Entomopathogenic Nematode (Nematoda: Rhabditida) Survey and their Occurrence in Soil of District Lakki Marwat, Khyber Pakhtunkhwa Province, Pakistan
Entomopathogenic Nematode (Nematoda: Rhabditida) Survey and their Occurrence in Soil of District Lakki Marwat, Khyber Pakhtunkhwa Province, Pakistan
Samreen Khan*, Salma Javed, Tabassum Ara Khanum, Nasira Kazi
Abstract | Entomopathogenic nematodes are one of the perfect biological control agents against many insect pests all around the world. In this regard, to collect data about entomopathogenic nematodes regarding their presence and distribution in remote areas such as one of the southern district Lakki Marwat of Khyber Pakhtunkhwa province. Extensive surveys carried out in the months of October 2019 to February 2020. Total 73 soil samples retrieved from multiple fruits, vegetables, ornamental flowers, medicinal plants and other commercially important trees from different four sites of district Lakki Marwat and subsequently assessed the presence of EPNs using last instar larva of greater wax moth Galleria mellonella L. as baited host. After completion of requisite process, resultantly the corresponding recovery rate of EPNs was found 10.9% which includes only genus Steinernema comprising two isolates of S. balochiense (24%), two isolates of S. siamkayai (24%) and four unidentified Steinernema spp (52%). However, genus Heterorhabditis was not identified from collected soil samples. Characterization of these previously reported isolates made possible by using morphometric scrutiny. The recovered nematodes further compared with original strains. This research established occurrence result of EPNs from four sites of Lakki Marwat district. In future, these discovered nematodes may be applied in captioned district as biocontrol agents against insect pests of agricultural systems. One of the alternative method against insecticides for better management of plant health under greenhouse and field circumstances in integrated pest management program. Being potential of these nematodes as biopesticides may be distributed to farmers to reduce use of insecticides already in practice and to control hazardous effect of chemical pesticides on both crops and human beings.
Novelty Statement | The present research effort was elaborated to conduct surveys and to ascertain the evidences of entomopathogenic nematodes from one of the southern districts i.e., Lakki Marwat, Khyber Pakhtunkhwa province of Pakistan.
Article History
Received: July 20, 2021
Revised: December 08, 2021
Accepted: December 25, 2021
Published: January 03, 2022
Authors’ Contributions
SK planned the study, executed surveys, collected and analyzed the data and prepared the manuscript. SJ supervised research and thoroughly revised the manuscript. TAK guided in basic features of nematodes. NK scrutinized data.
Keywords
Entomopathogenic nematodes, Survey, Steinernema, Occurrence, District Lakki Marwat
Corresponding author: K. Samreen
To cite this article: Khan, S, Javed, J., Khanum, T.A. and Kazi, N., 2021. Entomopathogenic nematode (Nematoda: Rhabditida) Survey and their occurrence in soil of District Lakki Marwat, Khyber Pakhtunkhwa Province, Pakistan. Punjab Univ. J. Zool., 36(2): 205-216. https://dx.doi.org/10.17582/journal.pujz/2021.36.2.205.216
Introduction
Nematode and insects could be believed as utmost successful groups of invertebrate animals in the nature. Generally, one of the unique class of nematodes (entomopathogenic nematodes) under the family Heterorhabditidae and Steinernematidae are bacterial feeders, survive in soil and few of the same have phoretic connections with insects (Morris et al., 2020). Staggeredly, numbers of countries are being used successfully to control variety of agricultural and stored product insect pests (Aatif et al., 2019; Yuksel et al., 2019; Ahuja et al., 2020; Mokrini et al., 2020; Torrini et al., 2020; Salma et al., 2020; Dichusa et al., 2021) fall under the orders Lepidoptera, Coleoptera, and Diptera (Burnell and Stock, 2000). Essential parameters such as moisture, soil texture, potential of Hydrogen (PH) and organic matters are vital aspects that affecting the virulence, existence and perseverance of these nematodes (Sandhi et al., 2020).
Entomopathogenic nematodes upsurge predation success via inducing cadaver volatiles that fascinate healthy herbivores (Zhang et al., 2019). It penetrates into host via natural openings, injecting symbiotic bacteria as Xenorhabdus for Steinernematids and Photorhabdus for Heterorhabditids (Leite et al., 2019), reproduces quickly and ultimately responsible for the death of host within 24 to 48 hours due to septicemia, creating the situation amiable for growth and reproduction of nematodes (Chandra et al., 2019). Upon occurrence of food storage in the cadaver, thereafter, millions of EPNs emerged from dead insect body for the search of new hosts (Kaplan et al., 2020; Shapiro-Ilan et al., 2018) (Figure 1).
A native EPN species is more adaptive to local environmental conditions. The biocontrol tactics in combined pest mechanism are of rising attention in the background of documented hazard effects of chemical composition on ecologies and human health. Consequently, finding EPNs species and also defining their insecticidal efficiency is chief scientific consideration (Bhat et al., 2019; Askary and Abd-Elgawad, 2021).
Lakki Marwat is an important southern district of Khyber Pakhtunkhwa Province of Pakistan and geographically situated at 32-17 to 32-53 N latitude and 70-23 to 71-16 E longitude. The characteristics of area has sand dunes, burning heat and dry weather bearing hot summer and moderately cool winter. Soil profile is considered to be loamy sand, sandy clay and clay loam. Furthermore, temperature commonly noted from 27-42ºC in summer whereas 4-20ºC during winter season (Samreen et al., 2020). However, the area unfortunately facing acute shortage of water for agriculture and entirely depends upon rain water though, rare and recurrent rainfall usually arises in the months of July and August (ESA, 2012).
Pakistan containing high diversity of ecosystem and rich in fauna adapted to various agroclimatic region. The occurrence and distribution of entomopathogenic nematodes from different agricultural sites including efficiency against various insect pests have been studied from Pakistan. However, existence of EPNs in soil of district Lakki Marwat is relatively un-explored. In this regard, this study is designed for the first time to conduct surveys of entomopathogenic nematodes occurrence and identification from soil of district Lakki Marwat.
Materials and Methods
Research site
An extensive nematological surveys were executed in four sites/ villages of District Lakki Marwat, Khyber Pakhtunkhwa which were previously unexplored i.e., Aba Khel, Aghzar Khel, Sarai Naurang and Tajori.
Sampling material
For the study of entomopathogenic nematodes, a total of 73 soil samples (1000 gm from each sample) were collected around the roots of various fruits, vegetables, ornamental flowers, medicinal plants and other commercially important trees up to 25 cm depth with the help of garden tool i.e., hand trowel. All samples were retained/kept in zip lock plastic bags with labeling flags comprising of relevant information (host, locality, time and date of collection) (Figure 2A) and subsequently shifted safely to main entomopathogenic nematodes research laboratory at National Nematological Research Centre, University of Karachi, Pakistan for their analysis.
