Evaluation of the Health Risks Associated with the Seafood Quality of Pakistani Exports
Evaluation of the Health Risks Associated with the Seafood Quality of Pakistani Exports
Saima Majeed1*, Tayyaba Asif2, Aisha Qureshi3, Ayesha Umer3 and Naveed Ahmad3
1Department of Maritime Sciences, Bahria University Karachi, Karachi 75260 Pakistan
2Department of Applied Sciences, Hamdard University, Karachi, Pakistan
3Aquatic Diagnostics and Research Centre, Bahria University Karachi, Karachi 75260 Pakistan
ABSTRACT
The quality of seafood is one of the significant concerns to food processors, buyers, and general wellbeing specialists. This investigation attempted to research the universality of microbial quality and antibiotic chloramphenicol residue in export-oriented frozen and fresh seafood to verify and control contamination for public health safety and international trade. A total of 32 frozen and fresh seafood samples were randomly collected for microbiological analysis including crabs, shrimps, and tin-packed tuna fishes, collected from 10 diverse processing plants situated at the Karachi fish harbor, Pakistan. The examination contains the assurance of total viable aerobic count (TVAC) by the standard plate count method estimated under 5×105 colony forming unit /g, E. coli count was found below 5 MPN/g, yeast and mold count were found under 1000 colony forming unit /g, all analyzed seafood test results were observed under the average permissible limit. Explicit fish pathogens such as Salmonella spp., Vibrio cholerae, Vibrio parahaemolyticus, Listeria monocytogene, and Staphylococcus aureus were likewise inspected yet discovered missing in all the samples under examination. All the frozen seafood samples were judged as protected food from a microbiological perspective and methods are satisfactory limit quantified by the international commission of microbiological specification for food.
Article Information
The article was presented in 42nd Pakistan Congress of Zoology (International) held on 23-25th April 2024, organized by University of Azad Jammu & Kashmir, Muzaffarabad, Pakistan.
Authors’ Contribution
SM wrote the manuscript. TA and AQ edited the manuscript and tested microbiology. AU tested biochemistry. NA helped in over improvement of the study and manuscript.
Key words
Seafood quality, Microbial analysis, Food assessment, Food standards, Food safety
DOI: https://dx.doi.org/10.17582/ppcz/42.01.08
* Corresponding author: saima.bukc@bahria.edu.pk
1013-3461/2024/0001 $ 9.00/0
Copyright 2024 by the authors. Licensee Zoological Society of Pakistan.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Fish and fishery items are one of the indispensable food segments from olden times, it is viewed as a great source of income for the coastline communities, international traders, and trade workers from various nations around the world (Salik et al., 2015; Rashed et al., 2013). Pakistan’s fisheries face difficulties contribute discreetly to economic improvement and social advancement. However global demand for protein-rich fisheries products is rising, providing chances for ocean-facing countries such as Pakistan (Yousuf et al., 2008). The trade of frozen fish, dry fish, salted and dehydrated fish is growing gradually from Pakistan to an extensive range of international markets including Japan, Vietnam, China, Taiwan, Hong Kong, Indonesia, Egypt, Saudi Arabia, UAE, Malaysia, Sri Lanka, UK, Bahrain, Qatar, Singapore, Kuwait Thailand, South Korea, and Bangladesh, etc. Although there are 55 seafood processing plants in Pakistan. Therefore, preserving the quality of frozen fish and fisheries is important for its acceptance in global markets as well as for the well-being of consumers (Khan and Abbas, 2023).
Seafood is an essential component of a healthy and balanced diet because of its rich nutritional content. The World Health Organization emphasized its addition to diet as it helps to prevent different diseases. In Europe alone, more than 70 % of non-communicable health-related issues are associated with food that is consumed regularly. Consumption of seafood once or twice a week is recommended for maximum health benefits to human health (Liu and Ralston, 2021).
