Faunal and Habitat Distribution of Mosquitoes (Diptera: Culicidae) in Chakwal, Punjab, Pakistan
Research Article
Faunal and Habitat Distribution of Mosquitoes (Diptera: Culicidae) in Chakwal, Punjab, Pakistan
Arif Mehmood1*, Muhammad Naeem2, Abu Bakkar Muhammad Raza1, Muhammad Zeeshan Majeed1, Muhammad Irfan Ullah1, Muhammad Asam Riaz1, Ikram ul Haq1 and Waqas Raza1
1College of Agriculture, University of Sargodha, Sargodha, Pakistan; 2Pir Mehr Ali Shah, Arid Agriculture University, Rawalpindi, Pakistan.
Abstract | Mosquitoes have different preferences for habitats on the basis of their feeding habits and behavior. Spatial hotspots are the target areas in precise control. The diversity indices and habitat web structures are the tools which can be helpful in controlling disease vector in case of epidemic. In this study, microhabitats were specified, surveys were conducted to explore specified microhabitats in Chakwal district of Pothwar region, Punjab, Pakistan. After qualitative and quantitative sorting, checklist of mosquitoes along with diversity indices and quantitative habitat web were prepared, which point out the hotspots of different mosquito species. In this study, a total of 580 specimens, comprising twelve mosquito species belonging to five genera were collected, which were deposited in the Biosystematics Laboratory of Pir Mehr Ali Shah, Arid Agriculture University Rawalpindi. Results show that Park, Forest area, and scrapyard were the most abundant habitats respectively, while the least abundant habitat was crop area. Parks were found to be the richest habitat, while graveyards were the least rich habitat.
Received | January 15, 2024; Accepted | February 22, 2024; Published | April 26, 2024
*Correspondence | Arif Mehmood, College of Agriculture, University of Sargodha, Sargodha, Pakistan; Email: [email protected]
Citation | Mehmood, A., M. Naeem, A.B.M. Raza, M.Z. Majeed, M.I. Ullah, M.A. Riaz, I. Haq and W. Raza. 2024. Faunal and habitat distribution of mosquitoes (Diptera: Culicidae) in Chakwal, Punjab, Pakistan. Sarhad Journal of Agriculture, 40(2): 463-469.
DOI | https://dx.doi.org/10.17582/journal.sja/2024/40.2.463.469
Keywords | Habitat distribution, Mosquito, Species distribution, Habitat web, Pothwar, Diversity index, Culicidae
Copyright: 2024 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Mosquitoes (Diptera: Culicidae) are the most lethal organisms found on earth. These transmit lethal diseases in humans e.g. dengue, malaria, Zika virus, yellow fever, etc. Malaria causes 400,000 deaths annually, while dengue causes 40,000 deaths worldwide (WHO, 2021). Mosquitoes show preference for some particular hosts (Robert et al., 1996).
The population of mosquitoes is regulated by several factors, with ecological characteristics being particularly significant (Majeed et al., 2019; Ranasinghi and Amarasinghe, 2021). These characteristics are closely tied to the habitat, which also yields crucial insights into the insect’s preferred host. The population and diversity of mosquitoes are intricately linked to the varied biotic and abiotic features of the habitat (Reisen, 2010). The type of vegetation or land cover plays a pivotal role in influencing mosquito diversity (Moncayo et al., 2000; Rochlin et al., 2008).
The active periods for mosquitoes are dawn and dusk. Sunlight and the smokes from the kitchen make them fly from resting position (Dhimal et al., 2014).
Mosquitoes usually aggregate around the preferred host. It is not their habit to aggregate around the commonly available hosts. They prefer greater diversity of host, commonly the most prevalent species inhabited their habitats alone. Mosquitoes do not prefer to oviposit in shaded habitats (Banafshi et al., 2013; Reiskind et al., 2017).
Different environmental factors affect the spatial distribution of mosquitoes. These factors include the availability of hosts (Mehmood et al., 2016), vegetation, the resting place and oviposition sites (Suleman et al., 1993).