Recovery method of EPNs
Recovery of entomopathogenic nematodes from soil were ensured by Galleria trap method as suggested by Bedding and Akhurst, 1975. Approximately 500 gm of soil were put into sterilized plastic pots (28 x 16 x 8 cm) and then six Greater wax moth Galleria mellonella L. larva (Lepidoptera: Galleridae) were included in each pot (Figure 2B). Pots were enclosed with a fitted perforated cap, placed upside down and incubated for period of week at room temperature (25 ± 2 ºC). Furthermore, observations were noticed on daily basis. Dead larvae were removed and sanitized with distilled water for elimination of soil particles. Subsequently for isolation of 3rd stage juvenile’s, dead cadavers were shifted to White trap method (White, 1927) by utilizing small plastic containers (28 x 16 x 8 cm). Plastic cavity block (4.5 x 4.5 cm) was placed inverted at the bottom of container having filter paper on the top of cavity block. Distilled water was filled at the depth of 1 cm. Thereafter, containers were covered with lid and incubated at 30 ± 5 ºC. The progenies were migrated to the surrounding water that emerged from cadaver. Subsequently, progenies/ infective juveniles (IJs) collected on the fourth day of emergence were used for identification.
Collected IJs were further stored in a storage chamber maintained at temperature i.e., 17-20ºC in 100 ml flask having distilled water mixed with a drop of Triton X-100.
For life stages of adult nematodes, five G. mellonella were parasitized by infective juveniles in 10 ml beaker having moist sterilized soil. In 1 ml water suspension approximately 100 IJs was applied on the top of the soil. After that, beaker was wrapped with aluminum foil and incubated at 25ºC (Figure 2C-E). Dead larva was rinsed with distilled water and accordingly placed in petri dishes (Figure 2F). Hence, first and second generation of male and female were achieved by dissecting cadaver every day (up to 10 days) in Ringer’s solution under stereomicroscope (Homonick et al., 1997).
Processing of specimens for morphological study (light microscopy)
Obtained specimens were further heat killed, preserved in TAF, afterward proceeded with solution I (3-4 ml) containing 95% ethanol (20 ml), glycerin (1 ml), distilled water (79 ml) and kept in incubator at 35-40ºC for 12 hours. Thereafter, solution II (3-4 ml) (5 ml glycerin, 95 ml ethanol) was added and subsequently again placed in incubator at about 40ºC for next 3 hours (Seinhorst, 1959). For permanent mounting, the processed nematodes were transferred to microscopic glass slide (25.4 x 76.2 x 1 mm) having a drop of glycerin. A small glass bits were used to evade destruction of nematode bodies. Approximately, 10 IJs, male and female of each were morphologically identified according to Nguyen and Hunt (2007) and subsequently measured under compound microscope.
Cross section
Cross section of lateral lines was performed in a drop of glycerin on microscopic slide and then nematode was sliced with a razor blade or sharp needle under stereomicroscope. Subsequently, slices were transferred to a drop of melted glycerin jelly in the center of slide. Cover slip (19 mm) was placed with pieces of glass fiber to prevent the glycerin jelly from touching the slide. Thereafter, lateral lines were viewed/ studied under high resolution compound microscope (Homonick et al., 1997).
Data analysis
The occurrence percentage and relative frequency of nematodes was assessed as follows:
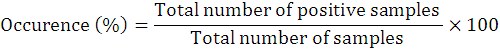
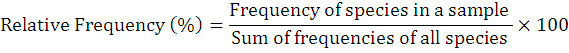
Results and Discussion
EPNs survey and their recovery rate
To explore the biodiversity related to entomopathogenic nematodes, 73 soil samples that resultantly revealed 8 positive samples (recovery rate is 10.9%). The current study reported first time EPNs surveys and occurrence in above cited four villages of district Lakki Marwat, Khyber Pakhtunkhwa province of Pakistan. The targeted areas for surveys and the specimens from where they were surfaced from its specific area are shown in map (Figure 3). Further, detailed information on EPNs status from each site is given in Table 1. During this survey, only genus Steinernema was surfaced comprising two isolates of Steinernema balochiense (Shahina et al., 2015) (LM-38 and LM-17) (24%), two isolates of Steinernema siamkayai (Stock et al., 1998) (LM-33 and LM-06) (24%) and four unidentified Steinernema spp. (52%) under the family Steinernematidae (Figure 4). These species were previously reported from Pakistan but during current survey these were surfaced from new geographical locations with some variations in their morphometry. Hence, after collected corresponding data the Steinernema balochiense (LM-38 and LM-17) was found as 3.4% from Sarai Naurang and 4.5% from Aghzar Khel, respectively. Contrarily, Steinernema siamkayai (LM-33 and LM-06) was found as 8.3% from Tajori and 10% from Aba Khel respectively. However, four unidentified Steinernema spp were originated only from village Sarai Naurang with 13.7%. Their vegetation type and relative frequencies was provided in Table 1. It has been assessed that the recovery rate of positive samples was greater in village Sarai Naurang (6.8%) followed by villages Aba Khel, Aghzar Khel and Tajori (1.3% each) or the recovery rate of EPNs from Sarai Naurang was five times greater as compared to other villages (Figure 5).
Life cycle
Life cycle with respect to temperature and humidity of current isolates Steinernema balochiense and Steinernema siamkayai are presented in Table 2. Both isolates LM-38 and LM-17 completed life cycle (IJs-IJs) in 9-10 days. Whereas LM-33 and LM-06 completed within 7-8 days as previous Pakistani isolate completed life cycle within 5-6 days and though the original strain of S. siamkayai from 8-10 days.
Morphological characterization and comparison with original strains
Key morphological characterization was made with respect to 3rd stage infective juveniles’ length. Upon conducted morphometric values/scrutiny, IJs of both current isolates belong to carpocapsae group as IJs length ≤ 600 µm (Nguyen and Hunt, 2007) and their lateral field which has six longitudinal ridges (Figure 2G). Next, these were further morphologically and morphometrically identified by first and second-generation males and females as Steinernema balochiense (Shahina et al., 2015) and Steinernema siamkayai (Stock et al., 1998) and subsequently each isolate compared between each other and presented in Tables 3, 4 and 5. Morphological similarities found in line with original strains but some variations have been detected in the following characters due to differences in geographical locations and other biotic or abiotic factors:
Table 1: Details of surveyed sites and entomopathogenic nematodes status.
|
Geographical origin |
EPNs STATUS |
||||||||
|
GPS coordinates |
Elevation (m) |
NS |
NPS |
Genus |
Species (Isolate code) |
Vegetation |
Occu-rrence % |
RF % |
|
|
Aba Khel |
32º34'09"N 70º49'39"E |
265 |
10 |
01 |
Steinernema |
S. siamkayai (LM-06) |
Pomegranate |
10 |
25 |
|
Aghzar Khel |
32º23'37"N 70º44'16"E |
471 |
22 |
01 |
Steinernema |
S. balochiense (LM-17) |
Sarkanda |
4.5 |
11.2 |
|
Sarai Naurang |
32º49'43"N 70º46'33"E |
299.10 |
29 |
01 |
Steinernema |
S. balochiense (LM-38) |
Paper flower |
3.4 |
8.5 |
|
04 |
Steinernema |
Steinernema spp. |
Sheesham, Karir, Guava, Grapes |
13.7 |
34.3 |
||||
|
Tajori |
32º37'51"N 70º34'59"E |
319 |
12 |
01 |
Steinernema |
S. siamkayai (LM-33) |
Date Palm |
8.3 |
20.8 |
NS, Number of samples; NPS, Number of positive samples; RF, Relative frequency.
