Etiology of Subclinical Bovine Mastitis In Biblian- Ecuador
Research Article
Etiology of Subclinical Bovine Mastitis In Biblian- Ecuador
Mercy Cuenca-Condoy1*, Lourdes Reinoso-García2, Juan González-Rojas2, Dionel García-Bracho3
1School of Veterinary Medicine, Academic Unit of Agricultural Sciences. Catholic University of Cuenca. Av. Las Américas, Cuenca 010101, Ecuador; 2Center for Research, Innovation and Technology Transfer (CIITT), Catholic University of Cuenca. Ricaurte 010162, Ecuador; 3School of Veterinary Medicine, University of Zulia. Maracaibo Venezuela.
Abstract | Mastitis is an inflammatory reaction of infectious, traumatic or toxic origin of the mammary gland tissue. It is a common pathology in dairy cows and of economic importance in the dairy industry worldwide. The objective of the study was to identify the microorganisms that cause subclinical bovine mastitis and their frequency in the Biblián-Ecuador canton. One thousand four hundred and forty mammary quarters were analyzed, coming from 360 Holstein cows in milk production. The California Mastitis Test (CMT) was used to qualitatively verify the increase in somatic cell count (SCC) in milk to indicate the presence of microorganisms causing bovine mastitis. Of the 1440 quarters studied, 175 were found positive by CMT, these were plated in duplicate and incubated at 37°C for 18 hours. The microorganisms were isolated and identified by MAL-DI-TOF. The analyses determined the presence of Staphylococcus aureus, Staphylococcus chromogenes, Staphylococcus haemolyticus, Staphylococcus xylosus, Staphylococcus epidermidis, Streptococcus uberis, Streptococcus agalactiae, Lactobacillus mali, Moraxella osloensis, Kocuria salsiccia, Bacillus weihenstephanensis, Corynebacterium xerosis, Macrococcus canis, Chryseobacterium bovis, Rothia endophytica, among others. However, Staphylococcus chromogenes, Lactobacillus mali and Staphylococcus aureus were the most frequent bacteria in cases of subclinical bovine mastitis, reaching 23.4, 10.9 and 9.5% presence, respectively; Likewise, it was found that S. chromogenes and L. mali are more frequent in mechanical milking systems; S. aureus and S. uberis are present in equal frequency in mechanical and manual milking; Acinetobacter iwoffi and Kocuria salsiccia were only recorded in mechanical milking of dairy herds in Biblián -Ecuador. The study results suggested to improve management and hygiene in dairy farms of study area.
Keywords | Bovine, Mastitis, Etiology, Biblián - Ecuador.
Received | October 23 2023; Accepted | December 27, 2023; Published | March 20, 2024
*Correspondence | Mercy Cuenca-Condoy, School of Veterinary Medicine, Academic Unit of Agricultural Sciences. Catholic University of Cuenca. Av. Las Américas, Cuenca 010101, Ecuador; Email: [email protected]
Citation | Cuenca M, Reinoso L, González J, García D (2024). Etiology of subclinical bovine mastitis in biblian- ecuador. J. Anim. Health Prod. 12(1): 100-107.
DOI | http://dx.doi.org/10.17582/journal.jahp/2024/12.1.100.107
ISSN | 2308-2801

Copyright: 2024 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
INTRODUCTION
Mastitis is the most common disease affecting dairy cattle (Ruíz et al., 2016; Ji et al., 2022). It presents inflammation of the mammary gland, changes in the glandular tissue and chemical composition of milk (López, 2014). Therefore, it is highly prevalent and causes severe economic losses challenging to estimate (Chaves et al., 2017; Neculai et al., 2021; Maldonado et al., 2022; Aken et al., 2022; Demil et al., 2022) ranging from 170.00 to 400.00 $/cow/year (Araúz, 2011). For example, China reports losses range from 12,000 to 76,000 USD/farm/month (He et al., 2019), Egypt annually losses of 21,933,258.6 LE are reported (Azooz et al., 2020) due to decreased production and loss of quality (Year and Ramírez, 2016), therapeutic costs, discarding of young animals with high genetics (Bedolla, 2008; Bonifaz and Conlago, 2016; Mera et al., 2017) and negative effect on fertility (Pedroso and Roller, 2017), with gestation losses of 89% between day 30 – 60 of pregnancy (Miranda et al., 2019).
Mastitis is the costliest of the infectious diseases of dairy cattle (Fernandez et al., 2012; Cuéllar, 2020; Puerto et al., 2021) affecting 16% of gross income and up to 20 -25% loss of total production (Vissio et al., 2015). The subclinical form constitutes 70 to 75% of total economic losses, and affects between 15 and 40% of the mammary quarters (Araúz, 2011; Bobbo et al., 2017), costing $ 108-252/cow/year (Aken et al., 2022). It is caused by multiple animal-dependent and independent factors (Mora et al., 2015); infectious agents are the most important aetiological agents (Martínez et al., 2015; Dalanezi et al., 2020), considering trauma, injuries, allergies, toxic substances and neoplasms as secondary causes (Bedolla, 2008; Peláez, 2015). However, a mineral deficit reduces the activity of immune cells, causing immunosuppression, which is a predisposing factor for udder inflammation (Sugrue et al., 2019).
