Evaluations of Phytochemical Contents, Antibacterial and Antioxidant Potential of Methanolic Leaves Extract of Tanacetum camphoratum Less. (Camphor tansy).
Research Article
Evaluations of Phytochemical Contents, Antibacterial and Antioxidant Potential of Methanolic Leaves Extract of Tanacetum camphoratum Less. (Camphor tansy).
Muhammad Ajmal Khan1, Muhammad Yahya2*, Ali Hazrat2, Javed Khan3, Saeed Jan4, Tabinda Nowsheen2 and Inam Ullah5
1Center of Biotechnology and Microbiology, University of Peshawar, Khyber Pakhtunkhwa, Pakistan; 2Department of Botany, University of Malakand, Chakdara Dir (Lower)-1800, Khyber Pakhtunkhwa, Pakistan; 3Department of Botany Hazara University of Mansehera, Khyber Pakhtunkhwa, Pakistan; 4Department of Pharmacy, University of Swabi, Khyber Pakhtunkhwa, Pakistan; 5Department of Zoology, Abdul Wali Khan University Mardan, Khyber Pakhtunkhwa, Pakistan.
Abstract | Tanacetum camphoratum is a rhizomatous perennial herb. The current study investigated the phytochemical constituents, antioxidants, and antibacterial activities of methanolic extracts of T. camphoratum leaves. The methanolic extract was quantitatively evaluated for alkaloids, flavonoids and terpenoids contents. The antibacterial activity of methanolic leaves extract of T. camphoratum was carried out against Gram-positive and Gram-negative bacterial strains through agar well diffusion assay (small wells were made with cork borer and 6μl of plant extract was added to the wells). The antioxidant activity of methanolic leaves extract was evaluated through a well established protocol 2,2-diphenyl-picrylhydrazyl hydrate (DPPH) at various concentrations (50, 100, 150, 200, and 250 µL). The phytochemical analysis of methanolic leaves extract of T. camphoratum showed 2.80% alkaloids, 4.90% flavonoids and 1.80% terpenoids content. The antibacterial activity of the methanolic extract showed high effectiveness against Gram-positive bacteria (MRSA) with 23 mm zone of inhibition (ZOI), whereas against Gram- negative bacteria, S. typhi, E. coli and P. aeruginosa, with ZOI observed was 22, 18 and 20 mm, respectively. Antioxidant activity evaluated by using different concentrations of methanolic extracts showed good activity at 250 μL through DPPH, which depicted the vibrancy in phenolic, flavonoids and terpenoids contents. This insightful study highlighted the potential usage of methanolic leaves extract of T. camphoratum as a broad-spectrum antibacterial agent and an antioxidant source in food and pharmaceutical industry.
Received | July 07, 2021; Accepted | December 31, 2021; Published | July 28, 2022
*Correspondence | Muhammad Yahya, Department of Botany, University of Malakand, Chakdara Dir (Lower)-1800 KP Pakistan; Email: m.yahyabotanist@gmail.com
Citation | Khan, M.A., M. Yahya, A. Hazrat, J. Khan, S. Jan, T. Nowsheen and I. Ullah. 2022. Evaluations of phytochemical contents, antibacterial and antioxidant potential of methanolic leaves extract of Tanacetum camphoratum Less. (Camphor tansy). Sarhad Journal of Agriculture, 38(3): 989-996.
DOI | https://dx.doi.org/10.17582/journal.sja/2022/38.3.989.996
Keywords | Alkaloids, Antibacterial, DPPH, Flavonoids, Tanacetum camphoratum, Terpenoids
Copyright: 2022 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
The growing evidence of multiple drug resistance (MDR) implies the quest for novel synthetic antibacterial and antioxidative drugs, from traditional sources having role in treatment of different disease (Waltenberger et al., 2016). The antibiotics which were effective against various ailment at the beginning of the 20th century are now less effective because bacteria had developed certain level of resistance (Zaman et al., 2017). Moreover, the high cost of conventional therapeutics and in current economic crises situation people are re-evaluating the healthcare options. According to WHO, 80% of the world’s population depends on herbal remedies for their basic health and about 3.5 billion people in developing countries rely on medical supplies and plant products to maintain their health (Khan and Ahmad, 2019).