Pakistan is home to different ethnic and cultural societies along the people here consume different processed and non-processed foods. For the proper quality control checks different food safety and food quality standards are implemented in the industry to reduce food fraud and provide better food security. Pakistan Standards and Quality Control Authority (PSQCA) and Pakistan Council of Scientific and Industrial Research (PCSIR) are working in these domains and provide food consultation, testing, and certification services. These organizations are working under government and provincial offices are working to regulate and enforce quality standards, ensuring safe food production and distribution, and correct labeling. These objectives could be achieved through inspections, testing, and penalties in case of non-conformity (Asghar et al., 2023). Food security is a perplexing subject, where fish and fishery items are commonly viewed as hazardous commodities concerning their pathogen contents, regular pollutants, and other possible impurities and adulterants (De-Almeida et al., 2019; Adebayo-Tayo et al., 2012). Fish and shellfish consumption might cause sickness because of contamination or inebriation, some of these illnesses have been explicitly connected with microbes, which are impervious to antibiotics. Proper preservation and storage during the processing of seafood are also important, it has been assessed that more than 80 million cases annually of fish-borne infirmities on antibiotic resistance in the USA, and the expense of these illnesses are in billions of dollars each year (Gnanambal et al., 2005). Foodborne pathogens, such as Vibrio spp. and Salmonella. spp. is normally present in oceanic conditions and might cause contamination (Hernández-Cortez et al., 2017; Osunla and Okoh, 2017). The third-highest number of shellfish-associated illnesses is caused by Vibrio cholerae, the infections result in septicemia and mild gastroenteritis while the occurrence of Salmonella contamination as a result of seafood consumption is still low (Novoslavskij et al., 2016). Microbes may contaminate the fish from outside during off-hand management, packing, and cutting, the major external sources of bacterial loads are crushed ice and salt. A portion of the original microbial populace present on the surface of shrimps and crabs known as Specific Spoilage Organisms (SSO) causes the decay by producing metabolites that are liable for off-flavors and the organoleptic rejection of the item. Such conditions are resolved generally by the temperature and environmental situations during storage (Gram and Huss, 1996).
Seafood is highly liable to both microbiological and synthetic deterioration because of its high-water content, neutral pH, relatively huge amounts of free amino acids, and naturalistic occurrence of autolytic enzymes (Saloko et al., 2014). The most serious seafood safety issues that result in contaminated products are related to microbial and particularly bacterial pathogens (Jeyasekaran et al., 2006). But it can also be the result of cross-contamination due to the inappropriate handling and storage of fish (Bonnell, 2012; Huss, 1994). HACCP principles are being implemented, and various educational programs have been developed for food handlers and the consumer. Microbiological methods are utilized to gauge bacterial number, fish freshness, and hygiene, and to evaluate the conceivable presence of microbial organisms of public health concern (Cruz‐Romero et al., 2008).
The fisheries sector in Pakistan has assumed a considerable part in the reduction of destitution and achievement of food security in the last few years, the fare of fish and fishery products from Pakistan has grown remarkably to earn foreign exchange. As indicated by the centre for disease prevention and control (CDC), from 1973 to 2006, a variety of bacteria, viruses, and parasites were responsible for seafood contamination. The defilement might also be caused by the icing and water applied for washing (Fleming et al., 2018). So, proper preservation of the accurate value of the products is observed as vital for attaining the desired achievement in the international market of seafood products. The most common seafood exported from Pakistan is brown prawn (Metapenaeus monoseros), flower prawn (Penaeus semisulcatus), pink shrimp (Metapenaeus dobsoni), tiger prawn (Penaeus.monodon), white prawn (Penaeus indicus) and karikadi (Parapenaeopsis stylifera), mud crab (Scylla serrata), blue crab (Portunus pelagius), ribbon fish (L. savala), mackerel (Rastrelliger kanagurta), tuna (Thunnus albacares).
The seafood industry of Pakistan costs around $1.2 billion among those exports worth $370 million per annum. It is estimated that 0.8 million people and their families rely on this industry still the major threats faced by the sector include overfishing, poor quality control of catch, nets used for fishing, and less storage and preservation facilities in the trawler as well as processing plants. Overfishing impacts badly on the population of fish as random fishing reduces the population while on the other side, poor quality control and fewer storage facilities damage the catch and increase the waste (Ilyas et al., 2022).
Materials and Methods
Preparation of media and glassware
All the chemicals and glassware used in the current study are of analytical grade and were purchased from Sigma Aldrich, Daejung, Merck, and BDH. The glassware was washed and rinsed with distilled water and was further dried and sterilized in a hot air oven at 160°C for 1 h as per the protocol (Adibe and Eze, 2004). Commercial dehydrated culture media were purchased from Oxoid and BDH and prepared as per the instructions of the manufacturer and were further sterilized in an autoclave at 121°C for 15 min. The wire loop was sterilized before using each time on Bacti-cinerator sterilizer.