The recognition of bovine mastitis’s aetiology is essential, considering that pathogenic microorganisms have overcome a series of hierarchical barriers that result in zoonotic transmission from cattle to humans through the consumption of raw milk and meat (De Jesus et al., 2020; Maity and Ambatipudi, 2020). On the other hand, several publications from different geographical environments describe the resistance of pathogens to antibiotics in recent decades due to the indiscriminate and irrational use of antibiotics to treat the disease (Gao et al., 2012; Klibi et al., 2018; Castro, et al., 2020; Cheng and Han, 2020; O’Dea et al., 2020; Goulart and Mellata, 2022; Naranjo and Slowey, 2022; De Oliveira et al., 2022) perhaps due to the lack of knowledge at the producer level for rapid and efficient tests for the recognition and sensitivity of pathogens to antimicrobials.
In Ecuador, there is scarce information on the incidence, prevalence and recognition of etiological agents (Reyad, 2015); however, it is clear that the pathology constitutes one of the causes of low productive yields in the dairy industry, registering 7.11 litres/cow (INEN, 2017), with a contribution of 10. 40% of the Gross Domestic Product (GDP) of the national total. These figures show that milk production in the country is critical for food security and sovereignty since milk is considered a complete food of national priority (Ruíz et al., 2016), with 75% of production going to the sale of fresh and industrial milk (González and Vidal, 2021). This represents a consumption of 110 litres/inhabitant/year, an amount that is still below the FAO and WHO recommendations of 180 litres per capita per year (CIL, 2021), 12.86% for the production of cheese, butter, yoghurt and other dairy products, 6.86% for family consumption, 3.67% for feeding calves raised in buckets, and 1.70% for other purposes (Campaña and Aguilar, 2021). It is therefore, current study was designed to identify the frequency and microorganisms that cause subclinical bovine mastitis in the Biblián-Ecuador.
MATERIALS AND METHODS
The study was carried out in Ecuador, Cañar province, Biblián canton, located at an average altitude of 2,608 meters above sea level, with temperatures ranging between 2 and 14ºC and annual rainfall between 750 and 1000 mm/year (PDOT-BIBLIAN 2014). Livestock farms are semi-intensive, with manual or mechanical milking and two milkings/day, with forage mixtures of perennial ryegrass, annual rye grass, blue grass, white clover and red clover.
Three hundred and sixty female Holstein Friesian crossbred cattle were selected between day 30 and 150 of production, with 2 to 3 calvings. The early detection of subclinical mastitis was carried out during the months of June, July and August, the reagent for the diagnosis of bovine subclinical mastitis (CMT) from the commercial company LIFE - Ecuador was used. Following the process described by (Mellenberg and Roth, 2022), the udder was washed and dried, the first jet of milk was discarded and 2 ml of milk from each mammary quarter was collected on the test paddle, mixing with the Mastitest®-CMT reagent in equal parts, and shaking the paddle with circular movements for 2 minutes. Finally, the reaction created between sodium alkylarylsulfonate and the number of somatic cells (SC) present in the milk (gelation) was observed, classifying this reaction according to the method described by Smith in 1990, cited by (Bedolla et al., 2007). Obtained qualitative results for each quarter (Traces, +, ++ and +++) were explained in Table 1, adjusted under the interpretation proposed by (Pastor and Bedolla, 2008).
Table 1: Interpretation of the California Mastitis Test.
| Interpretation cells/Ml | Reaction | Number of somatic cells |
|
Negative
Traces |
No evidence
Mild precipitation |
0-200.000
201.000-400.000 |
|
+
++ |
No gel formation
Thick mixture |
401.000 - 800.000
801.000 - 1'500.000 |
| +++ |
Central peak formation |
> a 1'500.000 |
Samples that tested positive for mastitis in the CMT test were recollected in triplicate
Samples that tested positive for mastitis in the CMT test were recollected in triplicate into sterile 10 ml Falcon tubes and identified before being transported in a refrigerator at 4°C for somatic cells count (SCC) and microbiological testing. The somatic cell count was performed using the Ekomilk Scan CS analyzer (Ekomilk brand, Ultra Pro model, serial: SN S00802306, Bulgaria), using the method recommended by (INEN, 2012).
The samples for microbiology were sown in Petri dishes in duplicate on nutrient agar and subsequently incubated at 37ºC for 24 hours, verifying the growth of bacterial colonies. Subsequently, the colonies were isolated by morphological difference and the colonies were purified in three rounds to ensure purity. Colonies were then plated for the last time on blood agar and incubated for 18 hours at 37°C for the identification process using the matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry technique. This technique has become a reference resource for the identification of microorganisms in clinical microbiology services because it is quick, simple and more reliable than the conventional biochemical identification technique (Barreiro et al., 2010; Vega et al., 2012; Cattani et al., 2015; Siller et al., 2017; Costa et al., 2022).
A descriptive observational analysis of the data was performed using simple absolute frequencies (number of cases) and relative frequencies (percentage), which are presented in frequency tables. The prevalence of microorganisms causing bovine subclinical mastitis was calculated as a proportion using as a numerator the number of positive cases for mastitis and as a denominator the number of isolates of each microorganism, using the formula described by (Rodríguez, 2020).