Plants have bioactive chemicals that are rich source of medicine for many ailments almost everywhere around the globe (Rakholiya et al., 2013). Medicinal plants possess phytochemicals like flavonoids, alkaloids, terpenoids and glycosides, which are highly effective to resist microbial agents. The bioactive compounds present in traditional plants due to its numerous effective properties have been favored over manufactured molecules (Koparde et al., 2019). Plants were the most well studied and proved as effective for antibacterial, anthelmintic, and antifungal growth (Badawy and Abdelgaleil, 2014; Chen et al., 2016). The essential oil derived from plants have been used in medicine for treatment of various diseases linked to digestive track (bloating, indigestion, stomach and intestinal ulcers), liver and gallbladder problems, amenorrhea, gout, colds, skin diseases and in aromatherapy and cosmetics (Babar et al., 2015). There is a worldwide understanding that any plant recognized for its bio efficacy should be investigated (Sofowora et al., 2013). In addition, authentic effects on a particular human disorder of any plant extract motivate us to check indigenous Plants with antioxidant and antibacterial potential (Shaojian et al., 2017).
Tanacetum camphoratum is basically an herb and belong to medicinal plants and member of Asteraceae. It is the cosmopolitan family of vascular plants and composed of 23,000 species, with 16,200 genera genera and 12 sub families (Essiett and Archibong, 2014). This Plant (T. camphoratum) is perennial aromatic and widely distributed in the northern hemisphere. The stem is thick low lying having 25cm long and feather like leaves up to 25cm. The genus Tanacetum has many medicinal benefits and used widely uas an expectorant, vermifuges, spasmolytic, cardiotonic, anti-spasmodic and antiseptic (Saini et al., 2020). The phytochemical screening, antioxidant potential and antibacterial activities of T. camphoratum were not investigated before. Therefore, current study focusses on evaluating the phytochemical constituents, antibacterial and antioxidant activities of methanolic leaf extract of T. camphoratum.
Materials and Methods
Collection of Plant materials
The plant materials were collected from District Kohistan and identification was done with the assistance of flora from Pakistan, taxonomists, various pictorial guides and also through literature (Nasir and Ali, 1972).
Preparation of plant extract
The electrical grinder was used to crush the plant leaves to dry powder and soaked (150g dry powder) in methanol (450ml) for three days. Filtration of the plant extract was then done according to the protocol explained before (Zamir et al., 2013). Briefly, solvent was first filter via cotton and then through What-mann No.1 filter paper). The methanol extract was evaporated in a rotary evaporator at 40°C - 45°C under reduced pressure followed by freeze drying at -30°C. The extract was kept at -20 °C for future use.
To perform the antibacterial activity, disc diffusion method was used. The different bacterial strain used in current study include one Gram-positive (methicillin resistant staphylococcus aurous (MRSA) and three Gram-negative bacteria (S. typhi, E. coli, P. aeruginosa). The identification of the bacterial strains was done with the help of expert microbiologist of University of Peshawar, Pakistan K.P. Broad spectrum antibiotic vancomycin obtained from Islamabad pharmaceutical company was used as a standard control. The bacterial strains were maintained in nutrient agar media and kept in refrigerator and sub-cultured before experiment to test the antibacterial potential of the extract.
Agar disc diffusion method were used to evaluate the antibacterial potential of the extract. Briefly, the bacterial culture (100 μl) having 107 CFU/ml was spread uniformly on petri dishes with glass spreader and incubate at 37°C. The petri dishes were divided into 4 parts. Under aseptic conditions with cork borer wells were formed and 6μl of plant extract was poured into each well with micropipette. The plates were incubated for 24hours. The strains sensitivity was assessed by measuring the zone of inhibition (Z0I). All the experiments were performed in triplicate and represented as mean ± S.D.
Quantitative test for alkaloids
For the quantitative analysis of alkaloids Harborn with slight modification was followed (Harborne, 1992). Briefly, 5 g of dry leaf powder was weighted and added into 200ml acetic acid for 4 hours. After this concentrated ammonium hydroxide was added and filtered. The precipitate is washed again with diluted ammonium hydroxide and filtered. Finally weighted according to given formula;
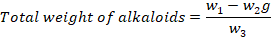
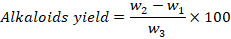
Where;
w1: Weight of Petri plate; w2: Alkaloids Petri plate weight; w3: Estimation initial plant sample weight.