Feeding behavior provides information regarding the hosts like birds, animals, and humans. Different species of mosquitoes prefer different types of habitats. Each habitat has its own specific physical characteristics, e.g. graveyards have less vegetation, thus low humidity and raised temperature. Parks and forest areas have high humidity and low temperature due to surplus vegetation. Animal sheds have high humidity and high temperature due to respiration of animals. Surroundings of streams, are cool as transpiration of water vapors is high (Aneidu, 1992). Humidity and temperature remain changing in houses due to the activities of humans. Precipitation directly affects the population of mosquitoes and the oviposition as precipitation creates the habitat for egg laying (Mehmood et al., 2017) as well as habitat for their larvae to grow (Vandyk and Rowley, 1995; Dhileepan, 1996; Lindblade et al., 1999; Web and Russell, 1999).
Some mosquitoes prefer to live in flatwoods, new fields, and salt marshes with low vegetation, a few prefer forested habitats, others live in the ecotone habitats and a few live in woodlands (Mehmood et al., 2022a) but come to open areas during nocturnal, diurnal, and crepuscular activities (Almiron and Brewer, 1994).
The involvement of humans also decides the habitats of mosquitoes. Some mosquitoes are human-loving and prefer to live in the vicinity of humans; others prefer to live near animals and birds (Mehmood et al., 2022b).
As male mosquitoes feed on the nectar from trees and flowers, they have preference for plants. The type of nectar and its availability alter the habitat distribution of mosquitoes. Reduction in the habitats reduces the growth and development of mosquito population. Changes or disturbance in the habitats causes the population of mosquitoes to change (Mehmood et al., 2022a).
Habitat distribution of mosquitoes provides a map of the mosquitoes in relation to their habitats, thus in case of any epidemic spread in an area, this map gives the information of hotspots of any mosquitoes in that region, making the control practices much easier.
Materials and Methods
The study area Chakwal is located in the southwest direction of capital city Islamabad, about 90 km away (Figure 1). Chakwal is mainly an agricultural area, but there are some cement factories present here. The microhabitats, which were specified for survey, included graveyards, scrap yards, parks, forest areas, crop areas, streams, houses, and animal sheds. The surveys were conducted on fortnightly basis. These habitats have different types of breeding places, including vegetation, water availability, scrap, discarded containers, bamboo stumps, tires, etc.
Graveyards are mostly dry places having fewer trees and grass. Scrap yards are dry and mostly open places with junk and many hiding places. Parks have much vegetation and human movement. Forest areas have tall trees, grasses, wildlife, and less human movement and disturbance. The houses are mostly dry having some earthen pots, human and animal activities. Animal sheds have animals, dung, human activities, and some water catchment area.
Both male and female specimens were captured using aerial net and dry ice traps (Mehmood et al., 2022a, b; Batzer et al., 2020; Barraud, 1934). Further sorted quantitative and qualitative sorting was done at Biosystematics Laboratory, Department of Entomology, PMAS Arid Agriculture University, Rawalpindi. Identification was done under the binocular stereoscope (Labomed CZM6). Ali et al. (2013), Qasim et al. (2014) and Tyagi et al. (2015) were followed for taxonomic identification. A checklist of butterflies of Chakwal region has been provided also (Table 1).
The habitat distribution was documented using webs created in Coral Draw X6, alongside diversity indices that were determined by PAST software (Riaz et al., 2022). To create the webs, the scale used was determined by the following formula.
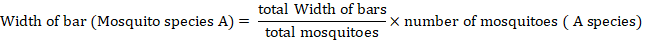
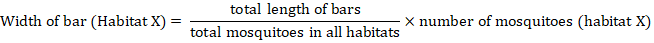
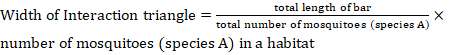
Results and Discussion
In this web (Figure 2), a total of 12 mosquito species belonging to genera Anopheles (6), Culex (3), Aedes (2), and Armigeres (1), comprising 580 specimens were recorded.