Table 2: Life cycle of current EPN isolate highlighted during study.
|
Species |
Temperature (ºC) |
Humidity (%) |
Life cycle (Days) |
|
S. balochiense (LM-38; LM-17) |
28.7-30 |
61-66 |
9-10 |
|
S. siamkayai (LM-33; LM-06) |
30-33.7 |
38-40 |
7-8 |
Table 3: Comparative morphometric data of 3rd stage infective juveniles of Steinernema balochiense and Steinernema siamkayai. All measurements are in µm in the form of Mean±SD and range in paranthesis.
|
Characters |
Steinernema balochiense (LM-38) |
Steinernema balochiense (LM-17) |
Seinernema siamkayai (LM-33) |
Seinernema siamkayai (LM-06) |
|
|
Infective Juveniles (IJs) |
|||||
|
L |
424.5±46.46 (330-485) |
466.7±33.38 (412-535) |
402.2±39.99 (358-452) |
458.8±14.7 (444-486) |
|
|
a |
19.2±1.12 (17.7-22) |
19.1±1.58 (17.1-23.2) |
19.0±0.53 (18.6-19.8) |
19.7±0.78 (18.5-20.5) |
|
|
b |
4.4±0.40 (3.6-4.9) |
4.6±0.27 (4.3-5.3) |
4.3±0.31(4.0-4.9) |
4.8±0.13 (4.7-5.1) |
|
|
c |
10.6±0.58 (9.8-12.1) |
10.3±1.06 (9.1-13) |
10.64±0.60 (10.1-11.6) |
10.6±0.18 (10.5-11) |
|
|
c' |
3.37±0.27 (3.0-3.7) |
3.6±0.37 (3-4.1) |
3.4±0.17 (3.2-3.6) |
3.5±0.19 (3.3-3.8) |
|
|
Body width |
21.9±1.64 (18-24) |
24.4±0.91 (23-26) |
21±2.52 (18-24) |
23.2±0.97 (22-24) |
|
|
Excretory pore (EP) |
35.2±2.6 (30-40) |
38.4±1.95 (35-41) |
33.6±0.8 (32-34) |
36.6±1.2 (35-38) |
|
|
Nerve ring (NR) |
72.2±1.83 (70-75) |
69.2±2.63 (65-75) |
56±4.56 (52-56) |
64.8±0.97(64-66) |
|
|
Esophagous (ES) |
94.9±3.93 (90-100) |
100.1±3.36 (92-105) |
90.8±4.48 (84-98) |
93.4±0.8 (92-94) |
|
|
Tail |
39.9±4.18 (30-45) |
45.3±3.63 (40-50) |
37.8±5.23 (32-44) |
42.8±1.6 (42-46) |
|
|
Anal body width |
11.7±1.00 (10-13) |
12.5±0.80 (11-14) |
10.8±0.97 (10-12) |
11.9±0.48 (11-12.5) |
|
|
D% |
37.0±2.52 (33.3-42.1) |
38.3±1.13 (36-40) |
36.9±1.24 (34.6-38) |
39±1.17 (38-40.8) |
|
|
E% |
88.6±4.63 (83.5-100) |
85.1±6.64 (76-97.5) |
90.3±11.09 (77.2-103) |
85.5±2.73 (82.6-90.4) |
|
|
H% |
37.3±9.63 (37-50) |
45.1±3.94 (39-50) |
39.2±6.26 (31.8-48.4) |
48.5±1.86 (47.6-52.3) |
|
|
Hyaline |
14.9±4.3 (10-20) |
21±3.28 (16-25) |
14.6±1.49 (12-16) |
20.8±0.97 (20-22) |
|
Note: a, body length/width; b, body length/esophagus; c, body length/tail; c, tail/anal width; D%, EP/ES x 100; E%, EP/TL x 100; H%, Hyaline/TL x 100.
Steinernema balochiense (LM-38 and LM-17)
IJs: They are different from original strain with respect to body length only.
1st and 2nd generation male: They are dissimilar from original strain by body width, esophagus, excretory pore, a and b ratios, SW%, D%, E% and anal width.
1st and 2nd generation female: They are different from original strain by body length, width, esophagus, excretory pore, D%, E%, b ratio, and tail length.
Steinernema siamkayai (LM-33 and LM-06)
IJs: They are different from original strain with respect to nerve ring position and E%.
1st and 2nd generation male: They are different from original strain by body length, esophagus, nerve ring, excretory pore, anal width, D%, SW% and spicule length.
Table 4: Comparative morphometric data of Steinernema balochiense (Isolates LM-38 and LM-17). All measurements are in µm in the form of Mean±SD and range in paranthesis.
|
Characters |
LM-38 |
LM-17 |
||||||
|
1st Gen. Male |
1st Gen. Female |
2nd Gen. Male |
2nd Gen. Female |
1st Gen. Male |
1st Gen. Female |
2nd Gen. Male |
2nd Gen. Female |
|
|
L |
1460±179.7 (1234-1716) |
4922.8±444.3 (4558-5756) |
1098.6± 213.12 (778–1414) |
1896.8± 127.2 (1708- 2060) |
1478.8± 119.5 (1296- 1610) |
4270± 670.7 (3469- 5212) |
791.8± 33.43 (741- 838) |
114.4± 387.2 (1175- 1497) |
|
Body width |
107±17.2 (88-130) |
282±19.64 (250-305) |
81±24.16 (45–115) |
95± 7.07 (85- 105) |
112.4± 11.27 (95- 127) |
153.6± 8.82 (140- 165) |
51.4± 4.40 (45- 58) |
69.6± 3.55 (65- 75) |
|
Excretory pore (EP) |
80±7.07 (70-90) |
17.4±11.53 (102-135) |
66.8± 7.75 (56–75) |
71± 8.60 (60- 85) |
66.6± 5.16 (60- 75) |
95.4± 6.5 (87- 105) |
58.4± 2.15 (55- 61) |
66.6± 4.02 (60- 72) |
|
Nerve ring (NR) |
83±20.3 (50-105) |
147.2±2.13 (144-150) |
90.8± 4.26 (85–97) |
119.8± 14.4 (99- 140) |
96.2± 12.56 (81- 115) |
130± 7.07 (120- 140) |
8.6± 4.67 (92- 105) |
118.6± 5.57 (110- 126) |
|
Esophagus (ES) |
140.2±7.35 (130-151) |
225±14.14 (205-245) |
134±10.67 (120–150) |
175.2± 7.67 (165- 187) |
159.4± 17.7 (136- 183) |
220.2± 23.0 (190- 251) |
125.2± 6.79 (116- 135) |
157.2± 9.55 (145- 171) |
|
Tail |
26.8±4.30 (20-23) |
44.6±5.81 (37-53) |
26.2± 3.54 (21–31) |
40.6± 1.85 (38- 43) |
28.6± 2.41 (25- 32) |
41.2± 8.18 (28- 52) |
23.8± 0.74 (23- 25) |
49.4± 3.26 (45- 54) |
|
Anal width |
37.8±5.74 (30-47) |
84.6±13.72 (69-104) |
32.2± 4.53 (26–39) |
38.4± 9.26 (26- 51) |
36± 2.28 (32- 40) |
50.4± 10.13 (40- 68) |
26± 0.89 (25- 27) |
27± 2.8 (23- 31) |
|
Spicule |
73±2.82 (69-77) |
- |
59±1.41 (57-61) |
- |
75.4± 1.01 (74- 77) |
- |
59.2± 1.72 (57- 62) |
- |
|
Guberna-culum |
51.2±1.16 (50-53) |
- |
43.2± 1.16 (42-45) |
- |
50.8± 3.12 (46- 55) |
- |
42± 1.41 (40- 44) |
- |
|
SW% |
183.8±10.94 (169-200) |
- |
145.2± 3.31 (140-149) |
- |
208.8± 18.35 (185- 234.3) |
- |
162.6± 1.85 (160- 165) |
- |
|
GS % |
66.3±2.85 (62.5-70) |
- |
63±2 (60-66) |
- |
65.94± 3.74 (61.3- 71.4) |
- |
67.5± 33.37 (63.4- 73.1) |
- |
|
Vulva |
- |
2649±185.7 (2395-2900) |
- |
1056.6± 77.13 (956- 1177) |
- |
2319.2± 334.0 (1945- 2851) |
- |