Quantitative test for flavonoids
The determination of flavonoids was done through Boham’s protocol (Kumar and Patra, 2017). Briefly, 10g of dry powder of leaves was taken in a beaker and 100ml of 80% methanol was added and filtered. The filtration process was repeated again and again, and the collected extract was transferred into Petri plates to dry completely. The water bath was then used to completely dry the powder and weighted by given equation.
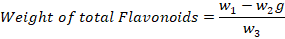
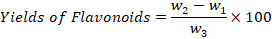
Where;
w1: Petri plate weight; w2: Flavonoids crucible weight; w3: Estimation initial weight of plant sample.
Quantitative test for terpenoids
The total terpenoids were estimated by following the protocol of Indumathi (Indumathi et al., 2014). Ten grams of dry powder of leaf extract was taken and soaked in 9ml of methanol for 24 hours. The solution was filtered and extracted with 10ml of petroleum ether. The ether extract was placed in pre-glass weighed plates and waited to dry out completely. When ether was evaporated completely (%) yield of total contents of terpenoids were measured by using the following formula.
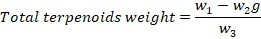
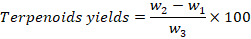
Where;
w1: weight of Petri plate; w2: Terpenoids crucible weight; w3: Estimations initial weight of plant sample.
Antioxidant activity
Quantitative antioxidant potential of leaf extract was performed with previous protocol with some modifications (Sengul et al., 2009). Briefly, DDPH was dissolved in 6.6ml of methanol in test tubes in dark. Different concentrations of methanolic leaves extract were taken in test tubes i.e. (50, 100, 150, 200, 250 µL) and 2mL of 1 mMDDPH solution was added. After that the antioxidant activity was recorded by observing the color change with different concentration of the plant extract. Ascorbic acid (AA) was used as a positive control.
Results and Discussion
Phytochemical contents
The methanolic leaves extract of T. camphoratum were evaluated quantitatively for the alkaloids, flavonoids and terpenoids contents. The results indicated that methanolic leaves extract contained 2.80% alkaloids content, 4.90% flavonoids and 1.80% terpenoids (Table 1). The presence of these effective secondary metabolites played a versatile role in treating many diseases which showed the medicinal value of T. camphoratum.
Antimicrobial activity
The antibacterial potential of methanolic extract of T. camphoratum was determined against Gram- positive bacteria Methicillin resistant staphylococcus aurous (MRSA), Gram-negative bacteria S. typhi, E. coli, P. aeruginosa. The methanolic fraction of T. camphoratum showed good antibacterial activity against Gram-positive stain MRSA (ZOI 23±1.113mm), followed by P. aeruginosa (22±1.1547mm), S. typhi (20±1.231mm), E. coli (18±2.3094mm). Vancomycin was used as a standard control which showed effectiveness against all strains having ZOI 17-18mm Table 1.
Table 1: Quantitative analysis secondary metabolites of Tanacetum camphoratum leaf extract.
|
SN |
Sec. metabolites |
Sec. metabolites in Grams |
Sec. Metabolites per gram of dried powder |
% Yield |
|
1 |
Alkaloids |
0.14 |
0.028 |
2.80 |
|
2 |
Flavonoids |
0.49 |
0.049 |
4.90 |
|
3 |
Terpenoids |
0.18 |
0.018 |
1.80 |
Table 2: Descriptive statistics for zone of inhibition of methanolic leaves extract of Tanacetum camphoratum against bacterial strains.
|
Bacterial Strains |
Extract ZOI (mm) |
Vancomycin ZOI (mm) |
|
MRSA |
23±1.113 |
18 |
|
S. typhi |
20±1.231 |
17 |
|
E. coli |
18±2.3094 |
17 |
|
P. aeruginosa |
22±1.1547 |
18 |
Each value represents the mean ± SD of three replicates.
The extract showed a dose dependent color change, shown the amount of extract reacting with DPPH.
Antioxidant activities
The methanolic fraction of T. camphoratum showed best antioxidant activity, when the DPPH concentration 150μL and the plant extract concentration 250μL was used which showed dark black color. These results demonstrated that the amount of extract will significantly affect the antioxidant activity represented by color changed in a dose dependent manner (Table 3).