Prevalence = 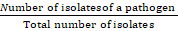 X 100
X 100
RESULTS
Of a total of 1440 mammary quarters analyzed,, 175 were detected by CMT field test and confirmed by somatic cell counting with subclinical bovine mastitis. Nineteen bacteria were identified responsible for MSB, including pathogenic, skinresident and environmental bacteria. Staphylococcus chromogenes is the most prevalent among the environmental microorganisms, with 23.4%. On the other hand, Staphylococcus aureus and Streptococcus agalactiae were the aetiological agents responsible for contagious bovine subclinical mastitis in the dairy herds of Biblián canton, with a prevalence of 9.5% and 0.5%, respectively, as shown in Table 2.
On the other hand, the study identified Staphylococcus chromogenes, Staphylococcus epidermidis and Kocuria salsiccia, resident bacteria of the skin and mucous membranes of both humans and mammals, which can be considered
Table 2: Etiology of bovine subclinical mastitis in Biblián-Ecuador
| Microorganisms | Isolated (Number) | Prevalence % |
|
Staphylococcus aureus
Streptococcus agalactiae
Streptococcus uberis
Staphylococcus chromogenes
Staphylococcus haemolyticus
Staphylococcus xylosus
Staphylococcus sciuri
Staphylococcus epidermidis
Lactobacillus mali
Moraxella osloensis
Kocuria salsiccia
Bacillus weihenstephanensis
Arthrobacter gandavensis
Acinetobacter iwoffi
Rothia terrae
Corynebacterium xerosis
Macrococcus canis
Chryseobacterium bovis
Rothia endophytica
|
13
1
10
32
4
2
2
9
15
4
11
2
1
11
1
4
1
12
2
137 |
9.5
0.7
7.3
23.4
2.9
1.5
1.5
6.6
10.9
2.9
8.0
1.5
0.7
8.0
0.7
2.9
0.7
8.8
1.5
100.0 |
contaminants when found in clinical samples. However, since they were found in a considerable percentage of the samples, they were considered positive. Likewise, Acinetobacter iwoffi was found, an environmental type bacterium resistant to commonly used disinfectants and adaptable to environmental conditions.
The study also recorded Lactobacillus mali as the etiological agent of bovine mastitis. Its presence in dairy cattle is due to the supplementation of the feed ration with silage inoculated with Lactobacillus strains to increase the amount of lactic acid and lower the pH more quickly, consequently decreasing plant respiration and enzymatic activity, increasing dry matter losses and digestibility.
Analyzing the bacterial frequency causing subclinical bovine mastitis in dairy herds in the Biblián canton, depending on the milking system, it was observed that S. chromogenes and L. mali are more frequent in mechanical milking systems; S. aureus and S. uberis are present with equal frequency in mechanical and manual milking; Acinetobacter iwoffi and Kocuria salsiccia were only recorded in mechanical milking systems.
DISCUSSION
Staphylococcus aureus is the bacterium that has been reported in most of the studies carried out in dairy farms in the highland region of Ecuador as responsible for bovine mastitis. Bermeo (2014) reported that Staphylococcus aureus is the most prevalent microorganism in dairy farms suffering from bovine mastitis in the province of Azuay (Caraguay, 2012). Thus Bermeo (2014) indicates that Staphylococcus aureus is the most prevalent microorganism in dairy farms suffering from bovine mastitis in the province of Azuay. Caraguay (2012) identified Staplycococcus sp. and Streptococcus sp. in dairy farms in the province of Loja-Ecuador as pathogens responsible for bovine mastitis. Bonifaz and Conlago (2016) identified Staphylococcus intermedius, Staphylococcus aureus, Streptococcus dysgalactiae, Staphylococcus epidermis, Escherichia coli, Micrococcus and Corynebacterium sp. as aetiological agents of bovine mastitis in dairy farms in Cayambe, Quito-Ecuador.
Likewise, Andrade and Sánchez (2018) pointed out S. aureus, Streptococcus sp. Bacillus sp. E. coli, Shigella sp. Klebsiella sp. and Enterobacter sp. in cattle farms in the province of Bolivar-Ecuador, as responsible for this pathology. Cuzco (2015) determined Staphylococcus aureus and Staphylococcus intermedius as aetiological agents of bovine mastitis in dairy farms in the province of Chimborazo-Ecuador.
Rodríguez and Muñoz (2017) refer in their study to Staphylococcus aureus as the most prevalent microorganism in cases of bovine mastitis in Trujillo - Peru. In Colombia, many reserachers (Sánchez et al., 2028; Calderón and Rodríguez, 2008; Andrade et al., 2014; Cruz et al., 2007) include S. aureus as the most prevalent bacterium in this pathology, with 31.1, 29.09, 6.0, and 42%, prevalence respectively.
There is no doubt that S. aureus and S. agalactiae are bacteria found involved in cases of contagious bovine mastitis. However, their prevalence differs between regions, countries and herds (Ruiz et al., 2011) depending on factors such as udder hygiene, barn size, milking parlour hygiene (Ramírez, 2015), animal age, milker’s hand washing and production volume (Ramírez et al., 2011) and lactation period, since the amount of lactoferrin (an enzyme that sequesters free ferric ions present in milk) is low at the beginning of the lactation phase and rises as this phase decreases. Thus, it can be stated that the prevalence of S. aureus in cases of bovine mastitis during the first months of lactation will be higher, considering that this pathogen has a substantial amount of iron in milk for its growth (Pereyra et al., 2014).
