Effect of Vitamins A, C and E on the Bio-Growth Performance and Captive Maturation of Lepidocephalichthys thermalis
Effect of Vitamins A, C and E on the Bio-Growth Performance and Captive Maturation of Lepidocephalichthys thermalis
Pankaj Gargotra1, Judith Betsy C1*, Cheryl Antony2 and Stephen Sampath Kumar J3
1TNJFU-Department of Aquaculture, Fisheries College and Research Institute, Tuticorin, Tamil Nadu, India.
2TNJFU- Department of Aquaculture, Dr. M.G.R. Fisheries College and Research Institute, Ponneri, Tamil Nadu, India.
3TNJFU- Directorate of Sustainable Aquaculture, Tamil Nadu Dr. J. Jayalalithaa Fisheries University, Nagapattinam, Tamil Nadu, India.
ABSTRACT
Loaches are highly nutritious and global demand has been recognized for only few species especially of ornamental importance. Lepidocephalichthys thermalis is an important food species of loach fetching high market price but there is limited data related to its nutritional requirements and reproductive biology. Therefore, a detailed study was carried out to analyze the effect of vitamin enriched diets on the captive maturation and breeding performance of L. thermalis. Vitamins A, C and E were incorporated at three different doses in basal feed containing 23% C.P and fed to brooders for a period of 90 days. Fecundity, GSI, gonad histology, hormone (cortisol and progesterone) variations, breeding performance and growth parameters were recorded. Significant differences in the growth and breeding performance of female brooder under different treatments were noted. Among the selected vitamins, incorporation of vitamin C at 150 mg /kg of diet resulted in maximum mean weight gain (2.51 ± 0.043 g), mean weight gain percentage (70.74 ± 1.219), mean DGR (0.16 ± 0.003) and mean SGR (3.13 ± 0.0037). Based on the histological observation, maximum maturity of L. thermalis was obtained when fed with diet incorporated with 200 mg/kg vitamin E. All the treatments recorded higher mean estimated growth and maturation parameters when compared to control and were significantly different (p<0.05). The results obtained were statistically analysed using One way ANOVA following Duncan multiple range test in SPSS- 22.0 to compare the significant differences in growth parameters among all treatments and discussion was made in the light of available literatures.
Article Information
Received 22 April 2022
Revised 15 July 2022
Accepted 20 August 2022
Available online 09 January 2023
(early access)
Published 29 January 2024
Authors’ Contribution
PG acquisition of data, data analysis and interpretation, preparation of manuscript. CJB design of the study, data analysis and interpretation, manuscript correction. CA design of the study. JSSK conception and design of the study, data analysis and manuscript correction.
Key words
Lepidocephalichthys thermalis, Vitamin, Fecundity, GSI, Gonad, Histology, Cortisol, Progesterone, Hormone
DOI: https://dx.doi.org/10.17582/journal.pjz/20220422140419
* Corresponding author: betsy@tnfu.ac.in
0030-9923/2024/0002-0781 $ 9.00/0
Copyright 2024 by the authors. Licensee Zoological Society of Pakistan.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Loaches are an important group of freshwater species that have global distribution. Lepidocephalichthys thermalis, Indian spiny loach inhabits various habitats ranging from hill streams to paddy fields, stream bottom, swamps and flooded fields (Kottelat, 1992; Havird and Page, 2010). Similar species has been reported from India, Indo-China, Myanmar, Nepal, Pakistan, Sri Lanka, Bangladesh, Malay Archipelago, Thailand and Vietnam (Jayaram, 1999). Mostly, these small loach fishes are used for aquarium purposes, but studies have also highlighted the nutritional importance of loach flesh (You et al., 2009). It contains high-quality protein, vitamins and is also known for medicinal values. Loach peptide is known to have an antioxidation and antifatigue effect (You et al., 2011). The richness of micronutrients in the aquatic products makes them highly acceptable as compared to animal protein and loaches are known for their nutritional and nutraceutical properties (You et al., 2009). However, culture techniques have not been standardized for loaches but wild-collected catch can fetch attractive market prices (USD 20). Despite being so valuable, information on its functional maturation, growth, morphology, biology, etc. are not much available.
Broodstock nutrition have profound effect on fecundity, gonad development, egg quality and larvae (Watanabe, 1985). However, information on the broodstock nutrition of loach is scanty. It is known that vitamin A is an essential dietary micronutrient for fish and known to play an important role in vision, reproduction, bone development, embryogenesis, growth and differentiation and maintenance of epithelial cells (Hernandez and Hardy, 2020). Vitamin C plays an important role in the biosynthesis of collagen, cartilage, bone formation (Wilson and Poe, 1973; Kraus et al., 2004; Darias et al., 2011), skin formation and growth (Barnes and Kodicek, 1972; Darias et al., 2011). Vitamin E is fat soluble, and its main function is to prevent peroxidation of polyunsaturated fatty acids of phospholipids and cholesterol in cellular and sub-cellular membranes thus protecting lipoproteins, biological membranes and lipid stores against oxidation (Bender, 1995; James et al., 2008). It is a potent antioxidant in reproduction (Riley and Behrman, 1991) and is widely distributed in animal tissues (Stocker et al., 1999). Hence, the present study was carried out to find out the effect of Vitamins A, C and E on the growth, captive maturation and breeding performance of L. thermalis.
Materials and Methods
Experimental animals
Wild collected specimens of L. thermalis from Parakkai, Kanyakumari district of Tamil Nadu, India (8.1519o N, 77.4544o E) was used in the experiment. They were brought to research centre at Kanyakumari Parakkai Centre for Sustainable Aquaculture, Parakkai and acclimatized for 2 weeks, and their length and weight were recorded immediately after they were stabilized.
Feed preparation
Feed was prepared using ground nut oil cake (GNOC), rice bran, wheat bran, corn flour and soy flour. Food grade vitamin A (West-coast pharmaceuticals, Ahmedabad, Gujarat), vitamin C (Avantor performance Materials India Ltd) and vitamin E (Procter and Gamble Health Ltd.) were procured. Doses of vitamins were finalized based on the previous studies on other species. Each vitamin was added at three different doses, vitamin A viz., 2000 I.U (T1), 4000 I.U (T2) and 6000 I.U (T3); vitamin C viz., 50 mg/kg (T4), 100 mg/kg (T5) and 150 mg/kg (T6) and vitamin E at 100 mg/kg (T7), 200 mg/kg (T8) and 400 mg/kg (T9) were selected for this study. The details of feed formulation and the proximate composition of the experimental feed is given in Table I.