Medicinal value of plants is due to their phytochemical constituents that play a major role in curing many infections, and the identification of these led to the discovery of novel drugs and improvements (Pakkirisamy et al., 2017). Plant based bioactive compounds have obvious physiological role in human body in disease condition. For example, gymnemic acid, an active plant based bioactive compound which normalize blood sugar by regenerate β-cells in diabetes and obesity (Mall et al., 2009). In line to this, current study evaluates the T. camphoratum methanolic leaves extract for secondary metabolites, antimicrobial agents, and qualitative antioxidant potential. Our phytochemical analysis revealed the presence of alkaloids, flavonoids and terpenoids contents in leaves extract. Studies have shown that these compounds present antioxidant, antibacterial, antidiabetic anti-inflammatory properties (Al-Owaisi et al., 2014; Basu and Pant, 2013). Plant derived alkaloids, flavonoids and terpenoids compound are considered natural antioxidants and play a major role in removal of disorder partially or totally. These compounds perform free radical scavenging activities by donating hydrogen molecules (Mayakrishnan et al., 2013).
The higher content of flavonoids (4.90%) in methanolic leaves extract of T. camphoratum account for numerous ranges of biological activities such as its use is manifested in antibacterial activity and in management of many diseases like oxidative stress, cardiovascular diseases, live dysfunction, cancer (Saini et al., 2020; Mohammed et al., 2013). This demonstrated that flavonoids, biological and pharmacological effects are dependent on their nature as pro-oxidants or antioxidants (Mihaela et al., 2009). Current study also demonstrated the bacterial suppression and antioxidant activity may be attributed to the presence of flavonoids. Alkaloids, another constituent identified in moderate amount in this study has been linked for centuries with medicinal use. Alkaloids main biological properties involve its toxic nature against
Table 3: Antioxidant Activity of Tanacetum camphoratum of leaf extract by qualitative method.
|
Plant |
Extract Conc (μL) |
DPPH Conc (μL) |
Extract Color |
+Control |
Ascorbic Acid Color |
|
T. camphoratum |
50 |
150 |
Black |
AA |
Slightly White |
|
100 |
150 |
Slightly light black |
AA |
Slightly White |
|
|
150 |
150 |
Slightly Dark black |
AA |
Slightly White |
|
|
200 |
150 |
Moderately black |
AA |
Slightly White |
|
|
250 |
150 |
dark black |
AA |
Slightly White |
+Control = Positive Control, AA represent Ascorbic Acid.
cell’s foreign organism, anti-asthmatic, anti-inflammation, neurologic disorders such as Alzheimer (Gopalakrishnan, 1979; Ganguly and Sainis, 2001; Stærk et al., 2002). The quantitative phytochemical analysis of plant extract showed the presence of terpenoids in low content (1.80%). Terpenoids played a major role as an insecticide and due to this many plants are being utilized in manufacturing pesticide (Stephane and Juleshttps, 2020). Plant based pesticide are less harmful compared to artificial pesticide. Variety of products containing terpenoids such as sprays, shampoo, lotions are widely used for many skin problems treatment (Khaleel et al., 2018).
Similarly, considerable antibacterial activity was showed by the methanolic leaves extract of T. camphoratum against all the test bacteria (MRSA, S. typhi, E. coli, P. aeruginosa). The extract showed antibacterial activity in order of MRSA> S. typhi >P. aeruginosa > E. coli. The antibacterial activity performed by the extract could be explained by the existence of many antibacterial agents. This observation is an agreement with the reports which stated antibacterial activities against a variety of bacterial strains such as E. coli, S. aureus, K. pneumonae, P. mirabilis of S. chinensis (Kannaiyanet al., 2012; Deepa and Bai, 2004). In current study the difference in sensitivity of Gram-positive and Gram-negative bacteria against the extract may be due to morphological variations between them. A similar study also reported less sensitivity of Gram-negative bacteria compared to Gram-positive bacteria against the ethanolic extract of E. elaterium (Felhi et al., 2017). The greater strength of drug resistance in Gram-negative bacteria was attributed due to the morphological complexity of its double membrane having lipoprotein and lipopolysaccharide. This complexity in structure act as barrier for antibacterial agents compared to Gram-positive bacteria. These results suggest the possible medicinal value of T. camphoratum in the treatment of bacterial infection.