752.2± 60.5 (671- 837) |
|
V% |
- |
51± 2.0 (48.8-54.2) |
- |
56.5± 0.43 (55.9- 57.1) |
- |
552± 1.49 (53.4- 57.6) |
- |
56.8± 0.81 (56- 58.2) |
|
a |
12.2±1.28 (10.9-14.3) |
22.8±4.16 (17.2-28.8) |
15.4± 2.50 (12–19.3) |
19.12± 0.60 (18.2- 20) |
12.16± 1.42 (10.4- 14.4) |
27.12± 2.94 (23.1- 31.5) |
14.78± 0.99 (13.5- 16.4) |
17.92± 1.06 (16.8- 19.8) |
|
b |
10.7±1.06 (9.2-12.2) |
24.0±1.18 (22.2-25.2) |
8.1± 1.33 (6.4–10.1) |
10.3± 0.60 (9.7- 11.4) |
9.18± 0.40 (8.7- 9.8) |
20.32± 1.96 (17.6- 23) |
6.04± 0.30 (5.6- 6.5) |
7.7± 1.03 (6.9- 9.6) |
|
c |
50.3±8.37 (38.5-63) |
122.5±17.9 (99-143.9) |
39.5± 3.56 (35.3–45.6) |
44.6± 2.09 (42.1- 47.9) |
52.32± 7.38 (43.2- 64.4) |
117.0± 17.8 (92- 140.5) |
42.2± 4.59 (36- 49.2) |
36.2± 6.8 (29.3- 47.7) |
|
D% |
54.9±3.20 (50.3-59.6) |
52.6±4.49 (47.4-60) |
48± 1.35 (46.2–50) |
43.5± 6.02 (34.3- 51.5) |
41.29± 7.45 (32.7- 53.5) |
47± 3.56 (41.8- 51.2) |
47.9± 2.74 (44- 51.7) |
43.8± 2.11 (41.3- 47.3) |
|
E% |
232.2±5.38 (225-240) |
174.7±16.72 (153.8-200) |
160.1±42.65 (100–220.8) |
153.4± 5.42 (145- 160) |
271.3± 27.82 (234.3-312.5) |
262.6± 41.01 (201.9-321.4) |
265.7± 3.36 (260.8-270) |
176.2 ±3.8 (170- 181) |
Note: SW%, spicule length/anal width x 100; GS %, gubernaculum length/spicule length x 100; V%, vulva length/body length x 1000.
1st and 2nd generation female: They are different from original strain by body length, width, esophagus, nerve ring, excretory pore, anal width and V%.
Search and identification of native entomopathogenic nematode species adapted to local agroclimatic condition, being as a biocontrol agent counter to major insect pests, display a critical component in developing integrated pest management (IPM) policy of a particular pest. The present investigations/surveys have been conducted to recover the native entomopathogenic nematodes species in various villages of district Lakki Marwat, KPK, Pakistan. In this current survey only genus Steinernema was surfaced from all positive samples from each selected area including two isolates of each Steinernema balochiense and Steinernema siamkayai with four unidentified Steinernema spp. Such result is supported to the fact that there is a greater diversity of Steinernematid species than Heterorhabditid worldwide (Nguyen et al., 2007; Hunt and Nguyen, 2016). The presence of higher proportion of nematodes in village Sarai Naurang than other villages is the fact of good irrigation system that providing better moisture level for survival and persistence of these nematodes in soil as moisture is the most crucial factor.
Table 5: Comparative morphometric data of Steinernema siamkayai (Isolates LM-33 and LM-06). All measurements are in µm in the form of Mean±SD and range in paranthesis.
|
Characters |
LM-33 |
LM-06 |
||||||
|
1st Gen. Male |
1st Gen. Female |
2nd Gen. Male |
2nd Gen. Female |
1st Gen. Male |
1st Gen. Female |
2nd Gen. Male |
2nd Gen. Female |
|
|
L |
1190±158.3 (992-1450) |
2529±282.8 (2215-2985) |
973.6± 29.18 (938–1020) |
2408±497.6 (1660-3000) |
1140±161.7 (900-1390) |
2624± 118.4 (2440- 2780) |
802± 68.79 (709–902) |
2486± 241.6 (2210- 2890) |
|
Body width |
81±8.60 (70-95) |
128.2±9.94 (111-140) |
54.4± 3.26 (50–59) |
130.6±20.21 (101-157) |
80.8±6.71 (70-90) |
122.8± 10.66 (107- 135) |
63.6± 2.41 (60–67) |
190± 0.07 (180 -200) |
|
Excretory pore (EP) |
61.6±3.55 (57-67) |
80±7.07 (70-90) |
56.2± 5.11 (49–64) |
68.8±6.24 (60-77) |
71.2±5.49 (62-78) |
71.4± 4.40 (65- 78) |
55.4± 3.55 (50–60) |
66.8± 4.3 (60- 73) |
|
Nerve ring (NR) |
104.8± 11.66 (89-120) |
125.8±16.4 (103-151) |
140± 7.07 (130–150) |
139±3.40 (134-144) |
105±3.40 (100-110) |
90.6± 7.96 (80- 103) |
128.6± 5.16 (122–137) |
121.4± 4.27 (115- 127) |
|
Esophagus (ES) |
134.4±17.92 (107-160) |
186±10.67 (170-200) |
148.8± 8.08 (137–160) |
189.2±19.64 (157-210) |
139±21.54 (110-165) |
142.4± 2.15 (139- 145) |
152.2± 12.15 (133–168) |
168± 10.29 (150- 180) |
|
Tail |
26.4±3.55 (21-31) |
28.4±5.53 (20-36) |
22.2± 1.72 (20–25) |
56±4 (50-61) |
32.4±2.41 (29-36) |
25.6± 3.72 (20- 30) |
19± 1.41 (17–21) |
46.6± 3.44 (42- 51) |
|
Anal body width |
51.2±6.27 (43-61) |
55.4±4.02 (50-62) |
27.2± 2.13 (24–30) |
36.8±2.56 (33-40) |
32.8±2.56 (30-35) |
62.2± 1.72 (60- 65) |
29± 2 (26–32) |
52.8± 4.7 (46- 60) |
|
Spicule |
64.8±3.12 (60-69) |
- |
55.2± 3.12 (51-60) |
- |
71±1.41 (69-73) |
- |
52.6± 1.85 (50-55) |
- |
|
Guberna-culum |
53.4±2.15 (50-56) |
- |
37.8± 1.72 (35-40) |
- |
53.2±1.72 (51-56) |
- |
38± 1.09 (37-40) |
- |
|
SW% |
1.54±0.10 (1.4-1.7) |
- |
1.54± 0.04 (1.5-1.6) |
- |
1.48±0.07 (1.4-1.6) |
- |
1.66± 0.04 (1.6-1.7) |
- |
|
GS % |
0.73±0.02 (0.71-0.77) |
- |
0.8± 0.08 (0.7-0.9) |
- |
0.70±0.007 (0.70-0.72) |
- |
0.8± 0.08 (0.7-0.9) |
- |
|
Vulva |
- |
1714.4±86.3 (1612-1860) |
- |
1206±155.0 (977-1399) |
- |
1601.8± 64.15 (1522-1697) |
- |
983.6± 54.80 (910-1058) |
|
V% |
- |
55± 3.40 (50-60) |
- |
54.8±3.12 (50-59) |
- |
53.2± 1.16 (52- 55) |
- |
55.4± 3.72 (50- 60) |
|
a |
12.2±0.74 (11-13) |
19.9±6.07 (12.5-28.8) |
16.4± 0.86 (15–17.5) |
16.6±1.85 (14-19) |
11.6±1.01 (10-13) |
18± 3.03 (14- 23) |
16.8±0.74 (16–18) |
18± 1.09 (17- 20) |
|
b |
12±2 (9-15) |
16.6±5.2 (7-22) |
8.24± 0.18 (8–8.5) |
13.4±2.15 (10-16) |
12.2±1.32 (10-14) |
13.6± 2.41 (10- 17) |
7.82±0.59 (7–8.8) |
10.2± 0.74 (9- 11) |
|
c |