Study area and sample collection
In the current study, different seafood samples were collected from 10 different processing plants located at nearby locations in Karachi Fish Harbor, Karachi, Pakistan. All samples were packed in isolated polystyrene boxes with ice and shifted to the laboratory within 1 h. All the samples were in a sterile container under aseptic conditions and kept on ice. These samples include blue crabs (Portunus pelagicus), mud crab (Scylla serrata), giant tiger prawn (Penaeus monodon), white prawn (Penaeus indicus), tongue sole fish (Cynoglossus broadhursti), cuttle fish (Sepia officinalis), ribbon fish (Lepturacanthus savala), mackerel (Rastrelliger kanagurta), tuna (Thunnus albacares). The collected samples were permitted to defrost at 4°C. The experiments were performed in triplicate for their microbiological flora analysis including total viable aerobic count (TVAC), total yeast and mold count (TYMC), and enumeration of E. coli. detection of Salmonella Spp., Vibrio cholera, Vibrio parahaemolytica, Listeria monocytogenes, and Staphylococcus aureus.
Sample processing
The sample was processed for microbiological testing by the method (Pomeranz, 2013). Ten gram raw and uncooked samples of crab meat and shrimp meat and 10 g cooked samples of fish were homogenized in 90 ml of phosphate buffered saline (1:10 dilution). These samples were further serially diluted in phosphate buffer saline up to 10-6.
The sterile sample was aseptically treated and minced properly followed by the transfer of 1 ml sample into 9 ml sterile saline and mixed properly. Serial dilution was prepared by transferring 1 ml sample from each tube to the next tube till 10-6.
Assessment of bacterial contents and enumeration of total aerobic microorganisms and yeast molds
The bacterial load of frozen food was assessed by the protocol of Sielaff et al. (2019). For the enumeration of total aerobic bacteria and total yeast and mold count, 1 ml of each sample was inoculated for total plate count agar (TPCA) and sabouraud dextrose agar (SDA) through pour plate technique. For total aerobic count, TPCA media plates were incubated at 37 °C for 2 days, and for the assessment of fungus, SDA media plates were incubated at 25°C for 5 days in the dark.
The calculation procedure for the total bacterial and fungal count was mentioned by (Rahimi et al., 2019). The total colonies were estimated and shown in colony-forming units per gram (CFU g-1).
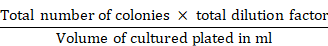
Isolation and identification of pathogenic strains
For the isolation of Coliforms, Salmonella spp., Vibrio spp., and Staphylococcus spp., samples were pre-enriched in a pre-enrichment broth of Butterfield’s phosphate-buffered water and lactose broth, respectively.
The 50 g sample was inoculated in 450 ml of buffer water and 25 g of sample in 225 ml of lactose broth and blended well, respectively. The samples were further incubated at 35 ± 2 °C for 6 to 8 h and 24 ± 2 h for Vibrio spp., Salmonella spp., Staphylococcus spp., and Coliforms, respectively. The 1.0 ml of sample for the isolation of Salmonella spp., was then further transferred into 10, 10 ml of pre-incubated RVS and T.T broth at 24 ± 2 h and 42 ± 2 °C, respectively. The 100 µl samples were streaked using a sterile glass spreader on pre-incubated plates of thiosulphate citrate bile salt sucrose (TCBS) agar, xylose lysine deoxycholate (XLD), mannitol salt agar (MSA), and MacConkey agar, EMB agar for Vibrio spp., Salmonella spp., Staphylococcus spp., and Coliforms, respectively. The plates were incubated at 37 °C for 24 to 48 h for Escherichia coli while coliform plates were incubated at 44.5°C for 24 h. While for the isolation of other pathogenic strains, plates were incubated at 37 °C overnight.
All isolates were purified through the four-plate streak method of streaking further purified cultures were sub-cultured and gram-staining was performed for confirmation. Identification of bacterial and fungal isolated strains was performed as described by El-Sayed et al. (2015), Ibrahim et al. (2014) and Eze et al. (2011).
Different biochemical tests were performed as recommended by bergey’s manual of systematic bacteriology (Vos et al., 2011) for identification of bacteria.