Similarly, methanolic fraction of T. camphoratum was also evaluated for the quantitative antioxidant activity by using DPPH radical scavenging assay. Antioxidants upon reaction with DPPH, which is a stable free radical, reduced to DPPH-H and as results absorbance decreases and color changed happened. The extent of changed in color revealed the scavenging potential of extract i.e. higher the extract, higher the color changed reflect good antioxidant potential. The methanolic fraction of T. camphoratum showed best antioxidant activity when we used higher concentration of extract (250μl) and DPPH concentration (150μl) with the appearance of dark black color. Our results are in line with another study in which they reported the antioxidant potential of T. macrophyllum and T. vulgare.
Conclusions and Recommendations
The plant selected (T. camphoratum) in current study is considered to be a rich source of many secondary compounds such as alkaloids, flavonoids, terpenoids. These compounds are responsible for antibacterial and antioxidants activities. The presence of terpenoids provide an additional advantage to T. camphoratum to be use in skin diseases. Further studies will be helpful for the identification of individual compound from the extract. The medicinal value of the plant was screened in a hope to isolate effective small molecules that could be effectively developed as a drug.
Acknowledgments
The authors are thankful to the department of botany Hazara University Mansehra, Pakistan and Department of Botany, University of Malakand, Chakadra Dir Lower to provide all lab work facilities during my research work.
Novelty Statement
This is the first study reported on the biological evaluation on T. camphoratum, collected from District Kohistan.
Author’s Contribution
Muhammad Yahya: Gave the idea and designed and developed th methodology, wrote and reviewed the manuscript.
Muhammad Ajmal Khan: Gave the idea and designed and developed th methodology, acquisition of the data analysed and interpretation of data, wrote and reviewed the manuscript.
Inam Ullah: Acquisition of the data.
Conflict of interest
The authors have declared no conflict of interest.
References
Agnihotri, V.K., S.G. Agarwal, P.L. Dhar, R.K. Thappa, B.K. Kapahi, R.K. Saxena, R.K. and G.N. Qazi. 2005. Essential oil composition of Mentha pulegium L. growing wild in the north-western Himalayas India. Flavour Fragr. J., 20(6): 607-610. https://doi.org/10.1002/ffj.1497
Agrawal, A. and M.M. Srivastava. 2008. Structural elucidation of bioactive principle of root of Boerhavia diffusa for its antifungal properties. Natl. Acad. Sci. Lett., 31(7-8): 215-219.
Al-Owaisi, M., N. Al-Hadiwi and S.A. Khan. 2014. GC-MS analysis, determination of total phenolics, flavonoid content and free radical scavenging activities of various crude extracts of Moringa peregrina (Forssk) Fiori leaves. Asian Pac. J. Trop. Biomed., 4(12): 964-970. https://doi.org/10.12980/APJTB.4.201414B295
Ali, B., Al-Wabel, N.A., Shams, S., Ahamad, A., Khan, S.A. and Anwar, F. 2015. Essential oils used in aromatherapy: A systemic review. Asian Pacific J. Trop. Biomed., 5(8): 601-611. https://doi.org/10.1016/j.apjtb.2015.05.007
Babar, A., N.A. Al-Wabel, S. Shams, A. Ahamad, S. A. Khan and F. Anwar. 2015. Essential oils used in aromatherapy: A systemic review. Asian Pac. J. Trop. Biomed., 5(8): 601-611.
Badawy, M.E. and S.A. Abdelgaleil. 2014. Composition and antimicrobial activity of essential oils isolated from Egyptian plants against plant pathogenic bacteria and fungi. Ind. Crops Prod., 52: 776-782. https://doi.org/10.1016/j.indcrop.2013.12.003
Basu, S. and M. Pant. 2013. Phytochemical evaluation and HPTLC profiling of extracts of Salacia oblonga. Int. J. Pharm. Sci., 4(4): 1409.
Chen, Z., B. He, J. Zhou, D. He, J. Deng and R. Zeng. 2016. Chemical compositions and antibacterial activities of essential oils extracted from Alpinia guilinensis against selected food borne pathogens. Ind. Crops Prod., 83: 607-613. https://doi.org/10.1016/j.indcrop.2015.12.063
Deepa, M.A. and V.N. Bai. 2004. Antibacterial activity of Salacia beddomei. Fitoterapia, 75 (6): 589-591. https://doi.org/10.1016/j.fitote.2004.04.011
Essiett, U.A. and Archibong, I.A. 2014. The taxonomic significance of certain anatomical variation in four genera of Asteraceae. Bull. Envron. Pharmacol. Life Sci., 3(5): 150-163.