41±5.40 (34-49) |
58±2.82 (54-62) |
32.6± 1.85 (30–35) |
56.8±2.56 (53-60) |
36.6±5.53 (29-45) |
54± 2.8 (50- 58) |
32± 3.4 (27–36) |
45.6± 3.44 (40 -50) |
|
D% |
0.54±0.03 (0.49-0.060) |
0.39±0.04 (0.33-0.46) |
0.44± 0.01 (0.42–0.47) |
0.34±0.04 (0.3-0.4) |
0.47±0.01 (0.45-0.50) |
0.34± 0.05 (0.30-0.42) |
0.46± 0.008 (0.45–0.47) |
0.37± 0.06 (0.3-0.4) |
|
E% |
2.02±0.51 (1.9-2.6) |
2.56±0.38 (2-3.1) |
2.26± 0.04 (2.2–2.3) |
1.28± 0.07 (1.2-1.4) |
2.28±0.07 (2.2-2.4) |
2.38± 0.23 (2.1- 2.7) |
2.2± 0.14 (2.0–2.4) |
1.36± 0.10 (1.2-1.5) |
Note: SW%, spicule length/anal width x 100; GS %, gubernaculum length/spicule length x 100; V%, vulva length/ body length x 100.
Steinernema balochiense originally isolated from Pakistan and described by morphologically, morphometrically as well as by DNA sequence polymorphisms by (Shahina et al., 2015) from guava (Psidium guajava L.) while Seinernema siamkayai was formerly identified and described by (Stock et al., 1998) from sandy clay loam soil under sweet tamarind (Tamarindus indicus L.) at Lohmsak district, Petchabun Province, Thailand. S. siamkayai is warm adapted and widely distributed nematode species in Pakistan. As per previously held record, from Pakistan the said species (S. siamkayai isolate Pak-157) was collected from soil samples of wheat from NWFP (Tabassum et al., 2007). Mehreen and Shahina (2013), studied detail intraspecific variations and phylogenetic relationship of twenty-eight S. siamkayai strains which were obtained from various areas of Pakistan by utilizing molecular markers and mitochondrial genes (12S mtDNA). Notwithstanding above, heat tolerance infectivity and reproduction were also measured at different temperatures under laboratory circumstances and concluded that they caused maximum mortality at high temperature zones.
Although, the first ever authentic report on entomopathogenic nematodes survey and their occurrence in Pakistan was conducted as well as studied with the utmost efforts of (Shahina and Maqbool, 1996). For further exploration, different habitats (agricultural fields, experimental plots, lawns, parks, turf grass and sea shores) were explored for soil sampling (500 samples) from Karachi, Thatta and Badin districts of Sindh which exhibit 95% genus Heterorhabditis and 5% genus Steinernema (Shahina et al., 1998). Subsequently, Tabassum et al. (2005) conducted an extensive survey from Dera Ghazi Khan, Bahawalpur, Vehari, Larkana, Sukkar, Nawab Shah, Hederabad, Tando Jam and Mirpurkhas areas of Sindh and Punjab, Pakistan and collected 603 samples with low i.e., 2.5% recovery rate for EPNs. Afterward frequent surveys have been collected time to time from different habitats of Pakistan and subsequently in these research periods, up till now fifteen species of EPNs including twelve species of Steinernema and three species of Heterorhabditis has been identified (Shahina et al., 2019). Our result found low recovery rate of nematodes which may be the influence of any climatic factor of this particular region. The result of low recovery is also coincided or similar to another research conducted throughout the world as (Abate et al., 2017) conducted survey in different sites of South Africa and accordingly found 4% EPNs from 28/640 soil samples. de Brida et al. (2017) isolated EPNs in 16 soil samples corresponding to 8% (16/201 samples) collected from agricultural sites with annual, fruit and forest crops in Brazil. A survey was conducted in nine provinces and three cities of Korea in which 23 soil samples (4.6%) were made optimistic for entomopathogenic nematodes out of 499 (Choo et al., 1995). Similarly, (Kour et al., 2020) detected EPNs from 35/478 sites (7.3%) around Viti Levu, Fiji Islands. Huma et al. (2019) first time explored six districts of Khyber Pakhtunkhwa, Pakistan including Swat, Kohat, Haripur, Chitral, Mardan, Peshawar and found positive vegetations i.e., 23.1% out of 108 samples from grassy land, tomato field, forest land and poplar trees land for EPNs (Heterorhabditis and Steinernema) existance. Another EPNs survey were also commenced from all over the world provided evidences on their occurrence (Kary et al., 2009; Valadas et al., 2013; Yuksel and Canhilal, 2019; Canhilal et al., 2016; Laznik et al., 2009; Edgington et al., 2010; Ma et al., 2010; Hazir et al., 2003; Chaerani and Indrayani, 2018; Dichusa et al., 2021). Global surveys were also supported for the presence of these valuable species and have to be commercially used for insect controlling. Devindrappa et al. (2019) isolated S. siamkayai from five districts of Uttar Pradesh, India with 8% retrieval from 150 samples and applied against soil dwelling stage pupae of Helicoverpa armigera (Hub.). Khatri-Chhetri et al. (2010) isolated S. siamkayai and S. abbasi from Nepal and tested their parasitic potential against 3rd instar larvae of Scarabaeid beetle (Chiloloba acuta) (Pokhrel et al., 2016). In another research, (Gowda et al., 2020) conducted survey from districts of Purvanchal and Bundelkhand region of India and found 2.3% rate for EPNs out of 130 soil samples. Later on, Spoladea recurvalis, Spodoptera litura, Spilosoma obliqua and Myllocerus subfaciatus were checked for mortality response by applied EPNs (S. siamkayai and Heterorhabditis indica).