Experimental setup
Small plastic tubs of 30-L capacity were used for the experiment and water level of 12 cm was maintained in the tubs throughout the experiment. The treatments were carried out in replicates along with control and each tank were stocked with 30 number of female fishes. Female brooders were fed with the experimental feed twice a day at ad libitum for a period of 60 days.
Assessment of growth performance
Growth parameters such as mean weight gain (MWG), daily growth rate (DGR), survival rate (SR) and specific growth rate (SGR) were calculated and documented carefully.
Table I. Feed formulation and proximate composition of the experimental diets (%).
|
Ingredients |
Diet (% of inclusion) |
|||||||||
|
C |
T1 |
T2 |
T3 |
T4 |
T5 |
T6 |
T7 |
T8 |
T9 |
|
|
GNOC |
20 |
20 |
20 |
20 |
20 |
20 |
20 |
20 |
20 |
20 |
|
Rice bran |
40 |
40 |
40 |
40 |
40 |
40 |
40 |
40 |
40 |
40 |
|
Corn flour |
10 |
10 |
10 |
10 |
10 |
10 |
10 |
10 |
10 |
10 |
|
Soy flour |
20 |
20 |
20 |
20 |
20 |
20 |
20 |
20 |
20 |
20 |
|
Wheat bran |
10 |
10 |
10 |
10 |
10 |
10 |
10 |
10 |
10 |
10 |
|
Vitamin A (I.U) |
0 |
2000 |
4000 |
6000 |
0 |
0 |
0 |
0 |
0 |
0 |
|
Vitamin C mg/kg |
0 |
0 |
0 |
0 |
50 |
100 |
150 |
0 |
0 |
0 |
|
Vitamin E mg/kg |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
100 |
200 |
400 |
|
Proximate composition |
||||||||||
|
Crude protein (%) |
22.72 |
22.70 |
22.73 |
22.69 |
22.79 |
22.80 |
22.78 |
22.78 |
22.77 |
22.77 |
|
Crude fibre (%) |
16.20 |
16.22 |
16.29 |
16.34 |
16.25 |
16.22 |
16.24 |
16.28 |
16.24 |
16.27 |
|
Moisture (%) |
6.37 |
6.4 |
6.39 |
6.41 |
6.42 |
6.42 |
6.41 |
6.41 |
6.43 |
6.41 |
|
Ash (%) |
8.66 |
8.64 |
8.65 |
8.62 |
8.61 |
8.62 |
8.61 |
8.64 |
8.62 |
8.61 |
|
Gross energy (Kcal/Kg) |
4106 |
4120 |
4100 |
4129 |
4124 |
4127 |
4126.5 |
4120.9 |
4126 |
4125.4 |
GNOC, ground nut oil cake.
Assessment of maturation
Ten fishes per treatment were dissected carefully, and bilobed gonads were abscised at the end of the feeding trial to assess the levels of maturation as explained below. The mean of all the recorded values were taken for the analysis.
Gonad histology
Histological sections were prepared, mounted and stained in histology lab and histological examinations were observed under microscope and documented carefully. Ovarian development was studied according to Kumari and Nair (1978a, b). The maturity stages of the ovary subjected to experiment were observed and estimated based on the proportions of each oocyte stage. Individual oocyte stage was counted and recorded to find out the maturity status of each particular fish in different treatments.
Gonadosomatic index (GSI) value
GSI was calculated during initial and final sampling. The GSI was estimated by using the following equation as suggested by Kumari and Nair (1978a, b).
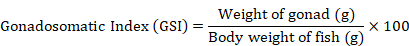
Absolute fecundity
Absolute fecundity was calculated as the total number of eggs present in fish ovary. Eggs were counted carefully from all treatments and the fecundity was recorded (Sarmento et al., 2018).
Hormone assays
Hormone assays were performed as described by Zhang et al. (2021). Gonad samples were homogenized in cold PBS (Phosphate buffer saline) and were centrifuged at 3000 rpm for 20 min in refrigerated centrifuge (4oC). The supernatants were collected and analyzed for cortisol and progesterone levels using Accubind ELISA kits.
Assessment of breeding performance
After the completion of 60 days of feeding experiment, males were introduced in the tanks at a ratio of 1:1 and were assessed for breeding performances for another 30 days. Number of spawns produced under different treatments were calculated carefully and were collected and stocked in separate tanks. Initial length and weight data of spawn produced were recorded carefully and were fed with powdered GNOC.
Statistical analysis
Growth parameters were subjected to F test to find out significant difference among treatments. One way ANOVA was used following Duncan multiple range test in SPSS- 22.0.
Results and Discussion
All the estimated mean growth and maturation parameters except hormone concentrations showed significant differences among the treatments as it can be seen in Tables II-IV. No mortality was observed in any of the treatments.
Effect of vitamin A on growth
Among the different incorporation levels of vitamin A, fishes fed with diet incorporated with 2000 I.U vitamin A (T1) recorded maximum weight gain (2.11 ± 0.008), weight gain % (59.48 ± 0.243), DGR (0.14 ± 0) and SGR (3.05 ± 0.061), while minimum values were reported from control (Table II).
Growth performance is the main criteria used to determine the optimum dietary requirement of vitamin A for fish (Hernandez and Hardy, 2020). Dietary vitamin A requirements determined for many fishes is in the range of 1,000 to 20,000 IU/kg, with freshwater species having slightly more requirement than marine species. Optimum dietary requirement recorded in the present study to attain maximum growth performance of L. thermalis female brooders is 2000 I.U which is understandable from the point that further increase in vitamin A concentration doesn’t influences the growth performance and moreover there are chances for hypervitaminosis as reported in few studies (Hernandez and Hardy, 2020). The level of vitamin A reported in the present study is in range with the optimum dietary requirements reported for other fishes such as, 2000-2500 I.U for trout and salmon, 1000-2000 I.U for carps and catfish and 1,000–2,000 I.U for channel catfish (Halver, 2002).
