrL, cm |
12.49 |
0.11 |
13.31 |
0.09 |
0.001 |
|
SL, cm |
7.60 |
0.10 |
7.85 |
0.08 |
0.06 |
|
BL, cm |
20.27 |
0.13 |
21.53 |
0.15 |
0.001 |
|
HB, cm |
30.67 |
0.16 |
32.17 |
0.06 |
0.001 |
|
HC, cm |
48.54 |
0.28 |
50.70 |
0.24 |
0.001 |
BW0 is birth weight; BW4 is body weight at 4 weeks; BW8 is body weight at 8 weeks; BW12 is body weight at 12 weeks; BW16 is body weight at 16 weeks; ADG4 is ADG at 0-4 weeks; ADG8 is ADG at 4-8 weeks; ADG12 is ADG at 8-12 weeks; ADG16 is ADG at 12-16 weeks; BrW is breast width; BrL is breast length; SL is shank length; BL is body length; HB is height toe to back and HC is height toe to comb; * is significant difference (P<0.05); ** is extremely significant difference (P < 0.01); ns is non-significant difference (P>0.05).
Phenotypic Correlations Amongst Growth Performance and Morphometric Traits in PH Chicken
Table 3 presents the correlation coefficients between growth performance and morphological traits in PH chickens. The correlations between body weights at different ages at week (BW0-BW16) ranged from 0.31 to 0.77, indicating a moderate positive correlation. This moderate positive relationship suggests that body weight measurements over time are consistently related, though not strongly, to one another. Additionally, the correlations between average daily gain (ADG) from ADG4 to ADG16 showed moderate to high positive correlations, ranging from 0.50 to 0.76. This indicates a stronger positive relationship between the growth rates over successive periods. In contrast, the correlations between growth performance traits and morphological characteristics were low, ranging from low to moderate levels. These findings suggest that while morphological traits have some predictive value for growth performance, their predictive power is limited. Correlation between growth performance and morphological traits, allowing for the selection of chickens with both superior growth and optimal morphological features. The moderate correlations between weight measures and growth rates highlight the potential of using these traits in selective breeding programs, although the lower correlations with morphological traits suggest that additional factors must be considered for comprehensive breeding strategies.
Sequence Variation and PCR-RFLP Analysis
The amplified PCR product of NPY gene in PH chicken, with PCR product sizes of 240 bp. Genotype pattern when cut with the restriction enzyme (DraI) exhibited 3 genotypes: genotype BB (240 bp), genotype Bb (240 bp, 161 bp, and 79 bp), and genotype bb (161 bp and 79 bp). Genotype frequencies and gene frequency values were calculated according to Falconer’s principle. Table 4 showed gene frequencies of gene B (0.28) lower than gene b (0.72). Genotype frequencies for Bb and BB were 0.30 and 0.13, respectively, the most common genotype for NPY was bb (0.57). Additionally, tests for HWE showed that the NPY genes did not meet the equilibrium assumption and did not adhere to HWE. The deviation from Hardy-Weinberg Equilibrium (HWE) indicates that the genetic structure of the population may be influenced by evolutionary factors or selective breeding practices. Therefore, the NPY gene not adhering to HWE suggests that there are evolutionary pressures or selective breeding strategies affecting the genetic makeup of the PH chicken population. This could include factors such as non-random mating or genetic drift. For the calculated PIC value of NPY gene was 0.32. According to these results, the NPY gene variation in PH chickens is moderate levels (Table 4). Polymorphism Information Content (PIC) is a measure used in genetics to quantify the informativeness of a genetic marker for linkage and association studies. It reflects the degree of polymorphism within a population by considering both the number and frequency of alleles. A higher PIC value indicates a more informative and diverse marker, while a lower value suggests less diversity and informativeness. PIC values can range from 0 (no polymorphism) to 1 (highly polymorphic).