Worldwide surveys demonstrated that EPNs found in 0.7–50 percent soil samples (Bruck, 2004; Pillay et al., 2009). Although extensive surveys professed only 2–11 percent EPN from greater than one thousand soil samples (Yoshida et al., 1998; Hazir et al., 2003; Hatting et al., 2009; Ma et al., 2010). Variation in mode of sampling, type of insect used for baiting as well as soil type can fluctuating the rate of EPNs existence. Not only these reasons but also vary by other parameters such as soil moisture, soil quality, potential of Hydrogen (pH), temperature and biotic factors (Cheruiyot et al., 2013). Non existing results of EPN doesn’t mean that they are entirely absent. However, they are enormously reliant on insect aggregation and have a tendency to be aggregated in soil more willingly than randomly distributed (Hominick, 2002; Campos-Herrera et al., 2013). More EPNs detection and their higher diversity may be increased by huge number of samples collected from more sites (Hominick, 2002; Erbas et al., 2014).
Though the main aim of the present survey was to collect the data about insect pathogenic nematodes which was restricted and implemented only in District Lakki Marwat for the first time in the history of previously conducted surveys in Pakistan.
Conclusions and Recommendations
Present survey conducted in four villages of District Lakki Marwat where no such research focused yet. In order to recover substantial diversity of native entomopathogenic nematodes species, numbers of soil samples were collected from agricultural sites and subsequently passed through soil trapping technique baited with host Galleria mellonella Linnaeus. Eight positive samples revealed species of carpocapsae group of nematodes belong to the genus Steinernema as Steinernema balochiense, a widespread Steinernema siamkayai and four Steinernema spp. with some morphometric variations from type specimens due to different agroclimatic environment of the district.
From present study it ascertained that environment of Lakki Marwat district rich in enormous diversity of entomopathogenic nematodes. This research is part of Ph.D. dissertation of principal author and based on data collection about EPNs distribution at district level. The recorded numbers of native species of entomopathogenic nematodes in few surveyed sites does not exemplify the full array of occurrence and may be possibility of presence of genus Heterorhabditis. In future, further research will explore occurrence, distribution, discoveries of new species and their insect controlling strategies against agricultural pest at captioned sites and other parts of district. These native beneficial nematodes be applied at laboratory and field level against insect’s pest which caused serious damage to major crops of the area including wheat, gram and other plantation. Hence, it is recommended that a sound policy may be designed and implemented at government level and the same be circulated to farmers to reduce the practice and hazardous effect of chemical pesticides on both crops and human beings.
Conflict of interest
The authors have declared no conflict of interest.
References
Aatif, H.M., Hanif, M.S., Ferhan, M., Raheel, M., Shakeel, Q., Ashraf, W., Irfan U.M. and Ali, S., 2019. Assessment of the entomopathogenic nematodes against maggots and pupae of the oriental fruit fly, Bactrocera dorsalis (Hendel) (Diptera: Tephritidae), under laboratory conditions. Egypt J. Biol. Pest Cont., 29: 1-5. https://doi.org/10.1186/s41938-019-0154-4
Abate, B.A., Slippers, B., Wingfield, M.J., Malan, A.P. and Hurley, B.P., 2017. Diversity of entomopathogenic nematodes and their symbiotic bacteria in south African plantations and indigenous forests. Nematology, 20: 1-17. https://doi.org/10.1163/15685411-00003144
Ahuja, A., Elango, K., Kumar, R., Sindhu, A.S. and Gangwar, S., 2020. Entomopathogenic nematodes as an alternative biological control agent against insect foes of crops. J. Exp. Biol. Agric. Sci., 8: 76–83. https://doi.org/10.18006/2020.8(2).76.83
Askary, T.H. and Abd-Elgawad, M.M.M., 2021. Opportunities and challenges of entomopathogenic nematodes as biocontrol agents in their tripartite interactions. Egypt J. Biol. Pest Contr., 31: 1-10. https://doi.org/10.1186/s41938-021-00391-9
Bedding, R.A. and Akhurst, R.J., 1975. A simple technique for the detection of insect parasitic rhabditid nematodes in soil. Nematology, 21: 109-110. https://doi.org/10.1163/187529275X00419
Bhat, A.H., Askary, T.H., Ahmad, M.J., Suman, A. and Chaubey, A.K., 2019. Description of Heterorhabditis bacteriophora (Nematoda: Heterorhabditidae) isolated from hilly areas of Kashmir valley. Egypt J. Biol. Pest Contr., 29: 1-7. https://doi.org/10.1186/s41938-019-0197-6
Bruck, D.J., 2004. Natural occurrence of entomopathogens in Pacific Northwest nursery soils and their virulence to the Black vine weevil, Otiorhynchus sulcatus (F.) (Coleoptera: Curculionidae). Environ. Entomol., 33: 1335–1343. https://doi.org/10.1603/0046-225X-33.5.1335
Burnell, A.M. and Stock, S.P., 2000. Heterorhabditis, Steinernema and their bacterial symbionts lethal pathogen of insects. Nematology, 2: 31-42. https://doi.org/10.1163/156854100508872
Campos-Herrera, R., Pathak, E., El-Borai, F.E., Stuart, R.J., Gutierrez, C., Rodriguez-Martin, J.A., Graham, J.H. and Duncan, L.W., 2013. Geospatial patterns of soil properties and the biological control potential of entomopathogenic nematodes in Florida citrus groves. Soil Biol. Biochem., 66: 163–174. https://doi.org/10.1016/j.soilbio.2013.07.011
Canhilal, R., Waeyenberge, L., Toktay, H., Bozbuga, R., Çetintas, R. and Imren, M., 2016. Distribution of Steinernematids and Heterorhabditids (Rhabditida: Steinernematidae and Heterorhabditidae) in the southern Anatolia region of Turkey. Egypt. J. Biol. Pest Contr., 26: 1-6.
Chaerani, H.P. and Indrayani, I.G.A.A., 2018. Isolation and molecular identification of entomopathogenic nematodes (Steinernema and Heterorhabditis) from east java and Bali. J. AgroBiogen., 14: 85–96. https://doi.org/10.21082/jbio.v14n2.2018.p85-95
Chandra, R.M., Lee, D. and Kim, Y., 2019. Host immunosuppression induced by Steinernema feltiae, an entomopathogenic nematode, through inhibition of eicosanoid biosynthesis. Insects, pp. 11. https://doi.org/10.3390/insects11010033
Cheruiyot, H.K., Ochuodho, J. and Njira, P., 2013. Assessment of the prevalence of entomopathogenic nematodes in vegetable fields in Uasin Gishu county, Kenya. Afr. Crop Sci. J., 11: 251–255.