Detection of Chloramphenicol residues in sea food samples
A total of 20 g of tissue samples were isolated from each processing plant sample and then transferred to the University of Karachi for analysis of chloramphenicol through HPLC (Akter-Mou et al., 2021). Ten grams of smashed seafood meat samples were mixed with equal volume of PBS buffer (pH = 6.88), shaken vigorously for 10 min and centrifuged for 5 min at 5500 rpm to collect the upper layer. Ethyl acetate (10 ml) was added, and centrifuged again. The extract was reduced with a nitrogen stream in a water bath at 45–50 °C. Two ml of methanol. 10 ml of 4% NaCl solution and 20 mL of hexane were added. The mixture was shaken for about 30 sec. The top layer of hexane was removed by aspiration and discarded. The aquens layer was analyzed by HPLC.
Results and Discussion
Bacterial development in frozen fish is one of the primary sources of food deterioration or contamination of fish. Thus, the microbial investigation of the frozen fish sample including processing water and ice works as a marker of fish quality assurance (Jacquin et al., 2020; Zeitoun and Mehana, 2014; Fisher et al., 2013). The main sign of spoilage as an off-flavor and unsavory taste of fish is a result of fungal contamination and may pose a public health risk and financial losses (Lund et al., 2000). The fungal infection of fish could be credited to improper hygiene during the catching, handling, manufacturing, storage, transportation, and marketing of fish. These findings were view reported (El-Ahl and Rasha, 2010). Many species of fungi may cause pulmonary infections, urinary tract infections, arthritis, osteomyelitis, dermatitis, endocarditis, meningitis, and eye infections (Hernández-Cortez et al., 2017). Fish caught in a polluted area may also raise the level of contamination (Ylitalo et al., 2012)
The current examination revealed that the entirety of the fish, shrimps, and crabs alongside the materials satisfy the guideline levels proposed by ICMSF which showed that aseptic and legitimate sterile conditions were kept up appropriately all through all means, for example, catching, landing, transportation, processing, management, and preservation.
The TVAC maximum microbiological limit for seafood is 5 × 105 cfu/g (Boyd and Tucker, 2012). The studied samples TVAC within the limit of 1.8 × 104 cfu/g which was lower than the maximum satisfactory limit as shown as in Figure 1A. Thus, all the samples meet the acceptable limit of TVAC specified by ICMSF which confirms the good quality of the frozen seafood. It also indicates that processing plants maintain a good temperature maintenance system which protects the seafood from spoilage.
Total yeast and mold count (TYMC) has a limit of 1 x 103. Yeast and mold were detected in the study at less than 1000 CFU/g, indicating that all seafood samples were within the permissible range for health and safety standards. This also suggests that the samples were taken from clean water and that food handlers and processors kept aseptic conditions throughout the processing (Fig. 1B).
E. coli in seafood is allowed in 1 MPN/g to 4 MPN/g. In the present study, E. coli loads of all the seafood samples were within the recommended limits the E. coli ranged from 1 MPN/g to 4 MPN/g showing that all the samples were received from pollution-free water which is probably a brackish water sample. The food processors and the controllers kept aseptic conditions and better temperature checks during overall processing as shown in Figure 1C.
In the 1930s the use of E. coli has been first reported as a sanitary indicator for fish samples (Gephart et al., 2017). E. coli is normally related to seafood contamination in the tropics. The lower number of E. coli can be valuable for identifying the accuracy of safety procedures during processing and handling (Hassan et al., 2007). There is a
complete absence of other pathogenic species in seafood-based Products. Pathogenic load absence of all the seafood samples suggested and represented that all the samples were received from pollution-free water, and the hygienic condition of the seafood was maintained after capturing. It also indicated that the seafood processors and the controllers kept aseptic conditions throughout the processing which ultimately resulted in healthy and free of contamination. Seafood microbial contamination risk associated with human disease in fresh seafood is not low. Storage and transportation in ice (0 °C) or freezing is the most effective prevalence of bacterial loads, cooking properly immediately before consumption is also an effective way of reducing hazards of fresh fish-borne disease. The most commonly recognized microbiological criteria for frozen raw fish are those set for aerobic plate counts (APC) at 25 °C, and E. coli proposed by the International Commission on Microbiological Specifications for Foods (ICMSF).