Table 3: Pearson correlation coefficient among growth performance and morphology traits in PH chickens.
|
traits |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
13 |
14 |
15 |
|
Growth |
|||||||||||||||
|
1_BW0 |
1.00 |
0.41* |
0.37** |
0.31** |
0.37** |
0.34** |
0.33** |
0.28** |
0.35** |
0.20** |
0.14* |
-0.12* |
0.28** |
0.09ns |
0.04 ns |
|
2_BW4 |
1.00 |
0.67** |
0.55** |
0.52** |
0.99** |
0.66** |
0.54** |
0.52** |
0.23** |
0.11 ns |
-0.15* |
0.29** |
0.14* |
0.02 ns |
|
|
3_BW8 |
1.00 |
0.77** |
0.63** |
0.66** |
0.99** |
0.76** |
0.63** |
0.30** |
0.14* |
-0.08 ns |
0.32** |
0.11 ns |
0.09 ns |
||
|
4_BW12 |
1.00 |
0.65** |
0.54** |
0.76** |
0.99** |
0.65** |
0.27** |
0.18** |
-0.10 ns |
0.38** |
0.09 ns |
0.03 ns |
|||
|
8_BW16 |
1.00 |
0.51** |
0.63** |
0.64** |
0.99** |
0.21** |
0.20** |
-0.02 ns |
0.32** |
0.16* |
0.14* |
||||
|
6_ADG4 |
1.00 |
0.65** |
0.53** |
0.50** |
0.22** |
0.10 ns |
-0.15* |
0.28** |
0.14* |
0.02 ns |
|||||
|
7_ADG8 |
1.00 |
0.76** |
0.62** |
0.30** |
0.13* |
-0.08 ns |
0.31** |
0.11 ns |
0.08 ns |
||||||
|
8_ADG12 |
1.00 |
0.64** |
0.26** |
0.18** |
-0.10 ns |
0.37** |
0.09 ns |
0.02 ns |
|||||||
|
9_ADG16 |
1.00 |
0.21** |
0.20** |
-0.02 ns |
0.31** |
0.16* |
0.14* |
||||||||
|
Morphology |
|||||||||||||||
|
10_Bre_W |
1.00 |
0.28** |
0.13* |
0.31** |
0.09 ns |
0.08 ns |
|||||||||
|
11_Bre_L |
1.00 |
-0.04 ns |
0.36** |
0.11 ns |
0.06 ns |
||||||||||
|
12_Shank_L |
1.00 |
-0.27* |
0.18** |
0.28** |
|||||||||||
|
13_Body_L |
1.00 |
0.17** |
0.11 ns |
||||||||||||
|
14_Hight1 |
1.00 |
0.71** |
|||||||||||||
|
15_Hight2 |
1.00 |
Table 4: Genotype and gene frequencies for polymorphisms.
|
Gene |
N |
Genotype frequency |
gene frequency |
Chi-Square |
PIC |
|||
|
BB |
Bb |
bb |
B |
b |
||||
|
NPY |
1.00 |
0.13 |
0.30 |
0.57 |
0.28 |
0.72 |
19.08 |
0.32 |
|
281 |
36 |
83 |
162 |
|||||
Association of NPY Haplotypes with Growth Performance and Morphology Traits in PH Chickens
The investigation of the relationships between polymorphisms in the candidate gene NPY and various growth performance and morphological traits in PH chickens revealed significant associations (Table 5). Notably, NPY gene polymorphisms were significantly associated with key growth performance parameters, including BW4, BW8, BW12, and BW16, as well as ADG4, ADG8, ADG12, and ADG16. The highly significant (P < 0.01) associations between NPY gene polymorphisms and BW12 and ADG12 were particularly noteworthy. It’s interesting to note that the Bb genotypes were associated with higher values of BW12 and ADG12 (1,245.58 g and 14.47 g/d, respectively) in comparison to the bb genotype (1,183.83 g and 13.74 g/d, respectively). However, in terms of BW12 and ADG12, there was no statistically significant difference between the chickens with the Bb and BB genotypes. In particular, PH chickens with the BB genotype in the NPY gene showed higher values for BW4 and ADG4 than PH chickens with the bb genotype, although there was no statistical difference between the BB and Bb genotype. In addition, there was no statistically significant difference in BW8, BW16, ADG8 and ADG16 between the Bb genotypes (815.67 g, 1,519.40 g, 14.03 g/d and 13.30 g/d, respectively) and the BB genotype (805.21 g, 1,496.67 g, 13.85 g/d and 13.10 g/d, respectively). Additionally, NPY polymorphism has been found to have effects on only the BL trait of PH chickens (P < 0.05). The mean BL value for PH chickens with the Bb genotype (21.10) and bb genotype (20.69) was significantly higher than that of PH chickens with the BB genotype (20.57) (P < 0.05).