Regarding the presence of Staphylococcus chromogenes, Staphylococcus epidermidis and Kocuria salsiccia, the finding coincides with those reported by Cabral et al. (2016). Tomazi et al. (2014) described S. chromogenes as one of the most prevalent microorganisms isolated from mastitis in dairy cows. Perhaps Kocuria spp. in bovine mastitis samples have been misidentified in clinical microbiology laboratories as coagulase-negative Staphylococcus (CoNS) based on their gram-reactive, catalase-positive and coagulase-negative properties (Kandi et al., 2016) and therefore, it is not reported as a pathogen of bovine mastitis.
Acinetobacter iwoffi was also present in raw milk samples from dairy cows with mastitis. The result coincides with the study of (Gurung et al., 2013), who found the bacterium in raw milk tanks from cows with bovine mastitis.
Mechanical milking systems exhibited a higher incidence of bacteria, such as S. aureus, S. chromogenes, L. mali, Acinetobacter iwoffi and Kocuria salsiccia. These results differ from those reported by Ávalos et al. (2022), who observed a greater presence of S. chromogenes, and S. aureus in mechanical goat milking systems. The difference could be attributed to inadequate milking practices, given that, in the dairy herds in the Biblián canton - Ecuador, producers use the same towel to dry the cows’ udders. This procedure represents an important risk factor for the transmission of these microorganisms.
S. aureus is found in manual and mechanical milking systems, being more common in the latter. Similar findings were reported by Faria et al. (2005), who observed the presence of S. aureus in both milking systems. Furthermore, these results are supported by Ruiz et al. (2011), who reported a higher incidence of Staphylococcus spp. and Streptococcus spp. in mechanical milking systems.
CONCLUSION
Cases of subclinical bovine mastitis in Biblián canton, province of Cañar-Ecuador, are caused mainly by Staphylococcus chromogenes, Lactobacillus malli and Staphylococcus aureus, reaching 23.4%, 10.9% and 9.5% of presence respectively, without discarding the importance of other bacteria such as Staphylococcus chromogenes, Staphylococcus haemolyticus, Staphylococcus xylosus, Staphylococcus epidermidis, Streptococcus uberis, Streptococcus agalactiae, Lactobacillus mali, Moraxella osloensis, Kocuria salsiccia, Bacillus weihenstephanensis, Corynebacterium xerosis, Macrococcus canis, Chryseobacterium bovis and Rothia endophytica.
ACKNOWLEDGEMENTs
Special thanks to the Center for Research, Innovation and Technology Transfer, of the Catholic University of Cuenca, for allowing the development of part of the research.
To the Ministries of Agriculture and Livestock of Cañar-Ecuador, for the support provided in collecting samples.
CONFLICT OF INTEREST
We certify that there is no conflict of interest with any financial, personal, or other relationships with other people or organization related to the material discussed in the manuscript.
novelty statement
The study managed to determine the etiology of subclinical bovine mastitis and its frequency in mechanical and manual milking systems in the Biblián canton, province of Cañar, Ecuador.
AUTHORS CONTRIBUTIONS
Experiments were designed by LRG and JCG, and the experiments were performed by MCC. Data analysis was accomplished by MCC and DGB. The manuscript was written by MCC and JCG.
REFERENCES
Aken A., Hoop D., Friedli K., Mann S. (2022). Udder health, veterinary costs, and antibiotic usage in free stall compared with tie stall dairy housing systems: An optimized matching approach in Switzerland. Res. Vet. Sci. 152: 333-353. (Journal) https://doi.org/10.1016/j.rvsc.2022.08.021
Andrade C., Sánchez A. (2018). Estudio Clínico, Microbiológico, y Estimación Económica de Mastitis Bovina, en la Cooperativa de Producción Agropecuaria “El Salinerito,” provincia Bolivar-Ecuador. Universidad de las Fuerzas Armadas. [Online] https://repositorio.espe.edu.ec/bitstream/21000/14267/1/T-IASA%20I-005437.pdf (Internet article)
Andrade R., Caro Z., Dallos A. (2014). Prevalencia de mastitis subclínica bovina y su etiología infecciosa en fincas lecheras del altiplano boyacense (Colombia). Revista Científica, FCV-LUZ. XXIV(4):305 - 310. (Journal)
Araúz E. (2011). La mastitis subclínica y su influencia en la producción, calidad y economía lechera y medidas de manejo estratégico para su prevencion y control apropiado. Engormix. [Online]. https://www.engormix.com/lecheria/mastitis-infecciones-ubre/mastitis-subclinica-influencia-produccion_f13544/