Mosquito communities are affected by the type of habitats and the hosts available. These are also affected by the abundance of the hosts. Within an area mosquitoes gather around their preferred hosts (Robert et al., 1996). Dhimal et al. (2014) found that Culex prefers streams, water tank and seepage water drums for breeding, while Anopheles prefer paddy fields, seepage, tree holes, and water tanks, Aedes likes to breed in discarded tires, plastic drums, tree holes, stream and water tank (Dhimal et al., 2014).
In the district Chakwal, Culex vagans was in the highest percentage (20.79%), while the lowest percentage (1.86%) was shown by Armigeres kuchingensis followed by Culex tenuipalpis (2.1%), Culex theileri (3.03%), Culex pluvialis (3.03%), Armigeres obturbans (3.03%), Culex seniori (4.9%), Anopheles stephensi (6.54%), Aedes aegypti (6.54%), Aedes albopictus (6.77%), Culex cornotus (7.71%), Anopheles splendidus (9.11%), Culex quinquefasciatus (11.44%) and Culex nilgiricus (13.08%).
Table 1: Checklist of mosquitoes found in Chakwal, Punjab, Pakistan during 2014-16 regarding habitat distribution.
|
Mosquito species |
Habitats |
|||||||
|
Animal shed |
Scrap yard |
Graveyard |
Park |
Forest area |
Crop area |
Residential area |
Stream |
|
|
Anopheles splendidus |
+ |
- |
- |
- |
- |
+ |
- |
- |
|
Anopheles culicifacies |
+ |
+ |
- |
- |
- |
+ |
- |
- |
|
Anopheles fluviatilis |
+ |
- |
- |
- |
- |
+ |
- |
+ |
|
Anopheles tesselatus |
+ |
- |
- |
+ |
- |
+ |
- |
- |
|
Armigeres obturbans |
- |
- |
- |
+ |
+ |
- |
- |
+ |
|
Anopheles stephensi |
+ |
- |
- |
- |
+ |
+ |
- |
+ |
|
Anopheles annularis |
- |
- |
- |
+ |
- |
+ |
+ |
+ |
|
Culex vagans |
- |
+ |
- |
+ |
+ |
- |
+ |
- |
|
Culex nilgiricus |
- |
+ |
- |
+ |
+ |
- |
+ |
- |
|
Lutzia vorax |
- |
+ |
- |
+ |
+ |
+ |
+ |
- |
|
Aedes aegypti |
- |
+ |
+ |
+ |
+ |
- |
+ |
+ |
|
Aedes albopictus |
- |
+ |
+ |
+ |
+ |
- |
+ |
+ |
Table 2: Diversity indices of mosquitoes found in Chakwal, Punjab, Pakistan during 2014-16.
|
Diversity indices |
Habitats |
|||||||
|
Animal shed |
Scrap yard |
Graveyard |
Park |
Forest area |
Crop area |
Residential area |
Stream |
|
|
Simpson index |
0.72 |
0.79 |
0.48 |
0.85 |
0.84 |
0.84 |
0.80 |
0.75 |
|
Shannon index |
1.42 |
1.65 |
0.68 |
2.0 |
1.90 |
1.9 |
1.69 |
1.53 |
|
Evenness |
0.83 |
0.87 |
0.98 |
0.92 |
0.96 |
0.95 |
0.90 |
0.77 |
Maximum Simpson index (0.85) was recorded in park, while the least (0.48) was recorded in graveyard, followed by animal shed (0.72), stream (0.75), scrap yard (0.79), residential area (0.80), crop area (0.84) and forest area (0.84) respectively. These results are supported by Mehmood et al. (2022a). Shanon index was recorded maximum (2.0) in park, while the least (0.68) in graveyard, followed by animal shed (1.47), stream (1.53), scrap yard (1.65), residential area (1.69), crop area (1.9), and forest area (1.9), respectively. Maximum evenness (0.98) was recorded in graveyard, while the least (0.77) in stream, followed by animal shed (0.83), scrap yard (0.87), residential area (0.90), park (0.92), crop area (0.95) and forest area (0.96) respectively (Table 2).