Native chickens play a crucial role in sustainable agriculture, biodiversity conservation, and cultural heritage preservation. These indigenous breeds possess unique genetic traits adapted to local environments, making them resilient to diseases, climate variations, and resource constraints (Wondmeneh et al., 2021). Additionally, native chickens often exhibit L-carnitine content, superior taste, texture, and nutritional quality compared to commercial breeds, contributing to their growing popularity among consumers seeking organic and traditional food options (Lengkidworraphiphat et al., 2021). Moreover, the production and consumption of native chicken meat and eggs support local economies, empower smallholder farmers, and foster community resilience against external market fluctuations and food insecurity (Adoligbe et al., 2020). Consumer preferences for native chicken products stem from their perceived authenticity and sustainable production practices, driving demand in both domestic and international markets. Thus, investing in the conservation and promotion of native chicken breeds is not only essential for preserving genetic diversity but also for promoting food security, rural livelihoods, and cultural heritage preservation.
Table 5: Least square mean of growth performance and morphology traits by genotype of NPY genes in PH chickens.
|
Trait |
Genotype |
P-value |
||
|
Growth performance |
BB |
Bb |
bb |
|
|
BW0, g |
29.35 |
29.92 |
29.40 |
ns |
|
BW4, g |
301.23a |
299.92 ab |
286.73 b |
* |
|
BW8, g |
805.21ab |
815.67a |
789.49b |
* |
|
BW12, g |
1,213.75AB |
1,245.58A |
1,183.83 B |
** |
|
BW16, g |
1,496.67 ab |
1,519.40 a |
1,467.38 b |
* |
|
ADG4, g/d |
9.71 a |
9.64 ab |
9.19 b |
* |
|
ADG8, g/d |
13.85 ab |
14.03 a |
13.53 b |
* |
|
ADG12, g/d |
14.10 AB |
14.47 A |
13.74 B |
** |
|
ADG16, g/d |
13.10 ab |
13.30 a |
12.84 b |
* |
|
Morphology |
||||
|
Brw, cm |
7.28 |
7.61 |
7.40 |
ns |
|
BrL, cm |
12.69 |
13.10 |
13.00 |
ns |
|
SL, cm |
7.75 |
7.69 |
7.69 |
ns |
|
BL, cm |
20.57 b |
21.10a |
20.69ab |
* |
|
HB, cm |
31.45 |
31.31 |
31.49 |
ns |
|
HC, cm |
49.94 |
49.16 |
49.89 |
ns |
BW0 is birth weight, BW4 is body weight at 4 weeks; BW8 is body weight at 8 weeks; BW12 is body weight at 12 weeks; BW16 is body weight at 16 weeks; ADG4 is ADG at 0-4 weeks; ADG8 is ADG at 4-8 weeks; ADG12 is ADG at 8-12 weeks; ADG16 is ADG at 12-16 weeks; BrW is breast width, BrL is breast length; SL is shank length; BL is body length; HB is height toe to back and HC is height toe to comb; * is significant difference (P<0.05); ** is extremely significant difference (P < 0.01); ns is non-significant difference (P>0.05).
The growth characteristics of chickens are of economic importance traits, influencing meat production efficiency and profitability (Promket et al., 2021; Chomchuen et al., 2022). Traits such as average daily gain, feed conversion ratio, body weight and growth rate are critical economic indicators, and their enhancement through selective breeding can significantly boost productivity. Heritability value of growth traits in chickens are generally moderate to high (0.32 – 0.42), indicating a strong genetic component and the potential for substantial genetic gains through selection (Chen et al., 2021; Haunshi et al., 2022). Selective breeding programs targeting these traits in native chicken breeds can lead to improved growth performance while preserving their adaptability and resilience to local conditions (Sapkota et al., 2020).
