Estimation of Peak Spawning Season, Length at Maturity (Lm) and Sex Ratio of Silver Pomfret (Pampus argenteus) in the Bay of Bengal, Bangladesh
Estimation of Peak Spawning Season, Length at Maturity (Lm) and Sex Ratio of Silver Pomfret (Pampus argenteus) in the Bay of Bengal, Bangladesh
Md. Abdullah Al-Mamun1,2, Al-Mamun2,3, Sanjay Kumar Mohanta2,3,
Md. Farhan Tazim2,3, Mohammed Rashed Parvej 2,3, Md. Sharif Uddin2,3, Suman Barua1,2 and Liu Qun1*
1College of Fisheries, Ocean University of China, Qingdao 266003, China
2Department of Fisheries, Ministry of Fisheries and Livestock, Dhaka 1217, Bangladesh
3Marine Fisheries Survey Management Unit, Agrabad, Chattogram, Bangladesh
ABSTRACT
The information on size at maturity (Lm), peak spawning season, and sex ratio is crucial for sustainable management for fisheries production. Our study in mers of 655 Silver Pomfret, Pampus argenteus, 366 females and 289 males, collected monthly between February 2019 and January 2020, provides insight into the characteristics of size at sexual maturity, peak spawning season, and sex ratio of the species. Monthly variation in the gonadosomatic index (GSI) values and histological observations has revealed two spawning peaks, in spring (April-May) and fall (September). The maximum GSI has accounted to 4.70±1.45 and 5.01±1.75, which corresponded to warmer water temperature 29.68ºC±0.60 in April and 29.72ºC±0.28 in May, respectively, over the Bay of Bengal. At 50% sexual maturity (Lm), males mature at standard length of 145 mm and females at 163 mm. During the peak spawning time, the sex ratio significantly differed from 0.5, with females dominating over males, according to a Chi-squared test (p<0.05). To ensure sustainable exploitation of this valuable species, implemention of the mesh size regulation for protecting this species less than 145 mm and seasonal closures in the Bay of Bengal (BoB), Bangladesh would be very effective.
Article Information
Received 30 June 2021
Revised 22 September 2022
Accepted 08 October 2022
Available online 01 March 2023
(early access)
Published 04 September 2023
Authors’ Contribution
MAA-M, AM, SKM, MFT, MRP, MSU and SB contributed to the sample collection and processing of the samples in the laboratory. MAA-M analyzed the data and wrote the preliminary draft under the supervision of QL. The submitted manuscript was edited by all of the contributors.
Key words
The Bay of Bengal, Spawning season, Sizes at sexual maturity (Lm), Sex ratio, Silver pomfret
DOI: https://dx.doi.org/10.17582/journal.pjz/20210630100624
* Corresponding author: [email protected]
0030-9923/2023/0005-2397 $ 9.00/0
Copyright 2023 by the authors. Licensee Zoological Society of Pakistan.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
INTRODUCTION
Since spawning aggregations are heavily exploited by fishermen, appropriate management schemes for protecting commercially important fishes are heavily reliant on reproductive biology information (Hardie et al., 2007). For the sustainable management of fisheries, reproductive biology knowledge of exploited fish stocks is critical (Kennedy et al., 2006; Tracey et al., 2007; Khatun et al., 2019). The rainy season in the tropics and sub-tropical areas is considered an important factor due to the fertility of the water and its significance for newly hatched larvae (Ganias et al., 2004; Park et al., 2006; Juchno et al., 2007; Lone et al., 2008a). To manage fisheries resources, knowledge about the patterns of life history, exploitation status, and habitat of an individual species is needed to help better understand fish population dynamics and sotck-recruitment relationship. For commercially valuable species, studies based on reproduction, spawning seasons, length at sexual maturity, fecundity and sex ratios are critical for a systematic assessment of stock status and population dynamics. Biological data on a species’ reproduction is important for determining the threshold for sustainable exploitation and management strategies and evaluating the potential for aquaculture growth (King, 2003; Jakobsen et al., 2009; Akhter et al., 2020b).