Choo, H.Y., Kaya, H.K. and Stock, S.P., 1995. Isolation of entomopathogenic nematodes (Steinernematidae and Heterorhabditidae) in Korea. Jpn. J. Nematol., 25: 44-51. https://doi.org/10.3725/jjn1993.25.1_44
de Brida, A.L., Rosa, J.M.O., de Oliveira, C.M.G., deCastro e Castro, B.M., Serrao, J.E., Zanuncio, J.C., Leite, L.G. and Wilcken, S.R.S., 2017. Entomopathogenic nematodes in agricultural areas in Brazil. Sci. Rep., 7: 45254. https://doi.org/10.1038/srep45254
Devindrappa, S.M.B., Singh, B. and Kumar, K., 2019. Identification and parasitic potential of Steinernema siamkayai against soil-dwelling stage of Helicoverpa armigera (Hub.) under laboratory condition. J. Entomol. Zool. Stud., 7: 493-498.
Dichusa, C.A., Ramos, Jr, R., Aryal, S., Sumaya, N.P.D. and Sumaya, N.H., 2021. Survey and identification of entomopathogenic nematodes in the province of Cotabato, Philippines, for biocontrol potential against the tobacco cutworm, Spodoptera litura (Fabricius) (Lepidoptera: Noctuidae). Egypt. J. Biol. Pest Contr., 31: 1-10. https://doi.org/10.1186/s41938-021-00390-w
Edgington, S., Buddie, A.G., Moore, D., France, A., Merino, L., Tymo, L.M. and Hunt, D.J., 2010. Diversity and distribution of entomopathogenic nematodes in Chile. Nematology, 12: 915-928. https://doi.org/10.1163/138855410X498897
Erbas, Z., Gokce, C., Hazir, S., Demirbag, Z. and Demir, I., 2014. Isolation and identification of entomopathogenic nematodes (Nematoda: Rhabditida) from the Eastern Black Sea region and their biocontrol potential against Melolontha melolontha (Coleoptera: Scarabaeidae) larvae. Turk. J. Agric. For., 38: 187–197. https://doi.org/10.3906/tar-1301-42
ESA, 2012. Khyber Pakhtunkhwa-Southern Area Development Project (KP-SADP) Dera Ismail Khan, Lakki Marwat and Tank Districts, Government of Khyber Pakhtunkhwa.
Gowda, M.T., Patil, J., Vijayakumar, R., Halder, J., Veereshkumar, Divekar, P.A., Rai, A.B. and Singh, J., 2020. Isolation, identification and biocontrol potential of entomopathogenic nematodes occurring in Purvanchal and Bundelkhand regions of Uttar Pradesh, India. Egypt. J. Biol. Pest Contr., 30: 1-11. https://doi.org/10.1186/s41938-020-00290-5
Hatting, J., Stock, S.P. and Hazir, S., 2009. Diversity and distribution of entomopathogenic nematodes (Steinernematidae, Heterorhabditidae) in South Africa. J. Inverteb. Pathol., 102: 120–128. https://doi.org/10.1016/j.jip.2009.07.003
Hazir, S., Keskin, P.N.S., Stock, H., Kaya, H. and Ozcan, S.S., 2003. Diversity and distribution of entomopathogenic nematodes (Rhabditida: Steinernematidae and Heterorhabditidae) in Turkey. Biodivers. Conserv., 12: 375–386.
Hominick, W.M., 2002. Biogeography. In: Gaugler, R. (ed.) Entomopathogenic Nematology, 1, Wallingford, UK, CABI Publishing, pp. 115–143. https://doi.org/10.1079/9780851995670.0115
Hominick, W.M., Briscoe, B.R., del Pino, F.G., Heng, J., Hunt, D.J., Kozodoy, E., Mracek, Z., Nguyen, K.B., Reid, A.P., Spiridonov, S., Stock, P., Sturhan, D., Waturu, C. and Yoshida, M., 1997. Biosystematics of entomopathogenic nematodes: Current status, protocols, and definitions. J. Helminthol., 71: 271–298. https://doi.org/10.1017/S0022149X00016096
Huma, Z., Saljoqi, A.R. and Ali, F., 2019. Entomopathogenic nematodes survey and identification from different districts of Khyber Pakhtunkhwa, Pakistan. Sarhad J. Agric., 35: 1129-1137. https://doi.org/10.17582/journal.sja/2019/35.4.1129.1137
Hunt, D.J. and Nguyen, K.B., 2016. Advances in entomopathogenic nematode taxonomy and phylogeny. Nematology Monographs and Perspectives 12 (Series Editors: D.J. Hunt and R.N. Perry). Leiden, The Netherlands, Brill.
Kaplan, F., Shapiro-Ilan, D. and Schiller, K.C., 2020. Dynamics of entomopathogenic nematode foraging and infectivity in microgravity. NPJ Microgravity, 6: 1-9. https://doi.org/10.1038/s41526-020-00110-y
Kary, N.E., Niknam, G., Griffin, C., Mohammadi, S.A., and Moghaddam, M., 2009. A survey of entomopathogenic nematodes of the families Steinernematidae and Heterorhabditidae (Nematoda: Rhabditida) in the north-west of Iran. Nematology, 11: 107-116. https://doi.org/10.1163/156854108X398453
Khatri-Chhetri, H.B., Waeyenberge, L., Manandhar, H.K. and Moens, M., 2010. Natural occurrence and distribution of entomopathogenic nematodes (Steinernematidae and Heterorhabditidae) in Nepal. J. Invertebr. Pathol., 103: 74-78. https://doi.org/10.1016/j.jip.2009.10.007
Kour, S., Khurma, U., Brodie, G., and Hazir, S., 2020. Natural occurrence and distribution of entomopathogenic nematodes (Steinernematidae, Heterorhabditidae) in Viti Levu, Fiji Islands. J. Nematol., 52: 1-17. https://doi.org/10.21307/jofnem-2020-017
Laznik, Z., Toth, T., Lakatos, T., Vidrih, M. and Trdan, S., 2009. First record of Steinernema feltiae (Filipjev) (Rhabditida: Steinernematidae) in Slovenia. Helminthology., 46: 2: 135–138. https://doi.org/10.2478/s11687-009-0026-7
Leite, L.G., de Almeida, J.E.M., Chacon-Orozco, J.G. and Delgado, C.Y., 2019. Entomopathogenic nematodes. In: Natural enemies of insect pests in neotropical agroecosystems. Springer Cham., pp. 213-221. https://doi.org/10.1007/978-3-030-24733-1_18
Ma, J., Shulong, C., Zou, Y., Xiuhua, L., Richou, H., De Clercq, P. and Moean, M., 2010. Natural occurrence of entomopathogenic nematodes in North China. Russ. J. Nematol., 18: 117- 126.
Mehreen, G. and Shahina, F., 2013. Intraspecific variations and phylogenetic relationships among heat tolerant strains of Steinernema siamkayai (Rhabditida: Steinernematidae) from Pakistan. Pak. J. Nematol., 31: 105-123.