This study found that the correlation between body weight (BW0 to BW16) and the BL trait ranged from 0.28 to 0.38, while the correlation between ADG4 - ADG16 and the BL trait ranged from 0.28 to 0.37. These moderate correlations are consistent with findings from several other studies. This indicates a consistent relationship between these growth performance and morphological traits in chickens. The moderate correlation suggests that while BL is a useful indicator, it should be used in conjunction with other traits for more effective selection in breeding programs. The morphological characteristics of indigenous chickens, such as body size, wing shape, leg structure, breast girth, keel length, shank length, and body length, exhibit a significant correlation with their growth traits, weight gain rate and carcass traits. Research indicates that these physical attributes can effectively indicate the growth potential of chickens. Selecting breeds based on morphological traits can be used as indirect selection for efficient, allowing to enhance the growth performance of indigenous chickens (Sam et al., 2019). The phenotypic characterization of native chickens makes it easier to combine advantageous and desired features with favorable conditions, enabling optimal growth and reproduction performance (Vilakazi et al., 2020). Another study by Bila and Tyasi (2022) used of multivariate principal component analysis (PCA) on the morphological characteristics of Ross 308 chickens demonstrates a clear relationship with body weight traits. Although studies have shown a correlation between morphological traits and growth performance in chickens, further research is needed in the PH chicken population to fully understand these relationships. Investigating how selective breeding can simultaneously optimize both morphological and performance traits is crucial. This approach can lead to the development of chicken breeds with enhanced growth efficiency and desirable physical characteristics.
Molecular breeding, particularly through marker assisted selection (MAS), offers significant advantages in improving growth traits of native chicken breeds. MAS utilizes molecular markers linked to desirable traits, allowing for the precise selection of individuals with superior genetic potential (Sharma et al., 2024). This method enhances the efficiency, reducing the generation interval by identifying and selecting for specific alleles associated with enhanced growth performance and accuracy of breeding programs compared to traditional selection methods (Budhlakoti et al., 2022). This study identified genetic markers influencing growth traits, aligning with research on Chinese native chicken populations. The research showed 14 SNP markers strongly associated with growth traits, indicating potential for enhanced selection and breeding strategies. The results underscore the importance of genetic markers in optimizing growth traits in poultry (Li et al., 2020). Furthermore, MAS for improving body weight in local chickens. In addition, it was also found that adaptive traits are maintained while improving economic performance (Helal and El-Gendy 2023; Mastrangelo et al., 2023).
This study examined the NPY gene using PCR-RFLP and discovered a 240 bp fragment with three distinct genotypes: BB (240 bp), Bb (240 bp, 161 bp, and 79 bp), and bb (161 bp and 79 bp). This indicates that the frequency of the b allele is higher than that of the B allele. The bb genotype predominance points to a possible selection advantage or past drift in favor of the b allele. This result is consistent with recent research showing the distribution of genotypes arising from the digestion of NPY gene in white Iraqi female chickens using the restriction enzyme (DraI). Moreover, the genotype frequency of the recessive homozygous genotype was higher than that of the dominant homozygous genotype of local white Iraqi chicken (Zubaidi et al., 2023). The predominance of the recessive homozygous genotype suggests potential implications for genetic diversity and breeding strategies. However, the study contradicts the findings of Padwar and Thakur (2021), which showed that genotype dominant homozygous was predominant in the Kadaknath and Jabalpur chicken populations (India native chickens) when exploring NPY gene polymorphism using PCR-RFLP assay. In contrast, our research revealed a higher frequency of the recessive homozygous genotype compared to the dominant homozygous form. This discrepancy highlights the variability in genetic distribution across different chicken breeds.
The research findings elucidating the relationship between the NPY gene and growth performance in PH chickens provide valuable insights into the genetic mechanisms underlying phenotypic traits. The observed association between the Bb genotype and higher body weight during weeks 8-16, as well as increased average daily gain (ADG8 - ADG16), but no significant difference with the BB genotype. According to earlier research, highlights the potential influence of the NPY gene on growth regulation in native Thai chicken (Sartsoongnoen et al., 2020; Padwar and Thakur, 2021). These results suggest that the Bb genotype may offer advantage in growth performance, emphasizing the importance of genetic markers in selective breeding programs aimed at improving growth traits in chickens.