Felhi, S., M. Saoudi, A. Daoud, H. Hajlaoui, M. Ncir, R. Chaabane, A.El Feki, N. Gharsallah, N. and Kadri, A. 2017. Investigation of phytochemical contents, in vitro antioxidant and antibacterial behavior and in vivo anti-inflammatory potential of Ecballium elaterium methanol fruits extract. Food Sci. Technol., 37(4): 558-566. https://doi.org/10.1590/1678-457x.26316
Ganguly, T. and K.B. Sainis. 2001. Inhibition of cellular immune responses by Tylophora indica in experimental models. Phytomedicine., 8 (5): 348-355. https://doi.org/10.1078/0944-7113-00055
Gopalakrishnan, C., Shankaranarayan, D., Kameswaran, L. and Natarajan, S. 1979. Pharmacological investigations of tylophorine, the major alkaloid of Tylophora indica. Ind. J. Med. Res. 69: 513–520.
Harborne, J.B. 1992. A guide to modern technique of plant analysis. Chapman and Hill, London. Phytochem. Met., 279.
Indumathi, C., G. Durgadevi, S. Nithyavani and P.K. Gayathri. 2014. Estimation of terpenoid content and its antimicrobial property in Enicostemma litorrale. Int. J. Chem. Technol. Res., 6(9): 4264-4267.
Ji, S., Fattahi, A., Raffel, N., Hoffmann, I., Beckmann, M. W., Dittrich, R. and Schrauder, M. 2017. Antioxidant effect of aqueous extract of four plants with therapeutic potential on gynecological diseases; Semen persicae, Leonurus cardiaca, Hedyotis diffusa, and Curcuma zedoaria. Eur. J. Med. Res., 22(1): 1-8. https://doi.org/10.1186/s40001-017-0293-6
Kannaiyan, M., V.N. Manuel, V. Raja, P. Thambidurai, S. Mickymaray and T. Nooruddin, T. 2012. Antimicrobial activity of the ethanolic and aqueous extracts of Salacia chinensis Linn. against human pathogens. Asian Pacific J. Trop. Dis., 2: S416-S420. https://doi.org/10.1016/S2222-1808(12)60194-7
Khaleel, C., Tabanca, N. and Buchbauer, G. 2018. Terpineol, a natural monoterpene: A review of its biological properties. Open Chem., 16(1): 349-361. https://doi.org/10.1515/chem-2018-0040
Khan, M.S.A. and Ahmad, I. 2019. Herbal medicine: current trends and future prospects. In New Look to phytomedicine (pp. 3-13): Academic Press. https://doi.org/10.1016/B978-0-12-814619-4.00001-X
Koparde, A.A., Doijad, R.C. and Magdum, C.S. 2019. Natural products in drug discovery. in Pharmacognosy-medicinal plants. IntechOpen.
Kumar, A. and S. Patra. 2017. Qualitative and quantitative analysis of secondary phytochemical in gymnema sylvestre. Indian J. Sci. Res., 12(2): 150-156.
Mall, G.K., P.K. Mishra and V. Prakash. 2009. Antidiabetic and hypolipidemic activity of Gymnema sylvestre in alloxan induced diabetic rats. Glob. J. Biotechnol. Biochem., 4(1): 37-42.
Mayakrishnan, V., S. Veluswamy, K.S. Sundaram, P. Kannappan and Abdullah, N. 2013. Free radical scavenging potential of Lagenaria siceraria (Molina) Standl fruits extract. Asian Pac. J. Trop. Med., 6(1): 20-26. https://doi.org/10.1016/S1995-7645(12)60195-3
Mihaela, I., Denisa, M., Luciana, N., Constanţa, G. and Eva, K. 2009. Flavonoids effect on peripheral blood mononuclear cells fluidity. Romanian J. Biophys., 19: 43-8.
Mohammed, H.A., S.K. Alshalmani and A.G. Abdellatif. 2013. Antioxidant and quantitative estimation of phenolic and flavonoids of three halophytic plants growing in Libya. J. Pharmacog. Photochem., 2 (3): 89-94.
Nasir, E. and Ali, S.I. 1972. Flora of Pakistan National Herbarium, NARC. Islamabad. Dept. Botany, Univ. Karachi, Karachi (Fascicles).