Silver pomfret, Pampus argenteus (Eugen, 1788), a member of the Stromateidae family, is one of the highly relished table fish at home and abroad, and most economically important marine fishes in the Bay of Bengal, Bangladesh, as well as some other countries in Asia like India, Korea, Kuwait, China, Thailand, Japan, and Malaysia (Shahidullah, 1986; Divya et al., 2017). The fisheries production of silver pomfret was totaled to 11004 metric tons in 2018-19, accounting for 1.67% of total marine catch (DoF, 2019). According to Bangladesh’s fisheries statistical yearbook, two relative species, silver pomfret and Chinese pomfret, Pampus cinensis, were reported in the trawl fishery. In 2019, silver pomfret took up as much as 65-75% of fisheries catch of pomfret group in Bangladesh.
Despite the species’ global commercial importance, few systematic studies on the life-history traits have been conducted. Pati (1981, 1982) investigated the maturation and fecundity of silver pomfret in the BoB’s Indian water. Lee and Jin (1989) studied the reproductive biology of this species in the East China Sea and Kuwait waters (Almatar et al., 2004; Lone et al., 2008a, b), where they presented the maturing pattern between habitats and the changes in the reproductive timing. There is no study on the size at sexual maturity, sex ratios and impact of sea surface temperature on the gonadal maturation for P. argenteus in the Bangladesh coast. However, Akhter et al. (2020a, b) reported on the seasonal variations with biological parameters and reproductive seasonality with gonadal maturation of silver pomfret. Hence, we investigated the reproductive biology of silver pomfret in Bangladesh marine water, including determination of the peak spawning season, length at 50 percent sexual maturity (Lm), sex ratio and impact of sea surface temperature (SST) on gonadal development of P. argenteus in the Bay of Bengal (BoB), Bangladesh coast.
MATERIALS AND METHODS
All fish samples used in present study were obtained directly from monthly visits of commercial fisheries delivery at fish-landing centers and nearby local markets, from February 2019 through January 2020. According to the local act, the industrial fishing trawlers fish above the 40-meter water depth and the artisanal boats fish within the 40-meter water depth. The samples of industrial fishing trawlers were collected from the Chattogram’s Fishery Ghat (20º 19́ 38.33́́ ́ N and 91º 50́ 50.15́ ́ E), while artisanal samples were collected from the Cox’s Bazar’s BFDC Ghat (21º 27́ 04.05́ ́ N and 91º 58́ 16.62́ ́ E), Chattogram’s Fishery Ghat.
The samples were measured to the nearest centimeter for standard length (SL), fork length (FL) and total length (TL). The gonads were extracted and weighed to the nearest gram. For histological classification, the gonads were examined visually and anatomically. The gonad tissue samples were processed for a normal fixation period for the histological studies. Males and females were morphometrically and later microscopically categorized according to the maturity scale by inspecting gonad samples in histological slides (Almatar et al., 2004; Lone et al., 2008a, b; Longenecker and Langston, 2018). Photographs were taken with a microscope (OLYMPUS sc180) at 10 and 40 magnifications for histological observation, and oocyte diameter was determined under the same microscope.
The following equation was used to calculate the gonado-somatic index (GSI):
GSI = (gonad weight/ gutted weight of fish × 100).
Peak spawning time was identified at the highest GSI values by analyzing the monthly GSI variation. All females having gonads at the stage of maturing or above with vitellogenis or above stage oocytes are considered as mature.
The percentage of mature males and females for each 1 cm SL class was analyzed from the samples obtained during the major reproductive (peak) period to estimate the size at 50 percent sexual maturity. The relationship between the proportion of mature individuals by length class was plotted after adjusted to a logistic curve by the King (1995) equation for the estimation of L50 value.
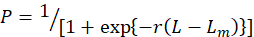
Here, P is the percentage of mature individuals, the slope of the curve is r, and the median of each size class is L. In this situation, even in the largest length class, the number of mature males and females was less than a hundred. According to King (1995) method, the raw data were modified to prevent an unreasonably high estimate of the Lm.
The number of specimens of each sex sampled each month was used to calculate the sex ratio. The chi-square (x2) study of the monthly sex ratio values was used to see whether there were any major deviations from the predicted 1:1 sex ratio for male and female fishes using the following formula.