One of the neuropeptides that is most widely expressed in the central and peripheral nervous systems is neuropeptide Y (NPY). In fact, it has been demonstrated that NPY regulates feed intake and energy homeostasis by stimulating appetite and feeding behavior in a variety of animals, including mice, rats, chickens, and rabbits. Recent research has revealed that the thyroid, heart, spleen, adrenal glands, white adipose tissue, muscle, and bone are among the peripheral tissues that release this neuropeptide and its receptors (Greene et al., 2022; Tran et al., 2023). In addition, the important effects of NPY on the importance economic characteristics of chicken were highlighted. Genetic variations in the NPY gene have been associated with differences in body weight, feed conversion ratio, and egg production (Padwar and Thakur, 2021; Promket et al., 2023; Zubaidi et al., 2023; Promket et al., 2024). Specifically, allelic variations that enhance NPY expression can lead to increased feed intake and improved growth rates, making NPY a valuable target for genetic selection in breeding programs.
NPY, which plays a significant role in various physiological processes including growth, appetite regulation, and energy homeostasis. NPY is predominantly produced in the hypothalamus, an area of the brain that regulates hunger and energy balance. When energy levels are low, NPY is released to stimulate appetite, encouraging food intake and subsequently increasing energy availability for growth processes. Moreover, NPY promotes the storage of energy in the form of fat by reducing energy expenditure. This ensures that there are sufficient energy reserves to support growth and other vital functions. NPY can influence the secretion of growth hormone (GH) from the pituitary gland. GH plays a crucial role in promoting growth and development, particularly in bones and muscles (Sener et al., 2022).
The experimental findings the relationship between the NPY gene and morphology in PH chickens. The observed association between the Bb genotype and increased body length (BL) compared to chickens with the BB genotype. Despite extensive research, little is understood about the morphological functions and expressed of NPY. It has been discovered that NPY is extensively expressed in the bone, white adipose tissue, and adrenal glands. This suggests that the neuropeptide may have a variety of physiological functions, such as adipogenesis, bone mass management, and energy metabolism (Dhamad et al., 2021). Since the bone is innervated, there may be a direct connection between it and activities mediated by the brain. In fact, as it is released by nerve fibers in the marrow and vascular canals, and with Y1 and Y2 receptors linked to bone homeostasis, the NPY system has recently emerged as one of the primary regulators of this significant process. NPY regulates bone mass differently at the hypothalamus and bone levels. While Y2 is essential for the central control of bone mass, studies of osteoblast-specific Y1 deletion in mice have demonstrated that this receptor is crucial for the activities of NPY directly at the bone. Remarkably, the effects of NPY in vivo differ from those observed in isolated mesenchymal stem/stromal cells (BMSCs) derived from bone marrow. In vivo, bone production decreases when hypothalamic NPY is elevated during fasting (Greene et al., 2022).
The integration of genomic tools and MAS in breeding programs has shown promising results in enhancing the growth characteristics of native chickens, contributing to increased productivity and sustainability in poultry production systems. Recent advances in genomic tools and breeding techniques have further facilitated the identification and selection of favorable alleles, accelerating the genetic improvement process. The accuracy of GEBV in chicken populations is clearly superior to pedigree-based EBV. This improvement can reach more than 50% for variables like feed intake that have somewhat strong heritability (Wolc et al., 2016). By leveraging genetic selection, it is possible to enhance the growth characteristics of native chickens, thereby optimizing their economic value and sustainability in smallholder farming systems.
CONCLUSIONS AND RECOMMENDATIONS
In conclusion, showed positive correlations that ranged from moderate to high between BW0 and BW16 and ADG4 and ADG16, indicating steady increase over time. The HBW group showed superior growth performance and morphology as compared to the LBW group, with exception of SL. The study on NPY gene polymorphisms in PH chickens revealed significant associations with growth performance and morphological traits. In particular, the Bb genotype was linked to greater body weight and ADG during weeks 4 to 16 in comparison to the bb genotype, suggesting a potential influence of the genotype on these traits. Furthermore, NPY polymorphism had an impact on BL; the Bb and bb genotypes demonstrated noticeably greater BL values in comparison to the BB genotype. These findings underscore the potential role of NPY gene variants in influencing growth performance and morphological traits in PH chickens. NPY gene polymorphisms have a significant effect on growth and morphology, which emphasizes their value as genetic markers in selective breeding schemes. This research provides valuable insights that can enhance productivity and efficiency in poultry farming, paving the way for future studies to explore additional genetic factors influencing these traits. Future research should focus on integrating NPY gene variants into broader genetic improvement strategies and examining their effects in other chicken breeds. Recommendations for practical applications include the incorporation of NPY polymorphisms into breeding programs to optimize growth traits and improve overall poultry production sustainability.