Ng, Y.P., Or, T.C.T. and Ip, N.Y. 2015. Plant alkaloids as drug leads for Alzheimer’s disease. Neurochem. Int., 89: pp.260-270. https://doi.org/10.1016/j.neuint.2015.07.018
Obafemi, C.A., Akinpelu, D.A., Taiwo, O.O. and Adeloye, A. 2006. Antimicrobial activity of solvent extracts of Terminalia catappa Linn leaves. Ife J. Sci., 8(1): 29-33. https://doi.org/10.4314/ijs.v8i1.32198
Pakkirisamy, M., Kalakandan, S.K. and Ravichandran, K. 2017. Phytochemical screening, GC-MS, FT-IR analysis of methanolic extract of Curcuma caesia Roxb (Black Turmeric). Pharmacogn. J., 9(6). https://doi.org/10.5530/pj.2017.6.149
Rakholiya, K., M. Kaneria, Desai, D. and S. Chanda. 2013. Antimicrobial activity of decoction extracts of residual parts (seed and peels) of Mangifera indica L. var. Kesar against pathogenic and food spoilage microorganism. Microbial pathogens and strategies for combating them: Science, Technology and Education. FORMATEX., 850-6.
Salamci, E., S. Kordali, R. Kotan, A. Cakir and Y. Kaya. 2007. Chemical compositions, antimicrobial and herbicidal effects of essential oils isolated from Turkish Tanacetum aucheranum and Tanacetum chiliophyllum var. chiliophyllum. Biochem. Syst. Ecol., 35(9): 569-581. https://doi.org/10.1016/j.bse.2007.03.012
Saini, I., Chauhan, J. and Kaushik, P. 2020. Medicinal value of domiciliary ornamental plants of the Asteraceae family. J. Young Pharm., 12(1): 3. https://doi.org/10.5530/jyp.2020.12.2
Sengul, M., H. Yildiz, N. Gungor, B. Cetin, Z. Eser and Ercisli. 2009. Total phenolic content, antioxidant, and antimicrobial activities of some medicinal plants. Pak. J. Pharm. Sci., 22(1).
Shaojian, J., A. Fattahi, N. Raffel, I. Hoffmann, M.W. Beckmann, R. Dittrich and M. Schrauder. 2017. Antioxidant effect of aqueous extract of four plants with therapeutic potential on gynecological diseases; Semen persicae, Leonurus cardiaca, Hedyotis diffusa, and Curcuma zedoaria. Eur. J. Med. Res., 22(1): 1-8.
Sofowora, A., Ogunbodede, E. and Onayade, A. 2013. The role and place of medicinal plants in the strategies for disease prevention. Afr. J. trad. Complem. Alter. Med., 10(5): 210-229. https://doi.org/10.4314/ajtcam.v10i5.2
Stærk, D., Lykkeberg, A.K., Christensen, J., Budnik, B.A., Abe, F. and Jaroszewski, J.W. 2002. In Vitro Cytotoxic Activity of Phenanthroindolizidine Alkaloids from Cynanchum v incetoxicum and Tylophora t anakae against Drug-Sensitive and Multidrug-Resistant Cancer Cells. J. Nat. Prod., 65(9): 1299-1302. https://doi.org/10.1021/np0106384
Stephane, F.F.Y. and Juleshttps, B.K.J. 2020. Terpenoids as important bioactive constituents of essential oils. In Essential Oils-Bioactive Compounds, New Perspectives and Applications. IntechOpen.
Stevens, P.F. 2008. Angiosperm Phylogeny Website http://www.mobot.org/MOBOT/research. APweb
Waltenberger, B., Mocan, A., Šmejkal, K., Heiss, E.H. and Atanasov, A.G. 2016. Natural products to counteract the epidemic of cardiovascular and metabolic disorders. Molecules., 21(6): 807. https://doi.org/10.3390/molecules21060807
Zaman, S.B., M.A. Hussain, R. Nye, V. Mehta, K.T. Mamun and N. Hossain. 2017. A review on antibiotic resistance: alarm bells are ringing. Cureus., 9(6). https://doi.org/10.7759/cureus.1403
Zamir, T., R. Farooqui, M.A. Rajput and K. Mustafa, 2013. In-Vitro Assessment of Antibacterial Activity of Methanol Extract of Brassica Oleracae against Selected Bacterias. Jlumhs., 12(03): 177.
To share on other social networks, click on any share button. What are these?