Effect of vit. A on maturation of L. thermalis
Fecundity from fishes fed with different doses of vitamin A was recorded in the range of 3985-5662 nos/fish (Table III). Maximum fecundity (5662 nos/fish) and G.S.I (21.60 ± 2.63) was recorded from fishes fed with 6000 I.U (T3) vitamin A (Table III). A direct relationship among mean estimated fecundity and GSI has been recorded with respect to dietary inclusion level of vitamin A in L. thermalis diets.
Maximum maturity based on the estimated maturity stages was recorded in T2 followed by T3 and T1 (Table IV and Fig. 1). Mean cortisol and progesterone concentrations recorded in the present study were in the range of 24.94± 0.04 to 25.08 ± 0.13 ng/ml and 2.57 ± 0.001 to 2.58 ± 0.003 ng/ml, respectively (Table III). Breeding was not observed in any of the treatment.
Few reports are available on the effect of vitamin A on fish reproduction (Furuita et al., 2003; Alsop and Vijayan, 2008) and the role of vitamin A in sexual maturation, reproduction and determining egg quality has been a controversial subject in past few decades (Izquierdo et al., 2001). In the present study, fishes fed with 2000 I.U vitamin A had maximum fecundity when compared to others, while maximum G.S.I was recorded with diet supplemented with 6000 I.U vitamin A. Maximum cortisol and progesterone concentration were recorded form the ovaries of fish fed with 2000 I.U and 6000 I.U vitamin A, respectively. In economic point of view, dietary supplementation of 2000 I.U vitamin A is sufficient enough to promote the maturation of L. thermalis female brooder.
It is reported that vitamin A can stimulate the development of the gonad, promote the maturation of the eggs, and prolong the spawning period (Wang et al., 2014). Bighead carp when fed with diet deficient with vitamin A had low fecundity (Santiago and Gonzal, 2000). The effective release of sufficient amount of retinol from tissues during breeding (Wolf, 1984) and the role of retinal in regulating the expression of various proteins related to onset of meiosis controls the breeding performance of fish (Ruivo et al., 2018). However, inclusion of high level of vitamin A in broodstock diet reported to have negative effect on the breeding performance of fish (Fontagné-Dicharry et al., 2010; Hernandez and Hardy, 2020) which may be the possible reason to explain low fecundity and G.S.I values in fishes fed with diet supplemented with 4000 I.U and 6000 I.U vitamin A, when compared with 2000 I.U vitamin A.
Although vitamin A enhanced the fecundity, GSI, cortisol and progesterone levels, it does not lead to spawning of the fishes. Only few studies have been conducted to see the effect of vitamin A supplementation in fish and majority of them has focused on larval development. Hence a clear picture about the influence of vitamin A on spawning is unavailable. It is also to be noted that dietary requirement of vitamin A based on spawning and breeding efficiency has not been standardized for any fish (Hernandez and Hardy, 2020). In the present study also vitamin A influenced maturation, but it did not contribute to the spawning of L. thermalis in captivity.
Effect of vitamin C on growth of L. thermalis
Among the selected doses of vitamin C, supplementation of 150 mg/kg (T6) vitamin C in the diets of L. thermalis female brooders recorded maximum weight gain (2.51 ± 0.04g), weight gain % (70.75 ± 1.22), DGR (0.16 ± 0.003) and SGR (3.13 ± 0.04) (Table II). This was in accordance to Zhou et al. (2012), who reported that juvenile cobia showed maximum weight gain, SGR, protein efficiency ratio and feed efficiency ratio when fed with diet supplemented with 193.4 mg/kg vitamin C.
Ibrahim et al. (2020) reported higher final body weight in Nile tilapia fingerlings when fed with feed containing 400 mg/kg of vitamin C compared to feed supplemented with 200 and 300 mg/kg of vitamin C. Betsy et al. (2021) mentioned that koi carp fed with vitamin C at 600 mg/kg diet exhibited higher growth performance when compared to 200 and 400 mg/ kg vitamin C and concluded that incorporation of vitamin C at higher concentration is necessary to enhance growth. The present study is also in line with this, and the higher concentration of vitamin C provided the best growth.
Effect of vit C on maturation of L. thermalis
Mean absolute fecundity was in the range of 3529.5 – 4858.3 nos/fish and G.S.I recorded from the fishes fed with different doses of vitamin C were in the range of 15.56 ± 2.90 to 20.12 ± 3.55 respectively (Table III). Maximum fecundity (4858.3 nos/fish) and G.S.I (20.12 ± 3.55) was recorded from fishes fed with 100 mg/kg (T5) and 150 mg/kg (T6) vitamin C, respectively.
Maximum maturity was recorded from fishes fed with 50 mg/kg (T4) (Tables IV and Fig. 3). The mean cortisol and progesterone concentrations were recorded in the range of 24.90 ± 0.01 – 25.02 ± 3.55 ng/ml and 2.56 ± 0.0 – 2.58 ± 0.002, respectively (Table III).
Only 48 number of spawns were collected from tanks stocked with fishes fed with 150 mg/kg (T6) vitamin C. Since these fishes hide in soft sand, there are possibilities that they may lay eggs under soft sand and hence, no eggs were physically observed in any of the treatments thus giving the reason that it was difficult to evaluate hatching and fertilization rates. Average initial length of spawns recorded in the present study was 0.6-0.7 cm.
Vitamin C is the leading micronutrient in enhancing the reproductive performance of fish due to the interactions between steroid hormones and catecholamines (Dabrowski and Ciereszko, 2001). Ascorbic acid can influence the reproductive performance of fish, under various factors such as age, size, stress and reproductive status (Toyama et al., 2000). Role of ascorbic acid in brood stock diet has been recorded in many species such as rainbow trout (Blom and Dabrowski, 1995), tilapia (Soliman et al., 1986) and gold fish (Xie et al., 2006).