ACKNOWLEDGMENTS
We gratefully thank the Agricultural Research Development Agency (Public Organization) and the Chiangmai Livestock Research and Breeding Center in Chiangmai, Thailand for providing the data and blood samples that served as the foundation for our investigation. The research work was financially supported by Mahasarakham University.
NOVELTY STATEMENT
This study presents novel insights into the role of neuropeptide Y (NPY) gene polymorphisms in influencing growth performance and morphology traits in PH chickens. The research highlights a significant association between NPY gene variants and key growth traits. The study also reveals a noteworthy link between NPY polymorphisms and body length, offering potential genetic markers for selective breeding. These findings suggest that incorporating NPY polymorphisms into breeding programs could enhance poultry productivity.
AUTHOR’S CONTRIBUTIONS
Jennarong Kammongkun: Collecting information on growth characteristics, collecting samples, analyzing data, coming up with study ideas, and editing the final data of the manuscript.
Doungnapa Promket: This process includes DNA Extraction and genotyping gene, data analysis and interpretation, paper writing, and approval of the final manuscript version.
Conflict of Interest
The authors declare no conflicts of interest.
REFERENCES
Adoligbe C, Arthur F, Richard OA, Nourou DA, Robert G, Marie CF, Guilherme JMR, Farougou S (2020). Native chicken farming: A tool for wealth creation and food security in Benin. Int. J. Livest. Prod., 11(4):146-162. https://doi.org/10.5897/IJLP2020.0716
Bila L, Tyasi TL (2022). Multivariate principal component analysis of morphological traits in Ross 308 broiler chicken breed. Asian J. Agric. Biol., 3: 1-7.
Budhlakoti N, Amar KK, Anil R, Chaturvedi K, Anuj K, Anjan KP, Uttam K, Rajeev RK, Philomin J, Mishra DC, Sundeep K (2022). Genomic Selection: A Tool for Accelerating the Efficiency of Molecular Breeding for Development of Climate-Resilient Crops. J. Front. Genet., 13: 1-17. https://doi.org/10.3389/fgene.2022.832153
Chen C, Zhiyong S, Yumao L, Peng L, Shouzhi W, Hui Z, Fan X, Huaishun G, Zhiping C, Hui L, Li L (2021). Estimation of the genetic parameters of traits relevant to feed efficiency: result from broiler lines divergent for high or low abdominal fat content. J. Poul. Sci., 100: 461–466. https://doi.org/10.1016/j.psj.2020.10.028
Chomchuen K, Veeraya T, Vibuntita C, Wuttigrai B (2022). Comparative study of phenotypes and genetics related to the growth performance of crossbred Thai indigenous (KKU1 vs. KKU2) chickens under hot and humid conditions. J. Vet Sci., 9: 1-12. https://doi.org/10.3390/vetsci9060263
Dhamad A, Marco Z, Elizabeth SG, Federico S, Sami D (2021). Neuropeptide Y and its receptors are expressed in chicken skeletal muscle and regulate mitochondrial function. J. Gen. Comp. Endocrinol., 310. https://doi.org/10.1016/j.ygcen.2021.113798
Dorota CS and Joanna B (2021). Growth Performance, Carcass Characteristics and Meat Quality of Organically Reared Broiler Chickens Depending on Sex. J. Anim., 11: 1-12. https://doi.org/10.3390/ani11113274
Falconer DS, and Mackay TFC (1996). Introduction to Quantitative Genetics, Ed 4. Longmans Green, Harlow, Essex, UK.
FAO (2012). Phenotypic characterization of animal genetic resources. Food and Agriculture Organization, Anim. Prod. Health Guidel., No. 11. Rome.
Furlong RF (2005). Insights into vertebrate evolution from the chicken genome sequence. J. Genome Biol., 6(2): 1-3. https://doi.org/10.1186/gb-2005-6-2-207
Gheyas A, Adriana VT, Adebabay K, Maria LJ, Tadelle D, Jacqueline S, Olivier H (2021). Integrated Environmental and Genomic Analysis Reveals the Drivers of Local Adaptation in African Indigenous Chickens. J. Mol. Biol. Evol., 38(10):4268–4285. https://doi.org/10.1093/molbev/msab156
Goodwin W, Linacre A, Hadi S (2011). An introduction to forensic genetics (Vol. 2), John Wiley Sons.