Anopheles annularis was recorded in habitats, including parks, crops, residential area and stream. The highest abundance was found in crop area, while the lowest in stream. Findings from this study are supported by the findings of Ilahi and Salman (2013) and Pal and Dutta (1992). Ilahi and Salman, Ali et al. (2013) and Lewanowski in 2013 had collected A. annularus from the river margins, springs and irrigation channels, which is similar to this study (Pal and Dutta, 1992; Fakoorziba and Vijayan, 2008).
Anopheles culicifacies was found in animal sheds, scrap yard and crop area. The highest abundance was observed in animal sheds, while the lowest in crop area. Findings are in partial accordance with Ali et al. (2013), and Pal and Dutta (1992). In addition to other habitats A. culicifacies was captured from animal sheds also, which depicts the zoophilic nature of his mosquito.
Anopheles fluviatilis was collected from animal sheds, crop area and stream. High abundance was recorded in crop area. Anopheles tesselatus was recorded in animal sheds, park and crop area. The low abundance was found in crop area. Anopheles splendidus was found in animal sheds and crop area. In this study, Anopheles splendidus was collected from houses and animal sheds, while Ilahi and Salman (2013) had collected from rice fields. The results are not in accordance with the findings of Ilahi and Salman (2013) it is because Anopheles splendidus like human and animal activity areas.
Culex vagans and Culex nilgiricus shared the same habitats, including scrap yard, park, forest area and residential areas. The abundance of Culex vagans was same in all the habitats. Culex nilgiricus was recorded in high abundance in scrap yard and forest area, while the low abundance was recorded in parks as was reported by Ali et al. (2013, 2015).
Lutzia vorax was recorded in scrap yard, park, forest area, crop area, and residential area. The low abundance was recorded in crop area; abundance was the same in the remaining habitats, which is in concordance with the findings of Tyagi et al. (2015).
Armigeres obturbans was recorded from seven different habitats, including Park, forest area, stream, animal shed, scrap yard and crop area. The results are in accordance with Rajput and Kulkarni (1990), Rajput and Singh (1990), Ilahi and Salman (2013). Partial accordance with Ali et al. (2015) was present as Ar. obturbans was not collected from houses, which may be due to the preference of high vegetation and humidity.
Aedes aegypti was recorded from six habitats, including stream, park, forest area, residential area, grave yard and scrap yard the abundance was found low in graveyard and scrap yard, where the humidity was low, vegetation was less and the human movement and activities were less. The results are in accordance with Rajput and Singh (1990), Barreera et al. (2011), Ilahi and Salman (2013) and Poveda et al. (1999).
Aedes albopictus was recorded from the graveyard, forest area, residential area, park, stream and scrap yard. The results are in concordance with Fakoorziba and Vijayan (2008) and Westby et al. (2021). A. albopictus was recorded in all the habitats observed except animal sheds and crop area. There was low abundance in stream, remaining habitats were having the same abundances. Fakoorziba and Vijayan (2008) had the same findings, but A. albopictus was found in residential areas and crops also due to the adaptive nature of A. albopictus.
Novelty Statement
District Chakwal has not been explored before this study for diversity and systematics of mosquitoes. This is the first study done for the exploration of the species distribution of mosquitoes. :
Author’s Contribution
Arif Mehmood: Conceived the idea, conducted research, arranged and analysed data and wrote the manuscript.
Muhammad Naeem: Conceived the idea, supervised the research, arranged and analysed data and provided resources.
Abu Bakkar Muhammad Raza, Muhammad Zeeshan Majeed, Muhammad Irfan Ullah, Muhammad Asam Riaz, Ikram ul Haq and Waqas Raza: Reviewed and edited the manuscript, and analysed data.
Conflict of interest
The authors have declared no conflict of interest.
References
Ali, N., K. Khan and A. Kausar. 2013. Study on mosquitoes of Swat Ranizai sub division of Malakand. Pak. J. Zool., 45(2): 503-510.