Table II. Bio-growth parameters (Mean ± S.E) of female L. thermalis brooders fed with Vitamin A, C and E at different concentrations observed during experimental period.
|
Parameter |
Treatments |
|||||||||
|
C |
T1 |
T2 |
T3 |
T4 |
T5 |
T6 |
T7 |
T8 |
T9 |
|
|
Weight gain (g) |
1i |
2.11c ± 0.008 |
1.54e ± 0.14 |
1.36g ± 0.02 |
2.17b ± 0.04 |
0.64j ± 0.03 |
2.51a ± 0.04 |
1.47f ± 0.14 |
1.02h ± 0.04 |
2.02d ± 0.21 |
|
Weight gain (%) |
28.1i |
59.48c ± 0.24 |
43.58e ± 4.06 |
38.51g ± 0.65 |
61.31b ± 1.13 |
18.24j ± 0.81 |
70.74a ± 1.22 |
41.47f ± 3.82 |
28.80h ± 1.22 |
56.95d ± 5.93 |
|
DGR |
0.07 I ± |
0.14 c ± 0 |
0.10 e ± 0.009 |
0.09 g ± 0.001 |
0.14 b ± 0.003 |
0.04 j ± 0.002 |
0.16 a ± 0.003 |
0.09 f ± 0.009 |
0.06 h ± 0.002 |
0.13 d ± 0.01 |
|
SGR |
0.07i ± |
3.05 c ± 0.06 |
2.25 e ± 0.17 |
1.74 g ± 0.05 |
2.63 b ± 0.01 |
2.09 j ± 0.08 |
3.13 a ± 0.04 |
2.80 f ± 0.27 |
2.47 h ± 0.02 |
2.98d ± 0.04 |
Data expressed as Mean ± SE; Mean values in the same row with different superscript differ significantly (p<0.05). One way ANOVA was used following Duncan multiple range test in SPSS- 22.0.
DGR, daily growth rate; SGR, specific growth rate.
Table III. Estimated maturation parameters of L. thermalis fed with vitamin A, C and E at three different concentrations.
|
Vitamins |
Concentration |
Mean body weight (g) |
Gonad weight (g) |
GSI |
Absolute fecundity (per gram body weight) |
Hormone level (ng/ml) |
||||
|
Initial |
Final |
Initial |
Final |
Initial |
Final |
Cortisol |
Progesterone |
|||
|
Control |
C |
0.74 ± 0.00 |
0.87 ± 0.00 |
0.001 ± 0.00 |
0.11 ± 0.00 |
0.14 ± 0.00 |
12.64 ±0.0 |
4172.4 |
24.97 ± 0.00 |
2.57 ± 0.000 |
|
A |
2000 I.U (T1) |
0.74 ± 0.00 |
1.25 ± 0.06 |
0.001 ± 0.00 |
0.20 ± 0.02 |
0.14 ± 0.00 |
16.20 ±1.21 |
3985 |
25.08 ± 0.13 |
2.57 ± 0.001 |
|
4000 I.U (T2) |
0.74 ± 0.00 |
0.99 ± 0.12 |
0.001 ± 0.00 |
0.18 ± 0.07 |
0.14 ± 0.00 |
18.48 ±4.92 |
4825 |
24.94 ± 0.04 |
2.58 ± 0.003 |
|
|
6000 I. U (T3) |
0.74 ± 0.00 |
0.85 ± 0.16 |
0.001 ± 0.00 |
0.18 ± 0.04 |
0.14 ± 0.00 |
21.60 ±2.63 |
5662 |
25.002 ± 0.02 |
2.58 ± 0.003 |
|
|
C |
50 mg/kg (T4) |
0.74 ± 0.00 |
1.10 ± 0.01 |
0.001 ± 0.00 |
0.20 ± 0.03 |
0.14 ± 0.00 |
17.91 ±3.12 |
3916.9 |
24.90 ± 0.01 |
2.57 ± 0.001 |
|
100 mg/kg (T5) |
0.74 ± 0.00 |
0.90 ± 0.23 |
0.001 |
0.14 ± 0.04 |
0.14 ± 0.00 |
15.56 ±2.90 |
4858.3 |
24.99 ± 0.12 |
2.57 ± 0.000 |
|
|
150 mg/kg (T6) |
0.74 ± 0.00 |
1.28 ± 0.01 |
0.001 ± 0.00 |
0.26 ± 0.04 |
0.14 ± 0.00 |
20.12 ±3.55 |
3529.5 |
25.02 ± 0.07 |
2.58 ± 0.000 |
|
|
E |
100 mg/kg (T7) |
0.74 ± 0.00 |
1.17 ± 0.04 |
0.001 ± 0.00 |
0.24 ± 0.07 |
0.14 ± 0.00 |
20.68 ±1.97 |
4142.1 |
24.97 ± 0.05 |
2.58 ± 0.002 |
|
200 mg/kg (T8) |
0.74 ± 0.00 |
1.05 ± 0.63 |
0.001 ± 0.00 |
0.20 ± 0.08 |
0.14 ± 0.00 |
19.05 ±6.83 |
5125 |
24.95 ± 0.13 |
2.57 ± 0.008 |
|
|
400 mg/kg (T9) |
0.74 ± 0.00 |
1.23 ± 0.21 |
0.001 ± 0.00 |
0.23 ± 0.06 |
0.14 ± 0.00 |
18.37 ±4.80 |
3733.5 |
24.97 ± 0.08 |
2.56 ± 0.000 |
|
Table IV. Histology observations of L. thermalis gonads fed with vitamin A, C and E at three different concentrations.