Regarding other pathogens none of the 32 seafood samples collected from Karachi fish harbor seafood processing industries had any of Salmonella spp., Vibrio cholerae, Vibrio parahaemolyticus, Listeria monocytogene, and Staphylococcus aureus. The microbiological quality of seafood in warm water areas is uncertain where fish are to be consumed raw and an increase of APC to levels in excess is usually indicative of unsatisfactory refrigeration, it may be necessary to test for pathogens like Salmonella and Vibrio spp. Proper processing, storage, and handling procedures of fish should be employed. Satisfactory dealing procedures before the consumption of fish are essential. Fish that physically indicate spoilage should not be retailed in the markets. In addition, manufacturing practices should be monitored by the trade authority to abate the risk of seafood products. An understanding of the etiologic agents and mechanisms of contamination that are agreeable to control is thus needed for the inhibition of seafood-associated infection outbreaks. There is a need to practice surveillance systems with pathogen-specific techniques to elude any future outbreaks.
Centers for disease prevention and control (CDC) revealed that the presence of profoundly pathogenic microorganisms i.e., Salmonella identified by the consumption of frozen raw tuna fish in 11 states of the USA and caused 18 outbreaks, 374 ailments, and 28 hospitalizations. The contamination was portrayed by diarrhea, fever, and intestinal cramps (Iwamoto et al., 2010). Pathogenic Vibrio causes hazardous foodborne diseases and is spoken to as a significant microbial representative of sporadic and epidemic human contamination (Espineira et al., 2010). Fish consumption isn’t a hazard because of ocean pollution by anthropocentric exercises and ecological factors (Hassan et al., 2018). The investigation of the microbial variety of fish is currently required as there is very little data available on the effect of water pollutants on fish life and its effect on human health since we need to handle new difficulties that will influence human well-being by consumption of contaminated fish, climate change and antimicrobial resistance. In this regard, few reports were going through with the emphasis on water quality and its effect on the health, and mental wellbeing of consumers (Marshall et al., 2019). The foundation of astute techniques toward fish security and quality affirmation inside the setting of such difficulties will add to sustainable development goals and one health.
However, in Pakistan, food-borne ailment from fresh or frozen fish consumption has not been reported and information on this issue is as yet deficient. The majority of the isolated genera are heat-labile and satisfactory cooking will inactivate it (Samarasekera et al., 2017; US Food and Drug Administration, 2011). While inappropriate dealing with and cross-pollution or crude fish dietary patterns may represent a well-being peril, particularly to susceptible populaces such as the immunosuppressed i.e., youngsters and older individuals.
High-pressure processing (HPP) has much applicability in seafood processing plants as it eliminates most seafood pathogens, such as Vibrio and Listeria spp., and slows down the growth of microorganisms responsible for food spoilage. As the water used in the system is cold water as a pressure medium, the sensory and nutritional properties of the seafood remain constant resulting in the development of clean-label seafood products. The same technology also helped to recover the maximum amount of edible meat from oysters, lobsters, crabs, mussels, clams, and scallops. HPP of seafood helps to confine the organoleptic and functional properties of seafood for an extended period, especially during refrigeration. It also helps to maintain a balance between safety, quality, processing efficiency, and regulatory compliance (Roobab et al., 2022).
In the present study chloramphenicol (CAP) residues of all the seafood samples were within the recommended limits of the European law ranging from 0 ppb to 0.1 ppb showing that all the samples were received from an antibiotics-free environment (Fig. 2).
The existence of chloramphenicol in food products is a violation of European law and many other countries, as it is related solely to the illegitimate practice of chloramphenicol in food production, very low residue levels pose a risk to consumer health, 0.3 ppb as a minimum required performance limit (MRPL) (Byrne, 2003).
This research revealed that the seafood under scrutiny from various processing plants in Karachi, Pakistan was of acceptable quality with low microbial load, and CAP residue might be because of a reduced amount of seawater pollution. So, can be concluded that the investigated frozen fishes, shrimps, and crabs were prepared with appropriately treated microorganism-free water and ice and kept up under great stockpiling conditions. Thus, these frozen fishes, shrimps, and crabs were qualified enough for trade additionally as human utilization according to a bacteriological perspective.
Conclusion
In conclusion, the microbiological quality in terms of microbial growth and antibiotic chloramphenicol CAP residue in exported frozen seafood from Pakistan was within the recommended limit of ICMSF. Hence, the examined frozen fishes were capable enough for export as well as for human consumption from the microbial and CAP residue point of view. The presence of viruses, parasites, viable but non-culturable (VBNC) state of the pathogenic bacteria, and biochemical parameters such as histamine risk might be a problem with frozen fish products which is the limitations of this study. It is revealed from the present finding that the seafood exported from Pakistan is well managed (Capturing, handling, freezing, and packing) according to the standard. To observe more rigorous quality standards of the trading countries, these quality parameters must be taken into consideration.