Ali, N., S. Noreen, K. Khan and S. Wahid. 2015. Population dynamics of mosquitoes and malaria vector incrimination in district Charsadda, Khyber Pakhtunkhwa (KP) Pakistan. Acta Trop., 141: 25-31. https://doi.org/10.1016/j.actatropica.2014.08.020
Almiron, W.R. and M.E. Brewer. 1994. Immature stages of mosquitoes (Diptera: Culicidae) collected during the autumn-winter period in Cordoba Province, Argentina. Memórias Do Instituto Oswaldo Cruz, 89(4): 625-628. https://doi.org/10.1590/S0074-02761994000400020
Aniedu, I., 1992. A comparative study of the distribution and seasonal abundance of malaria vectors in 3 ecologically distinct habitats in Baringo District, Kenya. J. Appl. Entomol., 114(1-5): 268-274. https://doi.org/10.1111/j.1439-0418.1992.tb01126.x
Banafshi, O., M. Abai, H. Ladonni, H. Bakhshi, H. Karami and S. Azari-Hamidian, 2013. The fauna and ecology of mosquito larvae (Diptera: Culicidae) in Western Iran. Turk. J. Zool., 37: 298-307. https://doi.org/10.3906/zoo-1206-12
Barraud, P.J., 1934. The fauna of British India, including Ceylon and Burma. Diptera, Family Culicidae. Tribe Megarhinini and Culicini. Taylor and Francis, London, pp. 455.
Barrera, R., M. Amador and A.J. MacKay. 2011. Population dynamics of Aedes aegypti and dengue as influenced by weather and human behavior in San Juan, Puerto Rico. PLoS Neglected Trop. Dis., 5(12): 1-9. https://doi.org/10.1371/journal.pntd.0001378
Batzer, D.P., A. Mehmood, D.G. Mead and D. Champagne, 2020. Phenology of Coquillettidia perturbans and Culiseta melanura (Diptera: Culicidae) in East-Central Georgia, USA: Implications for the ecology of Eastern Equine Encephalitis Virus. J. Entomol. Sci., 55(2): 156-162. https://doi.org/10.18474/0749-8004-55.2.156
Burkett-Cadena, N.D., C.J.W. McClure, R.A. Ligon, S.P. Graham, C. Guyer, G.E. Hill, S.S. Ditchkoff, M.D. Eubanks, H.K. Hassan and T.R. Unnasch, 2011. Host reproductive phenology drives seasonal patterns of host use in mosquitoes. PLoS One, 6(3): 1-7. https://doi.org/10.1371/journal.pone.0017681
Day, J.F., 2016. Mosquito oviposition behavior and vector control. Insects, 7(4): 1-22. https://doi.org/10.3390/insects7040065
Dhileepan, K., 1996. Mosquito seasonality and arboviral disease incidence in Murray valley, southeast Australia. Med. Vet. Entomol., 10(4): 375-384. https://doi.org/10.1111/j.1365-2915.1996.tb00760.x
Dhimal, M., B. Ahrens and U. Kuch. 2014. Species composition, seasonal occurrence, habitat preference and altitudinal distribution of malaria and other disease vectors in Eastern Nepal. Par. Vec., 7(540): 1-11. https://doi.org/10.1186/s13071-014-0540-4
Fakoorziba, M.R. and A. Vijayan. 2008. Breeding habitats of Culex tritaeniorhynchus (Diptera: Culicidae), a Japanese encephalitis vector, and associated mosquitoes in Mysore, India. J. Entomol. Res. Soc., 10(3): 1-9.
Ranasinghe, H.A.K. and L.D. Amarasinghe. 2020. Naturally occurring microbiota associated with mosquito breeding habitats and their effects on mosquito larvae. BioMed. Res. Intl. Article ID 4065315, 11. https://doi.org/10.1155/2020/4065315
Ifeoluwa, K.F., T.I.T.S.A. Emmanuel and O.A. Otubanjo. 2020. Seasonal abundance and larval habitats characterization of mosquito species in Lagos State, Nigeria. Sci. Afr., 10: e00656. https://doi.org/10.1016/j.sciaf.2020.e00656
Ilahi, I. and M. Suleman. 2013. Species composition and relative abundance of mosquitoes in Swat, Pakistan. Int. J. Innov. Appl. Stud., 2(4): 454-463.