Ávalos R., Palomares G., Díaz E., Medina N. (2022). Prevalencia de mastitis subclínica y determinación de los factores de riesgo en cabras ordeñadas de forma manual y mecanizada, en rebaños de Comondú, Baja California Sur, México. Acta Universitaria 32(e3268): p. 1-10. (Journal) https://doi.org/10.15174/au.2022.3268
Azooz M., El-Wakeel S., Yousef H. (2020). Financial and economic analyses of the impact of cattle mastitis on the profitability of Egyptian dairy farms. Vet. World.,13(9): 1750-1759. (Journal) https://doi.org/10.14202/vetworld.2020.1750-1759
Barreiro JR., Ferreira CR., Sanvido GB., Kostrzewa M., Maier T., Wegemann B., Böttcher V., Eberlin MN., Dos Santos MV. (2010). Identification of subclinical cow mastitis pathogens in milk by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. J. Dairy Sci. 93(12): 5661-5667. (Journal) https://doi.org/10.3168/jds.2010-3614
Bedolla C. (2008). Pérdidas económicas ocasionadas por la mastitis bovina en la industria lechera. REDVET., IX(4): p. 1-26. (Journal)
Bedolla C., Castañeda V., Wolter W. (2007). Métodos de detección de la mastitis bovina. REDVET. VIII (9): p. 1-17. (Journal)
Bermeo M. (2014). Incidencia de la Mastitis Subclínica Bovina, en el setor Soldados de la parroquía San Joaquin. Universidad del Azuay. [Online]. https://dspace.uazuay.edu.ec/bitstream/datos/3588/1/10272.pdf. (Internet article)
Bobbo T., Ruegg P., Stocco G., Fiore E., Gianesella M., Morgante M. Pasotto D., Bittante G., Cecchinato A. (2017). Associations between pathogen-specific cases of subclinical mastitis and milk yield, quality, protein composition, and cheese-making traits in dairy cows. J. Dairy Sci. 100(6): 4868-4883. (Journal) https://doi.org/10.3168/jds.2016-12353
Bonifaz N., Conlago F. (2016). Prevalencia e incidencia de mastitis bovina mediante la prueba de california mastitis test con identificación del agente etiológico,en paquiestancia, Ecuador. La Granja. 24(2): 43-52. (Journal) https://doi.org/10.17163/lgr.n24.2016.03
Cabral D., Lange C., Avellar P., Netto K., Vasconcelos M., Giambiagi M. (2016). Staphylococcus chromogenes, a Coagulase-Negative Staphylococcus Species That Can Clot Plasma. J. Clin. Microbiol. 54(5): 1372–1375. (Journal) https://doi.org/10.1128/JCM.03139-15
Calderón A., Rodríguez V. (2008). Prevalencia de mastitis bovina y su etiología infecciosa en sistemas especializados en producción de leche en el altiplano cundiboyacense (Colombia). Rev. Colomb. Cienc. Pecu. 21: p. 582-589. (Journal)
Campaña X., Aguilar P. (2021). Estudio de Mercado N° SCPM-IGT-INAC-002-2019 “Sector Lácteo” Versión Pública. Quito-Ecuador.
Caraguay M. (2012). Diagnóstico de Mastitis Subclínica por el método California Mastitis Test, Aislamiento, Identificación y Sensibilidad del germen en las ganaderías de la parroquia Chantaco del cantón Loja. Universidad Nacional de Loja. [Online]. http://dspace.unl.edu.ec/jspui/bitstream
/123456789/5389/1/DIAGN%C3%93STICO%20DE%20MASTITIS%
20SUBCL%C3%8DNICA%20POR%20%20EL%20M%C3%89TODO
%20CALIFORNIA.pdf (Internet article)
Castro V., Da Costa G., Guimaraes A., Heinemann M., Pereira A., Seles E. (2020). Relationship between virulence factors and antimicrobial resistance in Staphylococcus aureus from bovine mastitis. J. Global Antimicrob. Resist. 22: 792-802. (Journal) https://doi.org/10.1016/j.jgar.2020.06.010
Cattani M., Posse T., Hermes R., Kaufman S. (2015). Identificación rápida de microorganismos de frascos de hemocultivos por espectrometría de masas. Comparación de 2 procedimientos diagnósticos. Rev. Argent. Microbiol. 47(3): p. 190-195. (Journal) https://doi.org/10.1016/j.ram.2015.06.001
Chaves C., Vallejo D, Astaíza J., Benavides C., Chaves F. (2017). Hallazgos histopatológicos en la glándula mamaria de bovinos diagnosticados con mastitis clínica en la planta de beneficio del municipio de Ipiales, Colombia. Rev. Med. Vet., 33: p. 43-50. (Journal) https://doi.org/10.19052/mv.4050
Cheng W., Han S. (2020). Bovine mastitis: risk factors, therapeutic strategies, and alternative treatments - A review. Australasian J. Anim. Sci. 33(11): 1699–1713. (Journal) https://doi.org/10.5713/ajas.20.0156
Centro de la Industría Lactea del Ecuador -CIL. (2021). El sector lácteo ecuatoriano se reactiva con miras positivas para el 2022. [Online]. https://www.cil-ecuador.org/post/el-sector-l%C3%A1cteo-ecuatoriano-se-reactiva-con-miras-positivas-para-el-2022 (Internet article).