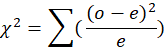
Where the observed frequency is o and the predicted frequency is e, critical value of chi-square is 3.84 in P>0.05 at 1 (one) degree of freedom.
RESULTS
Histological observation of ovaries in female
The silver pomfret ovaries are described as paired, L-shaped structures as described by Lone et al. (2008a). They are bound to the viscera on one side and the kidneys on the other. Silver pomfret oocyte development can be divided into six stages based on cytoplasmic cell characteristics (Murua et al., 2003; Liao and Chang, 2011; Brown-Peterson et al., 2011; Amparo et al., 2017; Longenecker and Langston, 2018) as defined below.
Primary growth
The youngest oocytes, known as the primary growth stage, are angular or irregularly shaped. The oocytes were strongly basophilic, had a prominent circular nucleus with several nucleoli, and were deeply stained purple with hematoxylin. All of the oocytes were either in the chromatin nucleolus or early peri-nucleolus stages (Fig. 1A). The oocytes had an average diameter of 40±5.79 μm (n=31). This stage was observed between July and September.
Yolk vesicle
The outline of the cells was smoother (less irregular) than those in the primary growth stage. Moderately dark staining cytoplasm was found with a few or many pale lipid vesicles (Fig. 1B). The nucleus became irregular in shape. The average oocyte diameter was 85.50±11.39 μm (n=35). This stage dominated in the gonads found in July and March.
Early vitellogenis
Uniform vitellin globules were observed in the cells, which stained moderately with toluidine blue or eosin. Usually the oocytes become about two-times larger than the previous stage. Globules first emerged on the periphery of the oocyte. Main growth and yolk vesicle stages had lighter cytoplasm. The zona radiate and follicular layer were thickened and clearly visible. The average diameter (n = 29) was 178.65 ± 33.3 μm. This stage was observed in various percentages in all of the months during the year (Fig. 1C).
The zona radiates were prominent and usually surrounded by a follicular cell layer.
Late vitellogenis
The vitellin globules became close to the nucleus at this stage. Yolk globules were mainly filled the cytoplasm, with some large oil droplets. The zona radiata became thicker compared to the previous stage. Blood vessels appeared on the ovary’s surface (Fig. 1D). In the late vitellogenis stage, the ova were yellowish in colour and spherical shape and they could be counted with the naked eye. The average diameter (n = 28) was 297.80 ± 33.8 μm. This period of ovarian development is known as the early-maturing stage, and it was observed in various percentages throughout the year, with the highest rates in January and February.
Maturation
The nuclear membrane was dissolved, and the nucleus cannot be observed. Vitellin coalesced into larger globules of different sizes within the cytoplasm. The lipid vesicles were also coalesced into larger droplets. Cell shape became irregular. The zona radiata became thin and pulled away from the follicle (Fig. 1E). The diameter of the oocyte reached the maximum size of 644.61 ± 41.37 μm (n = 30) during the oogenesis. Mature ovaries were found between April (72 %) and May (77%), with a peak in May, while the lowest occurrence (15%) was in July.
Post-spawning
With different morphological features, various types of post-ovulatory follicles were found after spawning. The post-ovulatory follicles had a huge follicular lumen, which had previously been filled by the oocyte. The ovary lost a lot of weight because of the egg release, and the ovaries became shrunken hollow sac-like structures. POFs were observed in the months of May, June, and October, which indicates spent fish.
Histological observation of maturity stages in male
During study of the testes development based on colour and histological analysis only three stages could be identified as the immature, maturing and mature (Murua et al., 2003; Liao and Chang, 2011; Brown-Peterson et al., 2011; Longenecker and Langston, 2018) as defined below.
Immature stage
The testes were small, transparent and pale yellow in color during the immature period. The majority of germ cells had progressed from spermatogonia to primary spermatocytes. The testes’ seminal lobules had a low number of spermatocytes but a large number of spermatogonia. Hematoxylin and eosin (H and E) staining revealed spherical spermatogonia. This stage was mostly visible in the months of July and August (Fig. 2A, B).