Khan, I.A., M.M.U. Din, S. Hussain, R. Akbar, M. Saeed, A. Farid, W. Fayaz and R.A. Shah. 2015. A study of mosquito fauna of District Upper Dir, Khyber Pakhtunkhwa, Pakistan. J. Entomol. Zool. Stud., 3(5): 455-458.
Lindblade, K.A., E.D. Walker, A.W. Onapa, J. Katungu and M.L. Wilson. 1999. Highland malaria in Uganda: prospective analysis of an epidemic associated with El Nino. Trans. R. Soc. Trop. Med. Hyg., 93(5): 480-487. https://doi.org/10.1016/S0035-9203(99)90344-9
Majeed, M.Z., I. Sarwar, M. Afzal, M.R. Khalid, M. Yahya and K. Shehzad. 2019. Differential composition of edaphic arthropods in different land-use types of district Sargodha (Punjab, Pakistan) and their relationship with soil physico-chemical and biological characteristics. Sarhad J. Agric., 35(4): 1071-1083. https://doi.org/10.17582/journal.sja/2019/35.4.1071.1083
Mehmood, A., M. Naeem, I. Bodlah and A. Mohsin, 2016. Systematics of anopheles and armigerus (Culicidae: Diptera) mosquitoes in the Pothwar Region, Punjab, Pakistan. Int. J. Mosq. Res., 3(5): 5-10.
Mehmood, A., Z. Iqbal, N. Fatima and M.S. Qureshi. 2017. Efficacy of plant extracts against the tree hole breeding mosquitoes. J. Biosci. Agric. Res., 13(2): 1130-1139. https://doi.org/10.18801/jbar.130217.138
Mehmood, A., M. Naeem and A. Mohsin, 2021. Seasonal occurrence and abundance of mosquitoes in Pothwar Plateau. Pak. J. Zool., 1-7: 2021.
Mehmood, A., M. Naeem, A.B.M. Raza, M.A. Riaz, M.Z. Majeed, N. Khan and W. Raza. 2022a. Species distribution, abundance and diversity of mosquitoes (Diptera: Culicidae) in district Jhelum (Punjab, Pakistan). Pak. J. Agric. Res., 35(3): 508-513. https://doi.org/10.17582/journal.pjar/2022/35.3.508.513
Mehmood, A., M.A. Riaz, A.B.M. Raza, W. Raza and M.Z. Majeed. 2022b. Spatial hotspots of mosquitoes (Diptera: Culicidae) in Attock, Pothwar, Punjab, Pakistan. Sarhad J. Agric., 38(5): 60-64. https://doi.org/10.17582/journal.sja/2022/38.5.60.64
Moncayo, A.C., J.D. Edman and J.T. Finn. 2000. Application of geographic information technology in determining risk of eastern equine encephalo-myelitis virus transmission. J. Am. Mosq. Cont. Assoc., 16(1): 28-35.
Pal, T.K. and R.K. Dutta, 1992. Anophelines (Diptera: Culicidae) of three districts (East Kameng, Lower Subansiri and Upper Subansiri) of Arunachal Pradesh and their perspective impact on Buman and Nonhuman hosts. Rec. Zool. Surv. Ind., 91(2): 189-202. https://doi.org/10.26515/rzsi/v91/i2/1992/160941
Poveda, G., N.E. Graham, P.R. Epstein, W. Rojas, I.D. Velez, M.L. Quinones and P. Martens, 1999. Climate and ENSOvariability associated to malaria and dengue fever in Colombia. Proc. Symp. Glob. Change Stud. Boston: Am. Meteorol. Soc., 10: 173-176.