Greene ES, Nedra A, Jalila SD, Sami D (2022). Avian Neuropeptide Y: Beyond Feed Intake Regulation Regulation. J. Vet. Sci., 9: 171. https://doi.org/10.3390/vetsci9040171
Haunshi S, Rajkumar U, Leslie LP, Kannaki R, Kandeepan G, Suresh D, Rudra NC (2022). Genetic parameters of growth traits, trend of production and reproduction traits, and meat quality status of Ghagus, an indigenous chicken of India. J. Trop. Anim. Health Prod., 54: 170- 179. https://doi.org/10.1007/s11250-022-03166-y
Helal MM, El-Gendy EA (2023). Marker-assisted selection for improving body weight in local chickens in Egypt. The J. Agric. Sci., 161: 135–147. https://doi.org/10.1017/S0021859623000060
Lengkidworraphiphat P, Rawiwan W, Thanaporn B, Arpamas C, Niraporn C, Sanchai J (2021). Taste-Active and Nutritional Components of Thai Native Chicken Meat: A Perspective of Consumer Satisfaction. J. Food Sci. Anim. Resour., 41(2): 237-246. https://doi.org/10.5851/kosfa.2020.e94
Li J, Long Z, Peng R, Ye W, Ling-Qian Y, Jin-Shan R, Xian-Xian Z, Yi-Ping L (2020). Genotype frequency distributions of 28 SNP markers in two commercial lines and five Chinese native chicken populations. J. BMC Genetics. 21(12): 2-10. https://doi.org/10.1186/s12863-020-0815-z
Mastrangelo S, Slim BJ, Francesco P, Filippo C, Filippo B, Emiliano L, Mauro P, Martino C (2023). Genome-wide mapping of signatures of selection using a high-density array identified candidate genes for growth traits and local adaptation in chickens J. Genet. Sel. Evol., 55(20): 1-16. https://doi.org/10.1186/s12711-023-00790-6
Mookprom S, Boonkum W, Kunhareang S, Siripanya S, Duangjinda M (2017). Genetic evaluation of egg production curve in Thai native chickens by random regression and spline models. J. Poult. Sci., 96: 274–281. https://doi.org/10.3382/ps/pew326
Padwar P, Thakur MS (2021). Association of neuropeptide-Y gene polymorphic variants with quantitative traits in Jabalpur colour and Kadaknath chicken. J. Indian Anim. Sci., 91(9): 729–732. https://doi.org/10.56093/ijans.v91i9.116461
Piorkowska K, Kacper Z, Katarzyna P, Joanna N, Katarzyna R, Natalia D, Joanna W, Dorota W (2020). Identification of candidate genes and regulatory factors related to growth rate through hypothalamus transcriptome analyses in broiler chickens. J. BMC Genomics, 21: 2-12. https://doi.org/10.1186/s12864-020-06884-5
Promket D, Pengmeesri K, Kammongkun J, Somchan T (2024). Identification of Melatonin Receptors Type C (MTNR1C) and Neuropeptide Y (NPY) Genes Related to Egg Production in Thai Indigenous Chickens. J. Adv. in Anim. Vet. Sci., 12(2): 206-215. https://doi.org/10.17582/journal.aavs/2024/12.2.206.215
Promket D, Pengmeesri K, Kammongkun J, Somchan T (2023). Polymorphism of the candidate genes and their association with egg production traits in Thai native chickens. J. Adv. in Anim. Vet. Sci., 11(4): 630-636. https://doi.org/10.17582/journal.aavs/2023/11.4.630.636
Promket D, Ruangwittayanusorn K (2021). The comparatives of growth and carcass performance of the Thai native chicken between economic selection (Chee KKU12) and natural selection (Chee N). Vet. Integr. Sci., 19(2): 247-257. https://doi.org/10.12982/VIS.2021.022
Rashid MA, Prabuddha M, Shakila F, Fazlul HB, Dongwon S, Jahangir A, Jun HL, Mohammad SAB (2020). Genetic diversity and population structure of indigenous chicken of Bangladesh using microsatellite markers. Asian-Australas J. Anim. Sci., 33: 1732-1740. https://doi.org/10.5713/ajas.20.0189