Maturing stage
The testes had rapidly grown in size during the maturation period. The majority of germ cells were spermatids. The testes were significantly larger and more transparent than those in the immature stage. the germinal epithelium can be seen all over the testes. The development of spermatocytes has begun. There were also, primary spermatocytes, secondary spermatogonia and secondary spermatocytes found. Some spermatogonia were very faint stained and seen with visible basophilic nucleoli. There were no spermatozoa in the lumen. This stage was found between September and December (Fig. 2C).
Mature stage
The testes were bigger in the mature stage than they were in the previous stage. The majority of germ cells were spermatozoa. The number of spermatocytes and spermatogonia ecreased as the number of spermatids increased. With some blood spots the testes were translucent and white at this time. During this time, sperms were released from the vent with a gentle pressure on the belly. Males were observed in this mature stage as larger proportion from March to June and September, with a peak in May (Fig. 2D, E).
Monthly changes in the GSI
All fish samples were included to account for monthly changes of GSI indexes for both males and females (Fig. 3A). In female, the maximum GSI values (3.51±1.79 to 5.01±1.75) were observed in April to June than in the other months, with a peak (5.01±1.75) in May. In the male, this value changed from 0.62±0.20 in March to 0.78 ±0.22 in May. It peaked for both sexes in May, indicating major spawning time. The secondary highest GSI values were observed in September 1.52 ± 0.97 and 0.20 ± 0.05 in females and males, respectively, indicating that silver pomfret may mature biannually in the Bay of Bengal, Bangladesh water (Fig. 3A). The majority of spawning fish with higher GSI of 4.70±1.45) and 5.01±1.75 corresponded to warmer temperature as of 29.68ºC ±0.60) and 29.72ºC ±0.28 over the Bay of Bengal, in April and May, respectively (Fig. 3B).
Group maturity rate
For the representation of monthly changes in female gonadal development, the above histologically observed stage I and II was considered as immature, stage III and IV as maturing, stage V and VI as mature and spent, respectively. In case of the male gonads, only three stages could be identified as the immature, maturing and mature. Figure 4 shows the monthly percentages of gonad growth stages for females and males from January to December. Both female and male reached at the highest maturity level, 77.27 % and 81.25%, respectively, in the month of May.
Spawning season
The mature oocytes of Pampus argenteus were present from January to Septebmer, with a larger number in April and May, according to the histological analysis. During the peak, the oocyte’s diameter reached its maximum size (644.61±41.37 μm). The GSI was higher in April and May than in other months, after gradual decreasing of the value from June, it raised again in September (Fig. 3A). The group maturity rate followed a similar trend (Fig. 4) in April and May. According to the results from the above mentioned methods, the major peak spawning periods of the silver pomfret in the Bay of Bengal Bangladesh water appeared to be in April-May, and a second minor peak in September.
The size at maturity
In the study, 253 females and 214 males of silver pomfret were collected during the main reproductive period. For female, the minimum, maximum and average size (SL) was observed 12, 24 and 18 cm, for the male, it was 11, 23 and 17 cm respectively. The relationship between SL and the percentage of mature females was expressed using a logistic function:
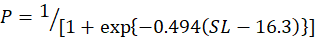
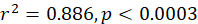
And for the male as follows:
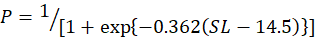
Males and females of this species were measured to be 14.5 and 16.3 cm in size at 50 % sexual maturity, respectively (Fig. 5A, B).
Sex ratio
The sex ratio data in relation to different months of the year revealed that the female prevailed in the majority of the months. The average female and male during the study period were 56% and 44%, respectively. During the peak spawning season of April and May, the number of females was significantly higher (P<0.05) than the males (Table I).