Costa LVD., Miranda RVDSL., Reis CMFD., Andrade JM., Cruz FV., Frazão AM., Fonseca ELD., Ramos JN., Brandão MLL., Vieira VV. (2022). MALDI-TOF MS database expansion for identification of Bacillus and related genera isolated from a pharmaceutical facility. J. Microbiolog. Methods. 203: 106625. (Journal) https://doi.org/10.1016/j.mimet.2022.106625
Cruz A., Estepa C., Hernández J., Sanabria J. (2007). Identificación de bacterias causantes de mastitis bovina y su resistencia ante algunos bacterianos. RevistaU.D.C.A.Actualidad & DivulgaciónCientífica. 10(1): 81-91. (Journal) https://doi.org/10.31910/rudca.v10.n1.2007.569
Cuéllar J. (2020). Mastitis bovina: enfermedad mundial. Veterinaria Digital. [Online]. https://www.veterinariadigital.com/articulos/mastitis-bovina-enfermedad-mundial/.
Cuzco G. (2015). Determinación de la Sensibilidad de CMT para el Diagnóstico de Mastitis Subclínica y su relación en cultivo de leche más antibiograma en la hacienda “El Boliche” Universidad Técnica de Ambato. [Online]. http://repositorio.uta.edu.ec/bitstream/123456789/18364/1/Tesis%2031%20Medicina%20Veterinaria%20y%20Zootecnia%20-CD%20343.pdf (Internet article)
Dalanezi F., Joaquim S., Guimaraes F., Guerra S., Lopes B., Schmidt E., Cerri R., Langoni H. (2020). Influence of pathogens causing clinical mastitis on reproductive variables of dairy cows. J. Dairy Sci. 103(4): 3648-3655. (Journal) https://doi.org/10.3168/jds.2019-16841
De Jesus G., Micheletti A., Padilha R., De Souza J., Alves F., Leal C., Garcez F. 27. Garcez W., Yoshida N. (2020). Antimicrobial Potential of Essential Oils from Cerrado Plants against Multidrug-Resistant Foodborne Microorganisms. Molecules. Molecules. 25(14). (Journal) https://doi.org/10.3390/molecules25143296
De Oliveira RP., Aragão BB., De Melo RPB., Da Silva DMS., De Carvalho RG., Juliano MA., Farias MPO., De Lira NSC., Mota RA. (2022). Bovine mastitis in northeastern Brazil: Occurrence of emergent bacteria and their phenotypic and genotypic profile of antimicrobial resistance. Comparat. Immunol. Microbiol. Infect. Dis. 85: 101802. (Journal) https://doi.org/10.1016/j.cimid.2022.101802
Demil E., Teshome L., Kerie Y., Habtamu A., Kumilachew W., Andualem T., Mekonnen S. (2022). Prevalence of subclinical mastitis, associated risk factors and antimicrobial susceptibility of the pathogens isolated from milk samples of dairy cows in Northwest Ethiopia. Prevent. Vet. Med., p. 105680. (Journal) https://doi.org/10.1016/j.prevetmed.2022.105680
Faría RJF, Valero- Leal K, D´Pool G, García UA, Allara CM, Morales D. Agentes bacterianos y contaje de célula somáticas de cuartos mestizos doble propósito ordeñados en forma manual o mecánica en cuatro fi ncas lecheras del estado Zulia, Venezuela. Rev. Cient Univ. Zulia. 2005; 15:64-67.
Fernandez O., Trujillo J., Peña J., Cerquera J., Granja Y. (2012). Mastitis bovina: generalidades y métodos de diagnostico. REDVET. 13(11): 1-20. (Journal)
Gao J., Yu FQ, Luo LP., He JZ., Hou RG., Zhang HQ., Li SM., Su JL., Han B. (2012). Antibiotic resistance of Streptococcus agalactiae from cows with mastitis. Vet. J. 194(3): 423-424. (Journal) https://doi.org/10.1016/j.tvjl.2012.04.020
González R., Vidal M. (2021). Mastitis bovina y calidad de la leche, un reto para la salud humana. Revista Universidad y Sociedad. 13(1): 89-96. (Journal)
Goulart D., Mellata M. (2022). Escherichia coli Mastitis in Dairy Cattle: Etiology, Diagnosis, and Treatment Challenges. Microbiol. Frontal. 13: 1-15. (Journal) https://doi.org/10.3389/fmicb.2022.928346
Gurung M., Nam HM., Tamang MD., Chae MH., Jang GC., Jung SC., Lim SK. (2013). Prevalence and antimicrobial susceptibility of Acinetobacter from raw bulk tank milk in Korea. J. Dairy. Sci. 96: p. 1997–2002. (Journal) https://doi.org/10.3168/jds.2012-5965
He W., Ma S., Lei L., He J., Li X., Tao J., Wang X., Song S., Wang Y., Wang Y., Shen J., Cai C., Wu C. (2019). Prevalence, etiology, and economic impact of clinical mastitis. Vet. Microbiol.., 242: p. 1-27. (Journal) https://doi.org/10.1016/j.vetmic.2019.108570
INSTITUTO ECUATORIANO DE NORMALIZACIÓN (INEN). (2012). Requisitos microbiológicos de la leche cruda tomada en hato. Quito-Ecuador. [Online]. https://www.gob.ec/sites/default/files/regulations/2018-10/Documento_BL%20NTE%20INEN%209%20Leche%20cruda%20Requisitos.pdf
Instituto Nacional de Estadisticas y Censos - INEN. (2017). Encuesta de superficie y producción agropecuaria continua. [Online]. http://www.ecuadorencifras.gob.ec/documentos/web-inec/Estadisticas_agropecuarias/espac/espac_2017/Informe_Ejecutivo_ESPAC_2017.pdf. (Internet article).