Qasim, M., M. Naeem and I. Bodlah. 2014. Mosquito (Diptera: Culicidae) of Murree Hills, Punjab, Pakistan. Pak. J. Zool., 46(2): 523-529.
Rajput, K.B. and S.M. Kulkarni. 1990. Records of Culicine mosquitoes from Bastar district (Madhya Pradesh) India (Diptera: Culicidae), Part·I genus Toxorhynchites, Tripteroides, Uranotaenia and Orthopodomyia. Rec. Zool. Sur. Ind., 87(1): 83-88. https://doi.org/10.26515/rzsi/v87/i1/1990/161456
Rajput, K.B. and T.K. Singh. 1990. Records of Anopheline mosquitoes collected from Manipur with Ecological Notes. Rec. Zool. Sur. Ind., 87(3): 197-206. https://doi.org/10.26515/rzsi/v87/i3/1990/161398
Reisen, W.K., 2010. Landscape epidemiology of vector-borne diseases. Ann. Rev. Entomol., 55: 461-483. https://doi.org/10.1146/annurev-ento-112408-085419
Reiskind, M.H., R.H. Griffin, M.S. Janairo and K.A. Hopperstad. 2017. Mosquitoes of field and forest: the scale of habitat segregation in a diverse mosquito assemblage. Med. Vet. Entomol., 31(1): 44-54. https://doi.org/10.1111/mve.12193
Riaz, M.F., A.B.M. Raza, M.Z. Majeed and T. Nazir. 2022. Diversity and species distribution of Aphids (Hemiptera: Aphididae) in district Sargodha (Punjab, Pakistan). Sarhad J. Agric., 38(2): 540-547. https://doi.org/10.17582/journal.sja/2022/38.2.540.547
Roberts, D.R., J.F. Paris, S. Manguin, R.E. Harbach, R. Woodruff, E. Rejmankova, J. Polanco, B. Wulls-chleger, and L.J. Legters. 1996. Predictions of malaria vector distribution in Belize based on multispectral satellite data. Am. J. Trop. Med. Hyg., 4(3): 304-308. https://doi.org/10.4269/ajtmh.1996.54.304
Rochlin, I., K. Harding, H.S. Ginsberg and S.R. Campbell, 2008. Comparative analysis of distribution and abundance of West Nile and eastern equine encephalomyelitis virus vectors in Suffolk County, New York, using human population density and land use/cover data. J. Med. Entomol., 45(3): 563-571. https://doi.org/10.1603/0022-2585(2008)45[563:CAODAA]2.0.CO;2
Service, M.W., 1981. Effects of wind on the behaviour and distribution of mosquitoes and blackflies. Int. J. Biometeorol., 24(4): 347-353. https://doi.org/10.1007/BF02250577
Suleman, M., K. Khan and S. Khan. 1993. Ecology of mosquitoes in Peshawar valley and adjoining areas: Species composition and relative abundance. Pak. J. Zool., 25(4): 321-328.
Tyagi, B.K., A. Munirathinam and A. Venkatesh. 2015. A catalogue of Indian mosquitoes. Int. J. Mosq. Res., 2(2): 50-97.
Vandyk, J.K. and W.A. Rowley. 1995. Response of Iowa mosquito populations to unusual precipitation patterns as measured by New Jersey light trap collections. J. Am. Mosq. Contr., 11(2): 200-205.
Webb, C.E. and R.C. Russell. 1999. Towards management of mosquitoes at Homebush Bay, Sydney, Australia. I. Seasonal activity and relative abundance of adults of Aedes vigilax, Culex sitiens, and other salt-marsh species, 1993-94 through 1997–98. J. Am. Mosq. Contr., 15(2): 242-249.
Westby, K.M., S.A. Adalsteinsson, E.G. Biro, A.J. Beckermann and K.A. Medley. 2021. Aedes albopictus populations and larval habitat characteristics across the landscape: Significant differences exist between urban and rural land use types. Insects, 12: 196. https://doi.org/10.3390/insects12030196
To share on other social networks, click on any share button. What are these?