|
Vitamin |
Concentrated |
Stages observed (nos) |
|||||
|
OG |
CNS |
PNS |
YVS |
PVS |
SVS |
||
|
Control |
C |
5 ± 0a |
7 ± 0a |
10 ± 0b |
3 ± 0d |
2 ± 0d |
3 ± 0h |
|
A |
2000 I.U (T1) |
3.5 ± 1.5d |
5 ± 3.0c |
5.0 ± 1.5g |
4 ± 0c |
2.5 ± 0.5c |
4.0 ± 0 f |
|
4000 I.U (T2) |
1.5 ± 1.5h |
3 ± 1.0e |
6.5 ± 4.5f |
4 ± 2.0c |
3.0 ± 1.0b |
6.5 ± 0.5b |
|
|
6000 I. U (T3) |
3.0 ± 1.0e |
3 ± 1.0e |
7.5 ± 1.5d |
5.5 ± 3.5b |
4.0 ± 1.0a |
4.0 ± 0f |
|
|
C |
50 mg/kg (T4) |
2.5 ± 0.5f |
2.5 ± 0.5f |
5 ± 1.0g |
4 ± 1.0c |
2.5 ± 0.5c |
6.0 ± 0c |
|
100 mg/kg (T5) |
4.4 ± 0.5c |
5.5 ± 0.5b |
11.0 ± 1.0a |
4 ± 0c |
3.0 ± 0b |
5.0 ± 0d |
|
|
150 mg/kg (T6) |
2.0 ± 2.0g |
2.0 ± 1.0g |
8.0 ± 3.0c |
6 ± 1.0a |
3.0 ± 1.0b |
4.5 ± 0.5 e |
|
|
E |
100 mg/kg (T7) |
0.00 ± 0.0i |
0.00 ± 0.0h |
2.0 ± 1.0i |
2.5 ± 1.5e |
2.0 ± 0d |
6.0 ± 0c |
|
200 mg/kg (T8) |
2.5 ± 0.5f |
2.5 ± 0.5f |
4.5 ± 1.5h |
3 ± 1.0d |
2.5 ± 0.5c |
7.0 ± 1.0a |
|
|
400 mg/kg (T9) |
4.5 ± 0.5b |
4.5 ± 0.5d |
7.0 ± 1.0e |
2.5 ± 0.5e |
2.0 ± 1.0d |
3.5 ± 1.5g |
|
Data expressed as Mean ± S.E.; Mean values in the same row with different superscript differ significantly (p<0.05). One-way ANOVA was used following Duncan multiple range test in SPSS- 22.0. OG, oogonia; CNS, chromatin nucleolus stage; PNS, perinucleolus stage; YVS, yolk vesicle stage; PVS, primary vitellogenin stage; SVS, secondary vitellogenin stage.
Blom and Dabrowski (1995) recommended 8 times higher dose of ascorbic acid than the recommended level (NRC, 1993) to optimize reproduction efficiency in salmonids. In the present study, brooders fed with 150 mg/kg diet exhibited high fecundity and G.S.I values as compared to 50 and 100 mg/kg supplementation. High concentration in ovaries during maturation, reflect its requirement during steroidogenesis in the ovarian follicle cells (Hilton et al., 1979). Results of the present study are in accordance with the dietary requirements of vitamin C in other fishes such as 100-150 mg/kg for trout, 100-150 mg/kg for salmon, 30-50 mg/kg for carps and 60 mg/kg for catfish (Halver, 1972, 2002).
Ascorbic acid plays an important role in steroidogenesis (Dabrowski and Ciereszko, 2001). Vitamin C influences the aromatization of androgens to estrogens, an important step in female reproduction (Waagbo et al., 1989). In the present study, fish fed with high concentration of vitamin C has shown high progesterone and cortisol values when compared to lower inclusion levels. High progesterone values with respect to high inclusion level of vitamin C is due to the ability of ascorbic acid to synthesize this hormone from ovarian cells (Luck et al., 1995). Further high fecundity, G.S.I and hormone values corresponding to high inclusion level of vitamin C is due to high requirements of ascorbic acid in adult stage as compared to juveniles due to active gonadal growth and transfer of ascorbic acid to eggs for embryonic development (Biswas and Deb, 1970).
Breeding was observed in fishes fed with 150 mg/kg vitamin C only. It has been reported that rainbow trout fed with ascorbic acid produced eggs with high hatching rate as compared to unfed species (Komarov and Knyazeva, 1984). It has been proved that presence of ascorbic acid during maturation of fish helps in prevention of free radicals’ oxidation resulting in high larval survival rate and fecundity as recorded in rainbow trout (Blom and Dabrowski, 1995). This may be the possible reason for the breeding response in fish fed with 150 mg/kg vitamin C feed.
Effect of vitamin E on growth of L. thermalis
Among the selected doses of vitamin E, maximum weight gain (2.02 ± 0.21g), weight gain % (56.95 ± 5.93), DGR (0.13 ± 0.01) and SGR (2.98 ± 0.04) were recorded in fish fed with diet supplemented with 400 mg/kg (T9) (Table II). The results of the present study are in accordance with the findings of Mehrad et al. (2012) and James et al. (2008) which showed that increase in dietary supplementation of vitamin E in the diets of zebra fish (500 mg/kg vitamin E) and gold fish (300 mg/kg vitamin E) results in significant increment in their growth performances. These results confirm that vitamin E supplementation is very much important for L. thermalis female brooders to maintain normal growth and physiological functions because growth is defined as function of both nutritional quality and the rate of consumption (Stickney, 2000).
Effect of vitamin E on maturation of L. thermalis
Fecundity and G.S.I recorded from the fishes fed with different doses of vitamin E were in the range 3733.5 – 5125 nos/fish and 18.37 ± 4.80 – 20.68 ± 1.97, respectively and the highest values were recorded from fishes fed with 100 mg/kg (T7) vitamin E (Table III). The results of present study with respect to the inclusion level of vitamin E are in accordance with the earlier studies (Syandri, 2004; James et al., 2008; Sarowar and Mollah, 2009). An indirect relationship between dietary inclusion level of vitamin E and mean estimated fecundity and GSI values has been recorded in the present study (Table III).
Based on the histological observations, maximum maturity was recorded when fishes fed with diet incorporated with 200 mg/kg (T8) vitamin E (Table IV and Fig. 3). The mean cortisol and progesterone concentrations were recorded in the range 24.95 ± 0.13–24.97 ± 0.05 ng/ml and 2.56 ± 0.0 – 2.57 ± 0.002 ng/ml respectively (Table III).
L. thermalis brooders fed with 400 mg/kg (T9) vitamin E alone exhibited breeding response. A total of 45 spawns were collected from tanks and the average initial length of spawns recorded in the present study was 0.6-0.7 cm which was similar to the treatment containing vitamin C at 150 mg/kg (T6). Fish fed with diet supplemented with 400 mg/kg vitamin E alone exhibited breeding response. Vitamin E has shown to influence the reproduction performance of fish by acting as an important antioxidant that helps in preventing the oxidation of PUFA in cellular and subcellular membranes (NRC, 1983).