Mokrini, F., Laasli, S., Benseddik, Y., Joutei, A.B., Blenzar, A., Lakhal, H., Sbaghi, M., Imren, M., Özer, G., Paulitz, T., Lahlali, R. and Dababat, A., 2020. Potential of Moroccan entomopathogenic nematodes for the control of the Mediterranean fruit fly Ceratitis capitata Wiedemann (Diptera: Tephritidae). Sci. Rep., 10: 1-11. https://doi.org/10.1038/s41598-020-76170-7
Morris, C., Malan, A.P., De Waal, J.Y. and Johnson, S., 2020. Laboratory bioassays on the susceptibility of trimen’s false tiger moth, Agoma trimenii (Lepidoptera: Agaristidae), to entomopathogenic nematodes and fungi. S. Afr. J. Enol. Vitic., 41: 183-188. https://doi.org/10.21548/41-2-4038
Nguyen, K.B. and Hunt, D.J., 2007. Entomopathogenic nematodes: Systematics, phylogeny and bacterial symbionts. Nematology monographs and perspectives, vol 5. Brill, Leiden, The Netherlands. https://doi.org/10.1163/ej.9789004152939.i-816
Nguyen, K.B., Hunt, D.J. and Mracek, Z.E., 2007. Steinernematidae: Species descriptions. In: Nguyen, K.B. and Hunt, D.J. (Eds). Entomopathogenic nematodes: systematics, phylogeny and bacterial symbionts. Nematology Monographs and Perspectives 5 (Series Editors: D.J. Hunt and R.N. Perry). Leiden, The Netherlands, Brill, pp. 121-609. https://doi.org/10.1163/ej.9789004152939.i-816
Pillay, U., Martin, L.A., Rutherford, R.S. and Berry, S.D., 2009. Entomopathogenic nematodes in sugarcane in South Africa. Proc. South Afr. Sugar Technol. Assoc., 82: 538–541.
Pokhrel, M., Thapa, R.B., Gharty, C.Y.D. and Sporleder, M., 2016. Efficacy of two entomopathogenic nematodes strains Steinernema siamkayai and S. abbasi against the 3rd instar larvae of Chiloloba acuta. J. Agric. Environ., 17: 73-81. https://doi.org/10.3126/aej.v17i0.19863
Salma, J., Tabassum, A.K. and Samreen, K., 2020. Biocontrol potential of entomopathogenic nematode species against Tribolium confusum (Jac.) (Coleoptera: Tenebrionidae) and Rhyzopertha dominica (Fab.) (Coleoptera: Bostrichidae) under laboratory conditions. Egypt J. Biol. Pest Contr., 30: 1-6. https://doi.org/10.1186/s41938-020-0206-9
Samreen, K., Salma, J. and Tabassum, A.K., 2020. Plant Parasitic nematode of genera Aphelenchus and Aphelenchoides (Nematoda: Aphelenchoidea) from District Lakki Marwat, Kyber Pakhtunkhwa, Pakistan. Pak. J. Phytopathol., 32: 169-178. https://doi.org/10.33866/phytopathol.030.02.0586
Sandhi, R.K., Pothula, R., Pothula, S.K., Adams, B.J. and Reddy, G.V.P., 2020. First record of native entomopathogenic nematodes from Montana agroecosystems. J. Nematol., 52: 1-11. https://doi.org/10.21307/jofnem-2020-060
Seinhorst, J.W., 1959. A rapid method for the transfer of nematode from fixative to anhydrous glycerin. Nematology, 4: 67-69. https://doi.org/10.1163/187529259X00381
Shahina, F. and Maqbool, M.A., 1996. Isolation of entomopathogenic nematodes (Heterorhabditidae and Steinernematidae) from Pakistan. Pak. J. Nematol., 14: 135-136.
Shahina, F., Anis, M., Zainab, S. and Maqbool, M.A., 1998. Entomopathogenic nematodes in soil samples collected form Sindh, Pakistan. Pak. J. Nematol., 16: 41-50.
Shahina, F., Nasira, K., Firoza, K. and Erum, Y.I., 2019. Overview of the nematode fauna of Pakistan. Pak. J. Nematol., 37: 171-243. https://doi.org/10.18681/pjn.v37.i02.p171-243
Shahina, F., Tabassum, K.A., Ali, S., Solangi, G.S., Mehreen, G. and Salma, J., 2015. Steinernema balochiense n. sp. (Rhabditida: Steinernematidae) a new entomopathogenic nematode from Pakistan. Zootaxa, 3904: 387-402. https://doi.org/10.11646/zootaxa.3904.3.4
Shapiro-Ilan, D.I., Hiltpold, I. and Lewis, E.E., 2018. Ecology of invertebrate pathogens: nematodes, In: Ecology of invertebrate diseases (eds. A.E. Hajek and D.I. Shapiro-Ilan). John Wiley and Sons, Hoboken, NJ. pp. 415–440. https://doi.org/10.1002/9781119256106.ch11
Stock, S.P., Somsook, V. and Reid, A.P., 1998. Steinernema siamkayai n. sp. (Rhabditida: Steinernematidae) an entomopathogenic nematode from Thailand. Syst. Parasitol., 41: 105–113. https://doi.org/10.1023/A:1006087017195
Tabassum, K.A., Kazmi, A.R. and Shahina, F., 2007. A redescription of Steinernema siamkayai (Stock, Somsook and Reid, 1998) (Rhabditida: Steinernematidae) from Pakistan. Int. J. Nematol., 17: 217-224.
Tabassum, K.A., Shahina, F. and Abid, B., 2005. Occurrence of entomopathogenic (EPN) nematodes in Pakistan. Pak. J. Nematol., 23: 99-102.
Torrini, G., Paoli, F., Mazza, G., Simoncini, S., Benvenuti, C., Strangi, A., Tarasco, E., Barzanti, G.P., Bosio, G., Cutino, I., Roversi, P.F. and Marianelli, L., 2020. Evaluation of Indigenous Entomopathogenic Nematodes as Potential Biocontrol Agents against Popillia japonica (Coleoptera: Scarabaeidae) in Northern Italy. Insects, 11: 1-15. https://doi.org/10.3390/insects11110804
Valadas, V., Laranjo, M., Mota, M. and Oliveira, S., 2013. A survey of entomopathogenic nematode species in continental Portugal. J. Helminthol., 88: 327–341. https://doi.org/10.1017/S0022149X13000217
White, G.F., 1927. A method for obtaining infective nematode larvae from cultures. Science, 66: 302–303. https://doi.org/10.1126/science.66.1709.302.b
Yoshida, M., Reid, A.P., Briscoe, B.R. and Hominick, W.M., 1998. Survey of entomopathogenic nematodes (Rhabditida: Steinernematidae and Heterorhabditidae) in Japan. Fundam. appl. Nematol., 21: 185–198.
Yuksel, E. and Canhilal, R., 2019. Isolation, identification, and pathogenicity of entomopathogenic nematodes occurring in Cappadocia Region, Central Turkey. Egypt. J. Biol. Pest Contr., 29: 1-7. https://doi.org/10.1186/s41938-019-0141-9
Yuksel, E., Canhilal, R. and Imren, M., 2019. Potential of four Turkish isolates of entomopathogenic nematodes against three major stored products insect pests. J. Stored Prod. Res., 83: 317-321. https://doi.org/10.1016/j.jspr.2019.08.003
Zhang, X., Machado, R. Ar., Doan, C.V., Arce, C.C., Hu, L. and Robert, C.A., 2019. Entomopathogenic nematodes increase predation success by inducing cadaver volatiles that attract healthy herbivores. Ecology, pp. 1-21. https://doi.org/10.7554/eLife.46668.024
To share on other social networks, click on any share button. What are these?