Ji Z., Ren W., Wu H., Zhang J, Yuan B. (2022). Exosomes in Mastitis—Research Status, Opportunities,and Challenges. Animals., 12(2881): 1-19. (Journal) https://doi.org/10.3390/ani12202881
Kandi V., Palange P., Vaish R., Bhatti A., Vinod C., Kandi M, Bhoomagiri M. (2016). Emerging Bacterial Infection: Identification and Clinical Significance of Kocuria Species. Cureus. 8(8): p. 1-6. (Journal) https://doi.org/10.7759/cureus.731
Klibi A., Maaroufi A., Torres C., Jouini A. (2018). Detection and characterization of methicillin-resistant and susceptible coagulase-negative staphylococci in milk from cows with clinical mastitis in Tunisia. Int. J. Antimicrob. Agents. 52(6): p. 930-935. (Journal) https://doi.org/10.1016/j.ijantimicag.2018.07.026
López J. (2014). Mamitis bovina: definición, etiología y epidemiología de la enfermedad. Ciencia Veterinaria.
Maity S, Ambatipudi K. (2020). Mammary microbial dysbiosis leads to the zoonosis of bovine mastitis: a One-Health perspective. Microbiol. Ecol. 97 (1: p. 1-17. (Journal) https://doi.org/10.1093/femsec/fiaa241
Maldonado D., Santos C., Quilapanta A., Mena L. (2022). Diagnóstico de Mastitis Subclínica Mediante Tres Métodos para el Control y Tratamiento en Bovinos de Leche Holstein. Dom. Cien., 8(1): p. 773-790. (Journal)
Martínez D., Cruz A., Millán A., Moreno G. (2015). Evaluación del estado de resistencia de agentes etiológicos de mastitis clínic y subclínica frente a algunos antimicrobianos utilizados en hembras bovinas del Municipio de Sotaquira (Boyacá - Colombia). Revista Científica, FCV-LUZ. XXV(3): p. 223-231. (Journal)
Mellenberg R., Roth C. (2022). Hoja de Información de la Prueba de Mastitis California (CMT). [Online]. https://www.udocz.com/apuntes/341361/hoja-de-informacion-de-la-prueba-de-mastitis-california-spanish. (Internet article).
Mera R., Muñoz M., Artieda J., Ortíz P., González R., Vega V. (2017). Mastitis bovina y su repercusión en la calidad de la leche. REDVET. 18(11): p. 1-16. (Journal)
Miranda S., Albuja C., Tríbulo H. (2019). Asociación entre la Mastitis Subclínica con la pérdida temprana de gestación en un hato de vacas lecheras. La Granja. 30(2): p. 48-56. (Journal) https://doi.org/10.17163/lgr.n30.2019.05
Mora M., Vargas. B., Romero J., Camacho J. (2015). Factores de riesgo para la incidencia de mastitis clínica en ganado lechero de Costa Rica. Agronomía Costarricense. 39(2): 77-89. (Journal) https://doi.org/10.15517/rac.v39i2.21777
Naranjo A., Slowey R. (2022). Invited review: Antimicrobial resistance in bovine mastitis pathogens: A review of genetic determinants and prevalence of resistance in European countries. J. Dairy Sci. 106(1):1-23 (Journal) https://doi.org/10.3168/jds.2022-22267
Neculai A., Ariton A., Mădescu B., Rîmbu C., Creangă S. (2021). Nanomaterials and Essential Oils as Candidates for Developing Novel Treatment Options for Bovine Mastitis. Animals., 11(1625): p. 1-21. (Journal) https://doi.org/10.3390/ani11061625
O’Dea M., Abraham R., Sahibzada S., Lee T., Jordan D., Laird T., Pang S., Buller N., Stegger M., W Coombs G., Trott D., Abraham S. (2020). Antimicrobial resistance and genomic insights into bovine mastitis-associated Staphylococcus aureus in Australia. Vet. Microbiol. 250: p. 108850. (Journal) https://doi.org/10.1016/j.vetmic.2020.108850
Pastor J., Bedolla J. (2008). Determinación de la prevalencia de mastitis bovina en el municipio de Tarímbaro, Michoacán, mediante la prueba de California. REDVET. 9(10): 1-35. (Journal)
PDOT-BIBLIAN. (2014). Plan de Desarrollo y Ordenamiento Territorial Cantón Biblián - Diagnostico . Gobierno Autónomo Descentralizado Municipal del Cantón Biblián (GAD - Biblián) (En línea) Disponible en: http://app.sni.gob.ec/sni-link/sni/PORTAL_SNI/data_sigad_plus/sigadplusdiagnostico/DIAGNOSTICO%20PDOT%20BIBLIAN%202014_14-11-2014.pdf
Pedroso R., Roller F. (2017). Mastitis, fertilidad y eficiencia de las biotecnologías de reproducción asistida en el trópico. Revista la Técnica - Producción y Salud Animal. p. 53-71. (Journal) https://doi.org/10.33936/la_tecnica.v0i17.694