Table I. Month wise sex ratio of Pampus argenteus during the study period.
|
Months |
Female |
Male |
Total |
%F |
%M |
x2 |
|
January |
24 |
20 |
44 |
54.55 |
45.45 |
0.36 |
|
February |
35 |
25 |
60 |
58.33 |
41.67 |
1.67 |
|
March |
25 |
22 |
47 |
53.19 |
46.81 |
0.19 |
|
April |
28 |
16 |
44 |
63.64 |
36.36 |
5.82* |
|
May |
44 |
25 |
69 |
63.77 |
36.23 |
5.23* |
|
June |
28 |
22 |
50 |
56 |
44 |
0.72 |
|
July |
30 |
25 |
55 |
54.55 |
45.45 |
0.45 |
|
August |
25 |
28 |
53 |
47.17 |
52.83 |
0.17 |
|
September |
40 |
29 |
69 |
57.97 |
42.03 |
1.75 |
|
October |
36 |
25 |
61 |
59.02 |
40.98 |
1.98 |
|
November |
25 |
28 |
53 |
47.17 |
52.83 |
0.17 |
|
December |
26 |
24 |
50 |
52 |
48 |
0.08 |
|
Total |
366 |
289 |
655 |
55.99 |
44.01 |
1.55 |
*significant (P<0.05), M, Male, F, Female.
DISCUSSION
The current research provides information on P. argenteus reproductive biology, including histological observation of gonad tissue, spawning season, size at maturity, and sex ratio. The simplest method for identifying spawning season is to apply GSI, whereas histological analysis is the most accurate and error-free method of determining gonadal maturity (West, 1990). Here, we associated with the GSI, egg diameter, and histology of both sexes to determine the spawning season for silver pomfret in the Bay of Bengal Bangladesh water.
Both males and females showed a consistent gonadal development pattern based on the estimated GSI and oocyte diameter. Furthermore, from March to June, histological observations revealed an increase in the rate of gonadal maturity in P. argenteus. Similar gonadal development patterns were observed for this species (Akhter et al., 2020b) in the Bay of Bengal Bangladesh. The group maturity rate in this study followed a similar trend of maturation from March to June (Fig. 4). Arocha and Barrios (2009) mentioned that the post-ovulatory follicles (POFs) is one of the most precise methods of distinguishing completely mature fish. In May, a number of POFs were observed within the ovaries. In October, few POFs were also found inside the ovaries, indicating that this species has a biannual spawning potential in this region. From March to June, the majority of mature spermatids was observed in males. These findings indicated harmonized gonadal maturity of male and female, in the Bay of Bengal Bangladesh water, with peak spawning in April-May and September.
The two spawning peaks found in this study are similar to those reported in previous studies in the same region. The breeding season for P. argenteus in the Bay of Bengal Orissa coast, India, is recorded to be February to August, with two spawning peaks in April and August, according to Pati (1982). In addition, Akhter et al. (2020b) recorded biannual spawning for this stock in the Bay of Bengal Bangladesh water in May–June and October. Hussain and Abdullah (1977) found that the breeding season in Kuwait waters runs from April to September, with two spawning peaks, one in April-May and the other in September, which is quite close to the findings of our research. According to Nekuru et al. (2013), the breeding season runs from April to September, with two spawning peaks in May and July in the Persian Gulf. However, Gopalan (1967) observed that its breeding season is from February to August, with a single spawning peak in the Arabian Sea in April-June. In silver pomfrets, water temperature can play an important role in determining breeding seasons. In the Bay of Bengal, monthly SST over a period of 24 years was reported by Chowdhury et al. (2012) with a peak in April-May and a sharp decrease from November. Another research (Lone et al., 2008a) on the seasonal variability, gross structure, and gonadal cycle of P. argenteus found the highest GSI in June (7.90±0.04), when the water temperature was at its highest level (30.60ºC). This corresponds to the major peak breading period (April-May), when the highest GSI was reported in the Bay of Bengal, Bangladesh water during the current analysis (Fig. 3B). As a result, rising water temperatures can act as a stimulant for P. argenteus spawning.
The scale of this species at 50 percent sexual maturity (Lm) was estimated to be 14.5 and 16.3 cm for males and females, respectively, in this report (Fig. 5A, B). In the Bay of Bengal, Indian water, Pati (1982) recorded 15 cm (male) and 17 cm (female) as Lm. In addition, Sivakami et al. (2003) estimated Lm for males and females to be 15 and 17 cm. Almatar et al. (2004) found the smallest mature male and female in Kuwait waters, with SL of 12.7 and 16.5 cm, respectively.