Progesterone concentration recorded from female brooders under different treatments were in the range of 2.563 ng/ml to 2.579 ng/ml and there is no significant difference among the treatments. Low level of progesterone recorded in present study is due to conversion of progesterone into metabolites such as ovarian maturation inducing steroid to initiate germinal vesicle breakdown as mentioned by Upadhyaya and Haider (1986). DeQuattro et al. (2012) reported that high levels of progesterone in fish act as endocrine disrupting chemical, thus affecting reproductive performance. In male fathead minnow’s brooders, it has been shown that exposure to 300 ng/L progesterone reduces the sperm motility (Murack et al., 2011). Wang et al. (2020) stated that exposure of fish to high progesterone concentration is associated with over expression of genes leading to disorders of endocrine system and the regulation of HPG axes-related gene expression and reported change in sex ratio, poor gonadal development and alterations in reproductive performance of crucian carp.
Cortisol levels from the ovaries of female brooders under different treatment were recorded in the range of 24.903 ng/ml to 25.083 ng/ml. High level of circulating cortisol in mature fish shows the activation of cortisol buffering capacity in gonad (Faught and Vijayan, 2018). High level of cortisol is needed during maturation stages as it is required to be taken up by the oocyte during vitellogenesis (Brooks et al., 1997) and is critical for early larval development (Hwang et al., 1992).
Cortisol concentrations has been recorded to increase and reach peak during spawning in the present study which was also reported by Carruth et al. (2000). High cortisol concentration during pre-spawning is required to mediate the inhibitory effects of stress on reproduction and give rise to possibility that cortisol buffering capacity is active at the level of the gonad, hence proper corticosteroid regulation during oogenesis is essential for ovarian development (Faught and Vijayan, 2018).
Better reproductive performance has been reported in Gold fish fed with 300 mg/kg Vitamin E (James et al., 2008). This was in accordance with the present study. High fecundity with large ova size and maximum G.S.I was reported in Cyprinus carpio fed with Vitamin E supplemented diet (Gupta et al., 1987). Poor breeding performances of fish under high level of vitamin E levels may be due to the antagonistic effect on growth and breeding performance of fish as reported by Sarowar and Mollah (2009).
Conclusion
Captive maturation and breeding of Indian spiny loach was tried successfully for the very first time. The results of the present study could be served important for similar species found globally. In the present study we can conclude that, incorporation of vitamin C and E at the rate of 150 mg/kg and 400 mg/kg diet respectively could result in the maximum breeding performance of L. thermalis in captivity. Also, it is important to note that, hormones have not played any significant roles in the captive breeding and maturation, thus we can say that use of hormone for captive breeding of similar species is not recommended.
Acknowledgment
The authors acknowledge the University authorities for the provisions made available for this study at KKPCeSA. Also, the SRF Research Fellowship provided by ICAR is acknowledged gratefully.
Funding information
The present work was carried out under ICAR-SRF fellowship.
Data availability statement
The data presented in this paper has not been shared with any other source.
Ethical statement
The experiment was conducted following the procedures of CPCSEA (Committee for the Purpose of Control and Supervision of Experiments on Animals), Ministry of Environment and Forests (Animal Welfare Division), Government of India on care and use of animals in scientific research.
IRB approval
The research committee of the University has approved the work without any objection.
Statement of conflict of interest
The authors have declared no conflict of interest.
References
Alsop, D., and Vijayan M.M., 2008. Development of the corticosteroid stress axis and receptor expression in zebrafish. Am. J. Physiol. Regul. Integr. Comp. Physiol., 294: 711-719. https://doi.org/10.1152/ajpregu.00671.2007
Barnes, M.J., and Kodicek, E., 1972. Biological hydroxylations and ascorbic acid with special regard to collagen metabolism. In: Vitamins and Hormones. Acad. Press, 30: 1-43. https://doi.org/10.1016/S0083-6729(08)60793-1
Bender, D.A., 1995. Nutritional biochemistry of the vitamins. Cambaridge University Press, New York, pp. 87–105.
Betsy, C.J., Sangavi, S., Ajith, J., Saravanan, M., and Sampath, K.J.S., 2021. Influence of antioxidants on the growth performance, gonadosomatic index and biochemical properties of gonad and fertilization success in koi carp (Cyprinus carpio L.). Aqua Res., https://doi.org/10.1111/are.15448
Biswas, N.A., Deb, C., 1970. In vitro studies on the effects of ascorbic acid and dehydroascorbic acid on Δ5-3β-hydroxysteroid dehydrogenase in toad testis. Endocrinology, 87: 170-173. https://doi.org/10.1210/endo-87-1-170
Blom, J.H., and Dabrowski, K., 1995. Dietary ascorbyl phosphate results in high ascorbic acid content in eggs of rainbow trout. Comp. Biochem. Physiol. A Physiol., 112: 75-79. https://doi.org/10.1016/0300-9629(95)00087-N
Brooks, S., Tyler, C.R., and Sumpter, J.P., 1997. Egg quality in fish: what makes a good egg? Rev. Fish Biol. Fish., 7: 387-416. https://doi.org/10.1023/A:1018400130692
Carruth, L.L., Dores, R.M., Maldonado, T.A., Norris, D.O., Ruth, T., and Jones, R.E., 2000. Elevation of plasma cortisol during the spawning migration of landlocked kokanee salmon (Oncorhynchus nerka kennerlyi). Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol., 127: 123-131. https://doi.org/10.1016/S0742-8413(00)00140-7