Declarations
Acknowledgments
The study was supported by the Aquatic Diagnostics and Research Centre. We are thankful to Bahria University Karachi Campus for providing Laboratory space and logistics.
Funding
The study received no funding.
Ethical statement
All applicable institutional, national and international guidelines for the care and use of animals were followed.
Statement of conflict of interest
The authors have declared no conflict of interest.
References
Adebayo-Tayo, A.C., Odu, N.N., Michael, M.U. and Okonko, I.O., 2012. Multi-drug resistant (MDR) organisms isolated from sea-foods in Uyo, south-southern Nigeria. J. Nat. Sci., 10: 61-70.
Adibe, N.C. and Eze, E.I., 2004. General laboratory techniques for tertiary institutions. Acad. Sci., 101: 17593-17598. https://doi.org/10.1073/pnas.0407921102
Akter Mou, S., Islam, R., Shoeds, M. and Nahar, N., 2021. Determinations of chloramphenicol in meat samples using liquid chromatography tandem mass spectrometry. Fd. Sci. Nutr., 9: 5670-5675. https://doi.org/10.1002/fsn3.2530
Asghar, W., Akhtar, A., Rahman, H.U., Sami, A. and Khalid, N., 2023. Global perspective on food fraud with special emphasis on the prevalence of food fraud practices and policies in Pakistan. World Fd. Policy, 9: 93-126. https://doi.org/10.1002/wfp2.12056
Bonnell, A.D., 2012. Quality assurance in seafood processing: A practical guide, Springer Science and Business Media, US.
Boyd, C.E. and Tucker, C.S., 2012. Pond aquaculture water quality management, Springer Science and Business Media, US.
Byrne, D., 2003. For the commission. The decision, C. 2003/181/EC of 13 March 2003 amending Decision 2002/657/EC as regards the setting of the minimum required performance limits (MRPLs) for certain residues in food of animal origin. Off. J. Eur. Commun., 71: 17-18.
Cruz-Romero, M., Kelly, A.L. and Kerry, J.P., 2008. Influence of packaging strategy on microbiological and biochemical changes in high-pressure-treated oysters (Crassostrea gigas). J. Sci. Fd. Agr., 88: 2713-2723. https://doi.org/10.1002/jsfa.3398
De-Almeida Couto, C.R., De-Assis Leite, D.C, Jurelevicius, D., Van-Elsas, J.D. and Seldin, L., 2019. Chemical and biological dispersants differently affect the bacterial communities of uncontaminated and oil-contaminated marine water. Braz. J. Microbiol., 51: 1-10. https://doi.org/10.1007/s42770-019-00153-8
El-Ahl, M.H.S. and Rasha, A., 2010. Studies on fungi in fish and fish products and their control. Dissertation, Cairo University.
El-Sayed, M.H., El-Aziz, Z.K.A. and Elbadawy, H.H., 2015. Evaluation of the microbial spoilage of Atlantic salmon (Salmo salar) fillets during the packaging processes and its control by preservatives. Int. J. Sci. Res. Sci. Eng. Technol., 1: 134-141.
Espineira, M., Atanassova, M., Vieites, J.M. and Santaclara, F.J., 2010. Validation of a method for the detection of five species, serogroups, biotypes, and virulence factors of Vibrio by multiplex PCR in fish and seafood. Fd. Microbiol., 27: 122-131. https://doi.org/10.1016/j.fm.2009.09.004
Eze, E.I., Echezona, B.C. and Uzodinma, E.C., 2011. Isolation and identification of pathogenic bacteria associated with frozen mackerel fish (Scomber scombrus) in a humid tropical environment. Afr. J. agric. Res., 6: 1947-1951.
Fisher, N.S., Beaugelin-Seiller, K., Hinton, T.G., Baumann, Z., Madigan, D.J. and Garnier-Laplace, J., 2013. Evaluation of radiation doses and associated risk from the Fukushima nuclear accident to marine biota and human consumers of seafood. Proc. natl. Acad. Sci., 110: 10670-10675. https://doi.org/10.1073/pnas.1221834110
Fleming, L.E., Katz, D., Bean, J.A. and Hammond, R., 2018. Epidemiology of seafood poisoning. In: Foodborne disease handbook: Volume IV seafood and environmental toxins. Taylor and Francis Group, Boca Raton.