The findings from the data on sex ratio related to different months of the year in the present study showed that the females were dominated in most of the months with a significant difference in peak season (Table I). Most earlier researchers (Oda and Namba, 1982; Dadzie et al., 2000; Ghosh et al., 2009) have reported female dominance in their studied populations. However, some studies (Almatar et al., 2004; Narges et al., 2007; Nasir, 2016) have reported male dominance in the natural populations of P. argenteus. In their studies, Sivakami et al. (2003) and Shi et al. (2006) found that the proportion of females and males in their populations was similar.
CONCLUSION
In the Bay of Bengal, Bangladesh water, the spawning peaks for silver pomfret are in April-May (major) and September (minor). This study suggests that during the main peak spawning period, the adults be protected by seasonal closure, resulting in a better breeding environment. Besides, the knowledge of size at 50% sexual maturity (Lm) and sex ratio, which have been studied for the first time in the Bangladesh coast, will help in the implementation of mesh size regulation to understand and forecast the annual changes that a population undergoes. Therefore, this study will help inform management strategies of this valuable species in the Bay of Bengal, Bangladesh water.
ACKNOWLEDGMENT
The authors would like to express appreciation to The Chinese Scholarship Council (CSC) and SOA (State Oceanic Administration) for their financial supports during this journey. The authors are grateful to the Marine Fisheries Survey Management Unit, Department of Fisheries, Government of Bangladesh, for making this work possible by ensuring the laboratory and other management supports to collect and process the samples. The authors also express sincere thanks to Dr. Zoarder Faruque Ahmed for his invaluable technical assistance, as well as all other scientists, and supportive staff who helped with sample collection and laboratory work.
Funding
This work is financially supported by the basic research fund (201562030) of the Ocean University of China.
Statement of conflicts of interest
The authors have declared no conflict of interest.
References
Akhter, F., Islam, M.M., Islam, M.A., and Zahangir, M.M., 2020a. Seasonal variations in some biological parameters (length-weight relationship, condition factor, hepatosomatic and gonadosomatic index) of silver and black pomfret (Pampus argenteus and Parastromateus niger) fromthe Bay of Bengal. J. Fish. Aquacult. Res., 4: 43–53.
Akhter, F., Islam, M.M., Iida, M., and Zahangir, M.M., 2020b. Reproductive seasonality and the gonadal maturation of silver pomfret Pampus argenteus in the Bay of Bengal, Bangladesh. J. Mar. Biol. Assoc. U. K., 100: 1155-1161. https://doi.org/10.1017/S0025315420000922
Almatar, S.M., Lone, K.P., Abu-Rezq, T.S., and Yousef, A.A., 2004. Spawning frequency, fecundity, egg weight and spawning type of silver pomfret, Pampus argenteus (Euphrasen) (Stromateidae), in Kuwait waters. J. appl. Ichthyol., 20: 176-188. https://doi.org/10.1111/j.1439-0426.2004.00546.x
Amparo, M.D.L.A.M., Cárdenas, R.S., Guevara, L.A.S. and Pérez, J.S.R., 2017. Gonadal development of Peprilus medius (Peters, 1869) (Perciformes: Stromateidae) from southeast of the Gulf of California, Mexico. Int. J. Morphol., 35: 56–61. https://doi.org/10.4067/S0717-95022017000100011
Arocha, F., and Barrios, A., 2009. Sex ratios, spawning seasonality, sexual maturity, and fecundity of white marlin (Tetrapturus albidus) from the western central Atlantic. Fish. Res., 95: 98–111. https://doi.org/10.1016/j.fishres.2008.08.010
Brown-Peterson, N.J., Wyanski, D.M., Saborido-Rey, F., Macewicz, B.J. and Lowerre-Barbieri, S.K., 2011. A standardized terminology for describing reproductive development in fishes. Mar. Coastal Fish., 3: 52-70. https://doi.org/10.1080/19425120.2011.555724
Chowdhury, S.R., Hossain, M.S., Md. Shamsuddoha and Khan, S.M.M.H., 2012. Coastal fishers’ livelihood in peril: Sea surface temperature and tropical cyclones in Bangladesh. Center for Participatory Research and Development, Dhaka. pp. 54.