Dabrowski, K., and Ciereszko, A., 2001. Ascorbic acid and reproduction in fish: Endocrine regulation and gamete quality. Aquacult. Res., 32: 623-638. https://doi.org/10.1046/j.1365-2109.2001.00598.x
Darias, M.J., Mazurais, D., Koumoundouros, G., Cahu, C.L., and Zambonino-Infant, J.L., 2011. Overview of vitamin D and C requirements in fish and their influence on the skeletal system. Aquaculture, 315: 49-60. https://doi.org/10.1016/j.aquaculture.2010.12.030
DeQuattro, Z.A., Peissig, E.J., Antkiewicz, D.S., Lundgren, E.J., Hedman, C.J., Hemming, J.D., and Barry, T.P., 2012. Effects of progesterone on reproduction and embryonic development in the fathead minnow (Pimephales promelas). Environ. Toxicol. Chem., 31: 851-856. https://doi.org/10.1002/etc.1754
Faught, E., and Vijayan, M.M., 2018. Maternal stress and fish reproduction: The role of cortisol revisited. Fish Fish., 19: 1016-1030. https://doi.org/10.1111/faf.12309
Fontagné-Dicharry, S., Lataillade, E., Surget, A., Brèque, J., Zambonino-Infante, J.L., and Kaushik, S.J., 2010. Effects of dietary vitamin A on broodstock performance, egg quality, early growth and retinoid nuclear receptor expression in rainbow trout (Oncorhynchus mykiss). Aquaculture, 303: 40-49. https://doi.org/10.1016/j.aquaculture.2010.03.009
Furuita, H., Tanaka, H., Yamamoto, T., Suzuki, N., and Takeuchi, T., 2003. Supplemental effect of vitamin A in diet on the reproductive performance and egg quality of the Japanese flounder Paralichthys olivaceus (T and S). Aquacul. Res., 34: 461-468. https://doi.org/10.1046/j.1365-2109.2003.00831.x
Gupta, S.D., Khan, H.A., and Bhowmick, R.M., 1987. Observations on the effect of vitamin E and growth hormone on the gonadal maturity of carps. J. Inland Fish. Soc. India, 19: 26-31. https://doi.org/10.2331/suisan.38.79
Halver, J.E., 1972. The role of ascorbic acid in fish disease and tissue repair. Bull. Japan Soc. Sci. Fish., 38: 79-92. https://doi.org/10.2331/suisan.38.79
Halver, J.E., 2002. The vitamins. In: Fish nutrition (eds. J.E. Halver and R.W. Hardy). Acad. Press, San Diego, CA: 20: 61–141. https://doi.org/10.1016/B978-012319652-1/50003-3
Havird, J.C. and Page, L.M., 2010. A revision of Lepidocephalichthys (Teleostei: Cobitidae) with descriptions of two new species from Thailand, Laos, Vietnam and Myanmar. Copeia, 1:137-159. https://doi.org/10.1643/CI-08-240
Hernandez, L.H., and Hardy, R.W., 2020. Vitamin A functions and requirements in fish. Aquacul. Res., https://doi.org/10.1111/are.14667
Hilton, J.W., Brown, R.G., and Slinger, S.J., 1979. The half-life and uptake of 14c-1-ascorbic acid in selected organs of rainbow trout (Salmo gairdneri). Comp. Biochem. Physiol. A Physiol., 62: 427-431. https://doi.org/10.1016/0300-9629(79)90080-X
Hwang, P.P., Wu, S.M., Lin, J.H., and Wu, L.S., 1992. Cortisol content of eggs and larvae of teleosts. Gen. Comp. Endocrinol., 86: 189-196. https://doi.org/10.1016/0016-6480(92)90101-O
Ibrahim, R.E., Ahmed, S.A., Amer, S.A., Al-Gabri, N.A., Ahmed, A.I., Abdel-Warith, A.W., Younis, E.S., and Metwally, A.E., 2020. Influence of vitamin C feed supplementation on the growth, antioxidant activity, immune status, tissue histomorphology, and disease resistance in Nile tilapia, Oreochromis niloticus. Aquacult. Rep., 18: 100545. https://doi.org/10.1016/j.aqrep.2020.100545
Izquierdo, M.S., Fernandez-Palacios, H., and Tacon, A.G., 2001. Effect of broodstock nutrition on reproductive performance of fish. Aquaculture, 197: 25-42. https://doi.org/10.1016/B978-0-444-50913-0.50006-0
James, R., Vasudhevan, I., and Sampath, K., 2008. Effect of dietary vitamin E on growth, fecundity, and leukocyte count in goldfish (Carassius auratus). https://doi.org/10.46989/001c.20485.
Jayaram, K.C., 2010. The freshwater fishes of the Indian region. 2nd Edition, Narendra Publishing House, Delhi.
Komarov, I.P., and Knyazeva, L.M., 1984. Effect of vitamin C-enriched feed on the physiological state of spawning rainbow trout. Aquac. Hydr. Ichtyol., 2: 43-48.
Kottelat, M., 1992. A synopsis of the Malayan species of Lepidocephalichthys, with descriptions of two new species (Teleostei: Cobitidae). Raffles Bull. Zool., 40: 201-220.
Kraus, V.B., Huebner, J.L., Stabler, T., Flahiff, C.M., Setton, L.A., Fink, C., Vilim, V., and Clark, A.G., 2004. Ascorbic acid increases the severity of spontaneous knee osteoarthritis in a guinea pig model. J. Am. Col. Rheumatol., 50: 1822-1831. https://doi.org/10.1002/art.20291
Kumari, S.D.R., and Nair, N.B., 1978a. Length-weight relationship of the loaches Noemacheilus triangularis Day and Lepidocephalus thermalis (Cuv. and Val). Matsya, 4: 52–58.
Kumari, S.R., and Nair, N.B., 1978b. Oogenesis in a tropical loach Lepidocephalus thermalis (Cuv. and Val.). In: Proceedings of the Indian Academy of Sciences, section B. PB 8005 CVRaman avenue, Bangalore 560080, India. Indian Acad. Sci., 88: 45. https://doi.org/10.1007/BF03179623.
Luck, M.R., Jeyaseelan, I., and Scholes, R.A., 1995. Ascorbic acid and fertility. Biol. Reprod., 52: 262-266. https://doi.org/10.1095/biolreprod52.2.262
Mehrad, B., Jafaryan, H., and Taati, M.M., 2012. Assessment of the effects of dietary vitamin E on growth performance and reproduction of zebrafish, Danio rerio (Pisces, Cyprinidae). J. Ocean Mar. Sci., 3: 1-7. http://www.academicjournals.org/JOMS; https://doi.org/10.5897/JOMS11.022
Murack, P.J., Parrish, J., and Barry, T.P., 2011. Effects of progesterone on sperm motility in fathead minnow (Pimephales promelas). Aquac. Toxicol., 104: 121-125. https://doi.org/10.1016/j.aquatox.2011.04.006
National Research Council, 1993. Nutrient requirements of fish and shrimp. National Academies Press, 1993.