Gephart, J.A., Troell, M., Henriksson, P.J., Beveridge, M.C., Verdegem, M., Metian, M. and Deutsch, L., 2017. The seafood gap in the food-water nexus literature issues surrounding freshwater use in seafood production chains. Adv. Water Resour., 110: 505-514. https://doi.org/10.1016/j.advwatres.2017.03.025
Gnanambal, M.E. and Patterson, J., 2005. Biochemical and microbiological quality of frozen fishes available in Tuticorin super markets. Fishery Technol., 42: 1-6.
Gram, L. and Huss, H.H., 1996. Microbiological spoilage of fish and fish products. Int. Fd. Microbiol., 33: 121-137. https://doi.org/10.1016/0168-1605(96)01134-8
Hassan, A.A., Hammad, A.M., El- Barawy, A.M. and Manal, A.H., 2007. Incidence of aflatoxigenic fungi in frozen and canned fishes and trials to inhibit aflatoxin production by use of some minor elements and lupinustermis seeds. Egypt. J. appl. Sci., 22: 351-360.
Hassan, R., Tecle, S., Adcock, B., Kellis, M., Weiss, J., Saupe, A. and Neil, K.P., 2018. Multistate outbreak of Salmonella paratyphi B Variant L (+) tartrate (+) and Salmonella Weltevreden infections linked to imported frozen raw tuna: USA, March–July 2015. Epidemiol. Infect., 146: 1461-1467. https://doi.org/10.1017/S0950268818001462
Hernández-Cortez, C., Palma-Martínez, I., Gonzalez-Avila, L.U., Guerrero-Mandujano, A., Solís, R.C., and Castro-Escarpulli, G., 2017. Food poisoning caused by bacteria (food toxins). Poisoning: From specific toxic agents to novel rapid and simplified techniques for analysis. pp. 33. https://doi.org/10.5772/intechopen.69953
Huss, H.H., 1994. Assurance of seafood quality (No. 334), Food and Agriculture Org.
Ibrahim, B.U., Baba, J. and Sheshi, M.S., 2014. Isolation and identification of bacteria associated with fresh and smoked fish (Clarias gariepinus) in Minna Metropolis, Niger State, Nigeria. Appl. environ. Microbiol., 2: 81-85.
Ilyas, F., Gillani, D.Q., Yasin, M., Iqbal, M.A., Javed, I., Ahmad, S. and Nabi, I., 2022. Impact of livestock and fisheries on economic growth: An empirical analysis from Pakistan. Sarhad J. Agric., 38:160-169. https://doi.org/10.17582/journal.sja/2022/38.1.160.169
Iwamoto, M., Ayers, T., Mahon, B.E. and Swerdlow, D.L., 2010. Epidemiology of seafood-associated infections in the United States. Clin. Microbiol. Rev., 23: 399–411. https://doi.org/10.1128/CMR.00059-09
Jacquin, L., Petitjean, Q., Côte, J., Laffaille, P. and Jean, S., 2020. Effects of pollution on fish behavior, personality, and cognition: Some research perspectives. Front. Ecol. Evol., 8: 86. https://doi.org/10.3389/fevo.2020.00086
Jeyasekaran, G., Ganesan, P., Anandaraj, R., Shakila, R.J. and Sukumar, D., 2006. Quantitative and qualitative studies on the bacteriological quality of Indian white shrimp (Penaeus indicus) stored in dry ice. Fd. Microbiol., 23: 526-533. https://doi.org/10.1016/j.fm.2005.09.009
Khan, M.W. and Abbas, G., 2023. Studies on the use of aquatic food in Pakistan. J. Zoo Sys., 1: 40-57. https://doi.org/10.56946/jzs.v1i2.246
Liu, C. and Ralston, N.V., 2021. Seafood and health: What you need to know? Adv. Fd. Nutr. Res., 97: 275-318. https://doi.org/10.1016/bs.afnr.2021.04.001
Lund, B., Baird-Parker, A.C., Baird-Parker, T.C., Gould, G.W. and Gould, G.W., 2000. Microbiological safety and quality of food. Springer Science and Business Media, US.