Dadzie, S., Abou-Seedo, F., and Al-Shallal, T., 2000. Reproductive biology of the silver pomfret, Pampus argenteus (Euphrasen), in Kuwait waters. J. appl. Ichthyol., 16: 247-253. https://doi.org/10.1046/j.1439-0426.2000.00237.x
Divya, P.R., Mohitha, C., Rahul, G.K., Shanis, C.P.R., Basheer, V.S., and Gopalakrishnan, A., 2017. Molecular based phylogenetic species recognition in the genus Pampus (Perciformes: Stromateidae) reveals hidden diversity in the Indian Ocean. Mol. Phylogenet. Evol., 109: 240–245. https://doi.org/10.1016/j.ympev.2016.12.030
DoF, Yearbook of Fisheries Statistics of Bangladesh, 2018-19. Fisheries resources survey system (FRSS). Department of Fisheries, Bangladesh. Minist. Fish. Livest., 36: 135. https://fisheries.portal.gov.bd/sites/default/files/files/fisheries.poc45c/2020-10-20-11-57-8df0b0e26d7d0134ea2c92ac6129702b.pdf (accessed on 9 March 2021).
Ganias, K., Somarakis, S., Machias, A., and Theodorou, A., 2004. Pattern of oocyte development and batch fecundity in the Mediterranean sardine. Fish. Res., 67: 13-23. https://doi.org/10.1016/j.fishres.2003.08.008
Ghosh, S., Mohanraj, G., Asokan, P.K., Dhokia, H., Zala, M.S., and Bhint, H.M., 2009. Fishery and stock estimates of the silver pomfret, Pampus argenteus (Euphrasen), landed by gill netters at Veraval. Indian J. Fish., 56: 177-182.
Gopalan, U.K., 1967. Studies on the maturity and spawning of silver pomfret, Pampus argenteus (Euphs.) in the Arabian Sea. Bull. natl. Inst. Sci. India, 38: 785-796.
Hardie, S.A., White, R.W., and Barmuta, L.A., 2007. Reproductive biology of the threatened golden galaxias Galaxias Auratus Johnston and the influence of lake hydrology. J. Fish Biol., 71:1820–1840. https://doi.org/10.1111/j.1095-8649.2007.01648.x
Hussain, N.A., and Abdullah M.A.S., 1977. The length-weight relationship, spawning season and food habits of six commercial fishes in Kuwaiti waters. Indian J. Fish., 24: 181-194.
Jakobsen, T., Fogarty, M.J., Megrey, B.A., and Moksness, E., 2009. Fish reproductive biology: Implications for assessment and management. Blackwell, Oxford. https://doi.org/10.1002/9781444312133
Juchno, D., Boron, A., and Golaszewski, J., 2007. Comparative morphology and histology of the ovaries of the spined loach Cobitis taenia L. and natural allopolyploids of Cobitis (Cobitidae). J. Fish Biol., 70: 1392-1411. https://doi.org/10.1111/j.1095-8649.2007.01419.x
Kennedy, A.J., Sutton, T.M., and Fisher, B.E., 2006. Reproductive biology of female shovelnose sturgeon in the upper Wabash River, Indiana. J. appl. Ichthyol., 22:177–182. https://doi.org/10.1111/j.1439-0426.2006.00745.x
Khatun, D., Hossain, M.Y., Nawer, F., Mostafa, A.A. and Al-Askar, A.A., 2019. Reproduction of Eutropiichthys vacha (Schilbeidae) in the Ganges River (NW Bangladesh) with special reference to potential influence of climate variability. Environ. Sci. Pollut. Res., 26: 10800-10815. https://doi.org/10.1007/s11356-019-04523-5
King, M., 1995. Fisheries biology, assessment and management. Fishing News Books, Oxford.
King, M., 2003. Fisheries biology, assessment and management. Oxford: Fishing News Books/Blackwell.
Lee, T.Y., and Jin, J.J., 1989. Studies on the biology of pomfrets, Pampus spp. in the Korean waters. Gonadal maturation and spawning. J. Korean Fish. Soc., 22: 266–280.
Liao, Y.Y., and Chang, Y.H., 2011. Reproductive biology of the needlefish Tylosurus acus melanotus in waters around Hsiao-Liu-Chiu Island, southwestern Taiwan. Zool. Stud., 50: 296-308.