Ruangwittayanusorn K, Doungnapa P, Kamonnate P, Jennarong K (2022). The association of dopamine receptor D2 (DRD2) and vasoactive intestinal peptide (VIP) polymorphisms on egg production in high egg strain of pradu hangdum Chiangmai chickens. Adv. Anim. Vet. Sci., 10: 213-218. https://doi.org/10.17582/journal.aavs/2022/10.2.212.218
Sam I M, Essien CA, Ukpanah UA, Ekpo JS (2019). Influence of Sex on Relationship Between Morphometric Trait Measurement and Carcass Traits in Broiler Chicken Raised in Humid Tropic. J. Anim. Vet. Adv., 18(11): 309-314. https://doi.org/10.36478/javaa.2019.309.314
Sapkota S, Mana RK, Naba RD, Nirajan B, Neena AG (2020). Selective Breeding to Improve Productive and Reproductive Performances and Survivability of Indigenous Sakini Chicken. J. Nepal Agric. Res. Counc., 6: 62-69. https://doi.org/10.3126/jnarc.v6i0.28116
Sartsoongnoen N, Li D, Wang Q (2021). The role of neuropeptide Y in regulating growth and reproductive traits in chickens. J. Poult. Sci., 100(9): 4567-4578.
SAS (2019). SAS/STAT User’s Guide; Version 9.4; SAS Inst. Inc.: Cary, NC, USA.
Sener K, Elif NA, Şule CC (2022). An Overview of Appetite Regulation Mechanisms. Koc. J. Sci. Eng., 5(2): 178-193. https://doi.org/10.34088/kojose.1091078
Sharma P, Shilpa D, Hadiya KK, George LB, Highland HN (2024). Overview of Marker-assisted Selection in Animal Breeding. J. Adv.Biol.Biotechnol., 27(5): 303-318. https://doi.org/10.9734/jabb/2024/v27i5790
Tran P, Mohamed ZE, Yuriko T, Ying W, Guofeng H, Vishwajit C, Mitsuhiro F (2023). Central Interaction Between l-Ornithine and Neuropeptide Y in the Regulation of Feeding Behavior of Neonatal Chicks. J. Poult. Sci., 60: 1-12. https://doi.org/10.2141/jpsa.2023004
Vilakazi BN, Ncobela CN, Kunene NW, Panella F (2020). Determining the morphological structure of indigenous chickens using multivariate principal component analysis of body measurements. J. Appl. Anim. Husb. Rural Develop., 13: 69-75:
Wang Y, Perot S, Ganrea C, Rodrigo AG, Anna W, Janet EF, Jack MD, Susan JL, Terra RK, Huaijun Z (2024). Genomic Regions and Candidate Genes Affecting Response to Heat Stress with Newcastle Virus Infection in Commercial Layer Chicks Using Chicken 600K Single Nucleotide Polymorphism Array. Int. J. Mol. Sci., 25: 1-17. https://doi.org/10.3390/ijms25052640
Wolc, A, Kranis A, Arango J, Settar P, Fulton JE, Sullivan NPO, Avendano A, Watson KA, Hickey JM, Campos G, Fernando RL, Garrick DJ, Dekkers JCM (2016). Implementation of genomic selection in the poultry industry. J. Animal Frontiers, 6: 23-31. http://dx.doi.org/10.2527/af.2016-0004.
Wondmeneh E, Temesgen A, Mekonnen D (2021). Indigenous chicken production systems and breeding practices: A review. J. Vet. Anim. Sci., 13: 100176.
Xu Y, Liu X, Fu J, Wang H, Wang J, Huang C, Prasanna BM, Olsen MS, Wang G, Zhang A (2020). Enhancing Genetic Gain through Genomic Selection: From Livestock to Plants. Plant Comm. 1, 100005. https://doi.org/10.1016/j.xplc.2019.100005
Zubaidi K, Rekabi M, Allaw A (2023). Effect of Polymorphism of the Neuropeptide Y (NPY) Gene on some Productive Traits of Iraqi Local White Chickens. Conf. Ser.: Earth Environ. Sci., 1252 012121. https://doi.org/10.1088/1755-1315/1252/1/012121
To share on other social networks, click on any share button. What are these?