Peláez M. (2015). Principales vulnerabilidades en la mastitis bovina en una Empresa Pecuaria Oriental de Cuba. REDVET. 16(5): 1-10. (Journal)
Pereyra E., Dallard B., Calvinho L. (2014). Aspectos de la respuesta inmune innata en las infecciones intramamarias causadas por Staphylococcus aureus en bovinos. Rev. Argent Microbiol. 46(4): 363-375. (Journal) https://doi.org/10.1016/S0325-7541(14)70096-3
Puerto M., Shepley E., Cue R., Warner D., Dubuc J., Vasseur E. (2021). The hidden cost of disease: I. Impact of the first incidence of mastitis on production and economic indicators of primiparous dairy cows. J. Dairy Sci. 104(7): p. 7932-7943. (Journal) https://doi.org/10.3168/jds.2020-19584
Ramírez J. (2015). Prevalencia y factores predisponentes a mastitis subclínica en establos lecheros de la provincia de Trujillo. CEDAMAZ. 5(1): p. 12 – 22. (Journal)
Ramírez N., Arroyave O., Cerón M., Jaramillo M., Cerón J., Palacio L. (2011). Factores asociados a mastitis en vacas de la microcuenca lechera del altiplano norte de Antioquia, Colombia. Rev. Med. Vet. (22): p. 31-42. (Journal) https://doi.org/10.19052/mv.562
Reyad S. (2015). Epidemiología molecular de la bacteriana mastitis en el ganado vacuno en la provincia de El Oro, Ecuador: Impacto económico y medidas de control. Informe Final de Actividades. Quito. (Internet article).
Rodríguez L. (2020). Prevalencia de mastitis subclínica en tambo lechero en Paraguay. Rev. Med. Vet. 40: p. 61-68. (Journal) https://doi.org/10.19052/mv.vol1.iss40.6
Rodríguez R., Muñoz E. (2017). Frecuencia y Susceptibilidad Antimicrobiana de Bacterias Causantes de Mastitis en Bovinos de un Establo de Trujillo, Perú. Rev. Inv. Vet. Perú. 28(4): 994-1001. (Journal) https://doi.org/10.15381/rivep.v28i4.13874
Ruiz A., Peña J, Remón D. (2016). Mastitis bovina en Cuba. Artículo de revisión. Rev. Prod. Anim., 28(2-3): 39-50. (Journal)
Ruiz A., Ponce P., Gomes G., Mota R., Sampaio E., Lucena E., Benone S. (2011). Prevalencia de mastitis subclínica bovina y microorganismos asociados: Comparación entre ordeño manual y mecánico, en Pernambuco, Brasil. Revista de Salud Animal. 33(1): p. 57-64. (Journal)
Sánchez M., Gutiérrez N., Posada I. (2018). Prevalencia de mastitis bovina en el Cañón de Anaime, región lechera de Colombia, incluyendo etiología y resistencia antimicrobiana. Rev. Inv. Vet. Perú. 29(1): 226-239. (Journal) https://doi.org/10.15381/rivep.v29i1.14084
Siller M., Hernández S., Sánchez F., González J., Muñoz J. (2017). Métodos rápidos de identificación de bacterias y hongos. Espectrometría de masas MALDI-TOF, medios cromogénicos. Enferm. Infecc. Microbiol. Clin. 5(5): p. 303–313. (Journal) https://doi.org/10.1016/j.eimc.2016.12.010
Sugrue I., Tobin C., Ross R., Stanton C., Hill C. (2019). Foodborne Pathogens and Zoonotic Diseases. In: Raw Milk: Balance between Hazards and Benefits, 1st ed, Nero L., De Carvalho A.F., Eds.; Academic Press: Cambridge, MA, USA. Volume 1, pp. 259–272. (Book) https://doi.org/10.1016/B978-0-12-810530-6.00012-2
Tomazi T., Gonçalves J., Barreiro J., D’ Campos P., Silva L., Eberlin M., Dos Santos M. (2014). Identification of coagulase-negative staphylococci from bovine intramammary infection by matrix-assisted laser desorption ionization–time of flight mass spectrometry. J. Clin. Microbiol. 52: p. 1658–1663. (Journal) https://doi.org/10.1128/JCM.03032-13
Vega S., Ferreira L, González M, Sánchez F, García M, García J, González J., Muñoz J. (2012). Eficacia de la espectrometría de masas MALDI-TOF en la identificación de bacterias anaerobias. Enferm. Infecc. Microbiol. Clin. 30(10): p. :597–601. (Journal) https://doi.org/10.1016/j.eimc.2012.03.002
Vissio C., Agüero D., Raspanti C., Odierno. L., Larriestra A. (2015). Pérdidas productivas y económicas diarias ocasionadas por la mastitis y erogaciones derivadas de su control en establecimientos lecheros de Córdoba, Argentina. Arch. Med. Vet. 47: p. 7-14. (Journal) https://doi.org/10.4067/S0301-732X2015000100003
Year G., Ramírez W. (2016). La Prevalencia de mastitis clínica en vacas mestizas Holstein x Cebú. REDVET. 17(3): 1-7. (Journal)
To share on other social networks, click on any share button. What are these?