Lone, K.P., Al-Ablani, S., and Almatar, S., 2008a. Oogenesis, histological gonadal cycle, seasonal variations and spawning season of female silver pomfret (Pampus argenteus, Euphrasen) from the spawning grounds of Kuwait. Pakistan J. Zool., 40: 379-407.
Lone, K.P., Al-Ablani, S. and Almatar, S., 2008b. Spermatogenesis, maturation, seasonal variation and spawning season of silver pomfret (Pampus argenteus, Euphrasen) collected from the natural spawning grounds of the offshore of Kuwait. Pakistan J. Zool., 40: 263–273.
Longenecker, K., and Langston, R., 2018. The jungle histology atlas of gonad stages in coral. Reef Fishes Second Edition.
Murua, H., Kraus, G., Saborido-Rey, F., Witthames, P.R.P.R., Thorsen, A. and Junquera, S., 2003. Procedures to estimate fecundity of marine fish species from field samples in relation to reproductive strategy. J. Northw. Atl. Fish. Sci., 33: 33-54. https://doi.org/10.2960/J.v33.a3
Narges, A., Preetha, K., Jasem, M., Gholam-reza, E., and Vahid, Y., 2007. Spawning season of Pampus argenteus (Euphrasen, 1788) in the Northwest of the Persian Gulf and its implications for management. Pak. J. biol. Sci., 10: 4551-4554. https://doi.org/10.3923/pjbs.2007.4551.4554
Nasir, N.A., 2016. Distribution of silver pomfret (Pampus argenteus) in Iraqi marine water. Mesopotamia Environ. J., 2: 67-77.
Nekuru, A., Imanpur, M. R., Taqizade, V., Shabani, A., and Momeni, M., 2013. A histological study of ovarian development and spawning peak of silver pomfret (Pampus argenteus, Euphrasen, 1788) in the Persian Gulf (Qeshm coastal waters). J. aquat. Ecol., 2: 52-61.
Oda, T., and Namba, Y., 1982. Attempt to artificial fertilization and rearing of larvae of silver pomfret, Pampus argenteus. Okayama Suishi Jiho, 56: 195-197.
Park, Y.J., Takemura, A., and Lee, Y.D., 2006. Annual and lunar-synchronized ovarian activity in two rabbit fish species in the Chuuk Lagoon, Micronesia. Fish. Sci., 72: 166-172. https://doi.org/10.1111/j.1444-2906.2006.01131.x
Pati, S., 1981. Fecundity of silver pomfret, Pampus argenteus (Euphrasen) from Bay of Bengal. Indian J. Mar. Sci., 10: 103–104.
Pati, S., 1982. Studies on the maturation spawning and migration of silver pomfret, Pampus argenteus (Euphrasen) from Bay of Bengal. Matsya, 8: 12-22.
Shahidhullah, M., 1986. Marine fisheries resources management in Bangladesh and current status of exploitation. Mar. Fish. Bull., 3: 22.
Shi, Z.H., Gao, L.J., Xie, Y.L., Luo, H.Z., Wang, H.P., and Chen, B., 2006. Comparison of reproductive characteristics between Pampus argenteus and Pampus cinereu in Zhoushan fishing ground. J. Fish. China, 30: 247-252. (In Chinese).
Sivakami, S., Vivekanandan, E., Raje, S.G., Shoba, J.K., and Rajkumar, U., 2003. Lizard fishes, Pomfrets and Bullseye. In: Status of exploited marine fishery resources of India (eds. M.M. Joseph and A.A. Jayaprakash). CMFRI, Kochi, India, pp. 141-157.
Tracey, S.R., Lyle, J., and Haddon, M., 2007. Reproductive biology and per recruit analyses of striped trumpeter (Latris lineata) from Tasmania, Australia: Implications for management. Fish Res., 84: 358–368. https://doi.org/10.1016/j.fishres.2006.11.025
West, G., 1990. Methods of assessing ovarian development in fishes: A review. Aust. J. Mar. Freshw. Res., 41: 199–222. https://doi.org/10.1071/MF9900199
To share on other social networks, click on any share button. What are these?