Riley, J.C., and Behrman, H.R., 1991. Oxygen radicals and reactive oxygen species in reproduction. Proc. Soc. exp. Biol. Med., 198: 781-791. https://doi.org/10.3181/00379727-198-43321C
Ruivo, R., Capitão, A., Castro, L.F., and Santos, M.M., 2018. The cycling gonad: retinoic-acid synthesis and degradation patterns during adult zebrafish Danio rerio oogenesis. J. Fish Biol., 92: 1051-1064. https://doi.org/10.1111/jfb.13564
Santiago, B.C., and Gonzal, A.C., 2000. Effect of prepared diet and vitamins A, E and C supplementation on the reproductive performance of cage-reared bighead carp Aristichthys nobilis (Richardson). J. appl. Ichthyol., 16: 8-13. https://doi.org/10.1046/j.1439-0426.2000.00137.x
Sarmento, N.L., Martins, E.F., Costa, D.C., Mattioli, C.C., da Costa Julio, G.S., Figueiredo, L.G., Luz, M.R, and Luz, R.K., 2018. Reproductive efficiency and egg and larvae quality of Nile tilapia fed different levels of vitamin C. Aquaculture, 482: 96-102. https://doi.org/10.1016/j.aquaculture.2017.08.035
Sarowar, M.N., and Mollah, M.F.A., 2009. Effects of dietary vitamin E on the growth and breeding performance of Ompok pabda. Bangladesh J. Fish. Res., 13: 105-114.
Soliman, A.K., Jauncey, K., and Roberts, R.J., 1986. The effect of dietary ascorbic acid supplementation on hatchability, survival rate and fry performance in Oreochromis mossambicus (Peters). Aquaculture, 59: 197-208. https://doi.org/10.1016/0044-8486(86)90004-9
Stickney, R.R., 2000. Encyclopedia of aquaculture. John Wiley and Sons, Inc. New York. pp. 960.
Stocker, A., Zimmer, S., Spycher, S.E., and Azzi, A., 1999. Identification of a novel cytosolic tocopherol-binding protein: Structure, specificity, and tissue distribution. IUBMB Life, 48: 49-55. https://doi.org/10.1080/713803478
Syandri, H., 2004. The use of vitamin E to increase the reproductive potential of garing fish (Tor douronensis). J. Dinamika Pertanian. 19: 141-151.
Toyama, G.N., Chain, J.E., and Cyrino, J.E.P., 2000. Vitamin C supplementation in diets for sexual reversal of Nile tilapia. Sci. Agricola, 57: 221-228. https://doi.org/10.1590/S0103-90162000000200005
Upadhyaya, N., and Haider, S., 1986. Germinal vesicle breakdown in oocytes of catfish, Mystus vittatus (Bloch): Relative in vitro effectiveness of estradiol-17β, androgens, corticosteroids, progesterone, and other pregnene derivatives. Gen. Comp. Endocrinol., 63: 70-76. https://doi.org/10.1016/0016-6480(86)90183-8
Waagbø, R., Thorsen, T., and Sandnes, K., 1989. Role of dietary ascorbic acid in vitellogenesis in rainbow trout (Salmo gairdneri). Aquaculture, 80: 301-314. https://doi.org/10.1016/0044-8486(89)90177-4
Wang, J., Li, B., Liu, X., Ma, J., Wang, S., and Zhang, L., 2014. Dietary vitamin A, ascorbic acid and α-tocopherol affect the gonad development and reproductive performance of starry flounder Platichthys stellatus broodstock. Chinese J. Ocean Limnol., 32: 326-333. https://doi.org/10.1007/s00343-014-3162-y
Wang, P., Sun, Q., Wan, R., Du, Q., and Xia, X., 2020. Progesterone affects the transcription of genes in the circadian rhythm signaling and hypothalamic-pituitary-gonadal axes and changes the sex ratio in crucian carp (Carassius auratus). Environ. Toxicol. Pharmacol., 77: 103378. https://doi.org/10.1016/j.etap.2020.103378
Watanabe, T., 1985. Importance of the study of broodstock nutrition for further development of aquaculture. In: Proceeding of international symposium on nutrition and feeding in fish. Academic Press. pp. 395-414.
Wilson, R.P., and Poe, W.E., 1973. Impaired collagen formation in the scorbutic channel catfish. J. Nutr., 103: 1359-1364. https://doi.org/10.1093/jn/103.9.1359
Wolf, G.E., 1984. Multiple functions of vitamin A. Physiol. Rev., 64: 873-937. https://doi.org/10.1152/physrev.1984.64.3.873
Xie, J.H., Yang, M.R. and Chen, Y.Y., 2006. The effect of vitamin C and E on the growth and reproduction of gold fish Carassius auratus red variety. J. Quanzhou Normal Univ. (Natural Science), 24: 84-90. https://doi.org/10.1007/s00343-014-3162-y
You, L., Zhao, M., Cui, C., Zhao, H., and Yang, B., 2009. Effect of degree of hydrolysis on the antioxidant activity of loach (Misgurnus anguillicaudatus) protein hydrolysates. Inn. Fd. Sci. Emerg. Technol., 10: 235-240. https://doi.org/10.1016/j.ifset.2008.08.007
You, L., Zhao, M., Regenstein, J.M., and Ren, J., 2011. In vitro antioxidant activity and in vivo anti-fatigue effect of loach (Misgurnus anguillicaudatus) peptides prepared by papain digestion. Fd. Chem., 24: 188-194. https://doi.org/10.1016/j.foodchem.2010.06.007
Zhang, Z., Bai, Q., Xu, X., and Zhang, X., 2021. Effects of the dominance hierarchy on social interactions, cortisol level, HPG-axis activities and reproductive success in the golden cuttlefish Sepia esculenta. Aquaculture, 533: 736059. https://doi.org/10.1016/j.aquaculture.2020.736059
Zhou, Q., Wang, L., Wang, H., Xie, F., and Wang, T., 2012. Effect of dietary vitamin C on the growth performance and innate immunity of juvenile cobia (Rachycentron canadum). Fish Shellf. Immunol., 2: 969-975. https://doi.org/10.1016/j.fsi.2012.01.024
To share on other social networks, click on any share button. What are these?