Marshall, K.H., Booth, H., Harrang, J., Lamba, K., Folley, A., Ching-Lee, M., Hannapel, E., Greene, V., Classon, A., Whitlock, L. and Shade, L., 2015. New product, old problem (s): multistate outbreak of Salmonella Paratyphi B variant L (+) tartrate (+) infections linked to raw sprouted nut butters. Epidemiol. Infect., 147: e20.
Novoslavskij, A., Terentjeva, M., Eizenberga, I., Valciņa, O., Bartkevičs, V. and Bērziņš, A., 2016. Major foodborne pathogens in fish and fish products: A review. Annls Microbiol., 66: 1-15. https://doi.org/10.1007/s13213-015-1102-5
Osunla, C.A., and Okoh, A.I., 2017. Vibrio pathogens: A public health concern in rural water resources in Sub-Saharan Africa. Int. J. environ. Res., 14: 1188. https://doi.org/10.3390/ijerph14101188
Pomeranz, Y., 2013. Food analysis: Theory and practice. Springer Science and Business Media, US.
Rahimi, S.M., Ebrahimi, M., Barikbin, B. and Zeinali, T., 2019. Evaluation of bacterial and fungal contamination of kitchens of Birjand University of Medical Sciences. BMC Res. Notes, 12: 1-6. https://doi.org/10.1186/s13104-019-4741-y
Rashed, N., Mrityunjoy, A., Tasnia, A., Das, K.K., Paul, L., Munshi, S.K. and Alam, M.Z., 2013. Microbiological study of major sea fish available in local markets of Dhaka city, Bangladesh. J. Microbiol. Biotechnol. Fd. Sci., 2: 2420-2430.
Roobab, U., Fidalgo, L.G., Arshad, R.N., Khan, A.W., Zeng, X.A., Bhat, Z.F. and Aadil, R.M., 2022. High-pressure processing of fish and shellfish products: Safety, quality, and research prospects. Comp. Rev. Fd. Sci. Fd. Saf., 21: 3297-3325. https://doi.org/10.1111/1541-4337.12977
Salik, K.M., Jahangir, S. and Ul Hasson, S., 2015. Climate change vulnerability and adaptation options for the coastal communities of Pakistan. Ocean Coast Manage., 112: 61-73. https://doi.org/10.1016/j.ocecoaman.2015.05.006
Saloko, S., Darmadji, P., Setiaji, B. and Pranoto, Y., 2014. Antioxidative and antimicrobial activities of liquid smoke nanocapsules using chitosan and maltodextrin and its application on tuna fish preservation. Fd. Biosci., 7: 71-79. https://doi.org/10.1016/j.fbio.2014.05.008
Samarasekera, K. and Abeygunawardena, S.I., 2017. Microbiology of seawater and sand in a selected bathing site of Sri Lanka–A study towards microbial quality assessment. Front. Environ. Sci., 3: 9-18. https://doi.org/10.11648/j.fem.20170301.12
Sielaff, A.C., Urbaniak, C., Mohan, G.B.M., Stepanov, V.G., Tran, Q., Wood, J.M. and Venkateswaran, K., 2019. Characterization of the total and viable bacterial and fungal communities associated with the International Space Station surfaces. Microbiome, 7: 1-21. https://doi.org/10.1186/s40168-019-0666-x
US Food and Drug Administration, 2011. Fresh and frozen seafood: Selecting and serving it safely.
Vos, P., Garrity, G., Jones, D., Krieg, N.R., Ludwig, W., Rainey, F.A. and Whitman, W.B., 2011. Bergey’s manual of systematic bacteriology: The firmicutes.Springer Science And Business Media, pp. 3.
Ylitalo, G.M., Krahn, M.M., Dickhoff, W.W., Stein, J.E., Walker, C.C., Lassitter, C.L. and Dickey, R.W., 2012. Federal seafood safety response to the deepwater horizon oil spill. Proc. natl. Acad. Sci., 109: 20274-20279. https://doi.org/10.1073/pnas.1108886109
Yousuf, A.H.M., Ahmed, M.K., Yeasmin, S., Ahsan, N., Rahman, M.M. and Islam, M.M., 2008. Prevalence of microbial load in shrimp, Penaeus monodon and prawn, Macrobrachium rosenbergii from Bangladesh. World J. agric. Sci., 4: 852-855.
Zeitoun, M.M. and Mehana, E.E., 2014. Impact of water pollution with heavy metals on fish health: Overview and updates. Glob. Vet., 12: 219-231.
To share on other social networks, click on any share button. What are these?








